Summary
In mammalian skin, Merkel cells are mechanoreceptor cells that are required for the perception of gentle touch. Recent evidence indicates that mature Merkel cells descend from the proliferative layer of skin epidermis; however, the stem cell niche for Merkel cell homeostasis has not been reported. Here, we provide the first genetic evidence for maintenance of mature Merkel cells during homeostasis by Krt17+ stem cells located in epidermal touch domes of hairy skin and in the tips of the rete ridges of glabrous skin. Lineage tracing analysis indicated that the entire pool of mature Merkel cells is turned over every 7–8 weeks in adult epidermis and that Krt17+ stem cells also maintain squamous differentiation in the touch dome and in glabrous skin. Finally, selective genetic ablation of Krt17+ touch dome keratinocytes indicates that these cells, and not mature Merkel cells, are primarily responsible for maintaining innervation of the Merkel cell-neurite complex.
Introduction
Our sense of touch enables numerous behaviors fundamental to human existence, allowing us to eat, communicate and survive (Barr and Sternberg, 1999; Bergman et al., 2000; Selden, 2004; Kaffman and Meaney, 2007; Lumpkin et al., 2010). In mammalian skin, Merkel cells are sensory mechanoreceptor cells that mediate the perception of light touch stimuli (Maricich et al., 2009). Because Merkel cells express both cytokeratins and neuroendocrine markers, their embryonic origin had been debated for more than four decades (Szeder et al., 2003; Morrison et al., 2009; Van Keymeulen et al., 2009). Initial reports using lineage-tracing analysis showed that Merkel cells were derived from Wnt1+ progenitors in the neural crest (Szeder et al., 2003); however, more recent evidence indicated that Merkel cells descend from the Krt14+ basal keratinocyte layer of the skin epithelium (Morrison et al., 2009; Van Keymeulen et al., 2009), which houses a number of epithelial stem and progenitor populations (Yan and Owens, 2008). In support of the latter studies, our laboratory previously identified a phenotypically distinct population of epidermal keratinocytes that reside along with Merkel cells in specialized skin structures termed touch domes (TDs) and are able to regenerate Merkel cells in surrogate assays (Woo et al., 2010). However, the identification and characterization of the stem cell niche for Merkel cell homeostasis as well as the kinetics of mature Merkel cell turnover have not been reported.
To address these issues, we generated a transgenic mouse model in our laboratory that selectively targets these TD keratinocytes based on their exclusive expression of cytokeratin Krt17. Using this mouse model in combination with other mutant alleles, we provide the first genetic evidence for maintenance of mature Merkel cells during homeostasis by Krt17+ keratinocytes located in epidermal TDs of hairy skin and in the tips of the rete ridges of glabrous skin. The identification of this novel stem cell niche may have important implications for age-related decline in Merkel cell numbers and Merkel cell carcinoma pathogenesis.
Results and Discussion
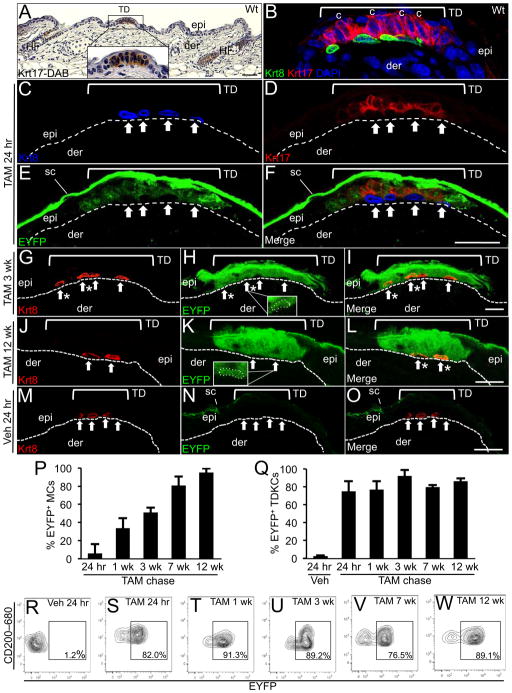
In the hairy skin of mice and humans, TDs are specialized structures in the epidermis that consist of unusual columnar keratinocytes in juxtaposition with neuroendocrine Merkel cells (Pinkus, 1902; Smith, 1977; Moll et al., 1996; Johnson et al., 2000; Johnson, 2001; Halata et al., 2003; Reinisch and Tschachler, 2005) (Figure 1A), which are identified by cytokeratin Krt8 expression (Figures 1B). We previously discovered a unique population of TD-residing keratinocytes that are able to give rise to Merkel cells in regenerative ex vivo assays (Woo et al., 2010). Microarray RNA profiling indicated that TD keratinocytes were phenotypically distinct to the remainder of interfollicular epidermal (IFE) cells (Woo et al., 2010) and identified cytokeratin Krt17 as a highly enriched transcript (8-fold) (Table S1). Here, we confirmed Krt17 as a selective marker of TD columnar keratinocytes that was not detected in Merkel cells or in the remainder of the IFE (Figures 1A, 1B, S1). In addition, Krt17 labeling tightly overlapped with expression of CD200 (Figure S2A), a previously established cell surface marker for TD keratinocytes (Woo et al., 2010). Based on these findings, we selected the Krt17 locus as a means to target TD keratinocytes and their progeny in adult epidermis. We utilized a BAC recombineering approach (Copeland et al., 2001; Liu et al., 2003) to generate a transgenic mouse model, K17CreERT2, in which a tamoxifen (TAM)-inducible Cre recombinase (CreERT2) (Feil et al., 1997) was inserted into the 3′ UTR of the Krt17 locus (Figures S2B and S2C). For validation, K17CreERT2 mice were crossed with R26REYFP reporter mice (Srinivas et al., 2001) and K17CreERT2;R26REYFP bigenic progeny were treated with TAM to induce EYFP expression (Figure S2D). We observed robust EYFP expression in TD keratinocytes in the epidermis (Figures 1D, 1E and 1F) and in Krt17+ cells in the hair follicle (HF) (Figures S3A, S3B and S3C), shortly after (24 hr) the last TAM administration. In the TD, no EYFP expression was detected in underlying Merkel cells or the surrounding IFE (Figures 1E and 1F). No EYFP was detected in TD keratinocytes from K17CreERT2;R26REYFP mice treated with vehicle (Figures 1M, 1N and 1O) rendering K17CreERT2 mice as an effective tool to selectively target Krt17-expressing skin lineages.
Fig. 1. Genetic pulse chase analysis in K17CreERT2;R26REYFP bigenic mice.
A, Wt histological mouse skin section labeled with Krt17 antibodies detected with DAB (brown). Krt17 is detected in HF outer root sheath cells and the TD (see 4.5X magnification inlay) but is absent in the remainder of the epidermis. Scale bar: 50 μm. B–O, Krt8, Krt17 and EYFP co-immunolabeling in dorsal skin sections from TAM- (B–L) or Veh-treated (M–O) K17CreERT2;R26REYFP bigenic mice. Labeling in the stratum corneum (sc) is due to non-specific binding of the GFP antibody. Arrows point out Merkel cells in each panel and asterisks designate EYFP+ Merkel cells (C–O). Hashed lines demarcate the epidermal-dermal border and brackets designate the borders of each TD with the surrounding IFE. C–F, G–I, J–L, and M–O represent the same field of view. P–Q, Bar graphs showing the average percentage of EYFP+ Merkel cells (P) or TD keratinocytes (Q) in K17CreERT2;R26REYFP skin sections following TAM or Veh treatment. Error bars show s.d. R–W, Representative CD200 (y-axis) and EYFP (x-axis) FACS contour plots showing the percentage of EYFP+ TD keratinocytes detected at 24 hr post Veh and each TAM chase time point. Abbreviations: epi, epidermis; der, dermis; HF, hair follicle; TD, touch dome; MC, Merkel cells; TDKCs, touch dome keratinocytes; c, columnar cells (panel B). Scale bar: 20 μm. See also Figures S1 and S2 and S3.
We first conducted genetic pulse chase studies in K17CreERT2;R26REYFP mice to investigate whether Krt17+ TD keratinocytes contribute to the Merkel lineage, as determined by the accumulation of EYFP+ Merkel cells over a 12-week period following TAM administration. Although few if any EYFP+ Merkel cells were detected 24 hr following the last TAM administration (Figures 1C, 1D, 1E, 1F and 1P), we observed a quantifiable increase in EYFP+ Merkel cells at 1 wk (33.6% ± 10.9) (Figure 1P) and 3 wk (50.7% ± 5.6) (Figures 1G, 1H, 1I and 1P) that continued until 7 wk post TAM at which time most Merkel cells were EYFP+ (80.7% ± 9.9) (Figure 1P). Virtually all Merkel cells remained EYFP+ (95.0% ± 4.5) at 12 wk post TAM (Figures 1J, 1K, 1L and 1P). These findings provide the first genetic evidence that the Merkel lineage descends from Krt17+ TD keratinocytes and, importantly, demonstrate that turnover of the entire mature Merkel cell pool requires approximately 7 to 8 wk in murine skin. Consistent with previous findings (Woo et al., 2010), these TD keratinocytes also appear to give rise to differentiated cells, as demonstrated by the EYFP labeling in the suprabasal layer above the TD (Figure 1I and 1L).
We then quantified the percentage of EYFP+ TD keratinocytes following TAM administration in K17CreERT2;R26REYFP mice by FACS analysis to assess the persistence of this cellular pool in the epidermis. TD keratinocytes were gated as α6 integrin+CD34−Sca-1+CD200+ epidermal cells as previously described (Woo et al., 2010) (Figures S3D, S3E and S3F), and the average percentage of EYFP+ TD keratinocytes was compared between each TAM chase time point (Figure 1Q). We observed efficient induction of EYFP+ TD keratinocytes at 24 hr post TAM (75.0% ± 11.4) (Figures 1Q and 1S) and EYFP induction in TD keratinocytes was maintained between 1 (77.0% ± 9.4) to 12 wk post TAM (92.4% ± 6.3) (Figures 1Q, 1T, 1U, 1V and 1W). No EYFP+ TD keratinocytes were detected in vehicle-treated K17CreERT2;R26REYFP mice (Figures 1Q and 1R). Interestingly, no EYFP+ cells were observed in the IFE outside of the touch dome borders at any chase time point (Figures 1E, 1H and 1K) indicating that the cellular input from TD keratinocytes is confined to the TD during homeostasis.
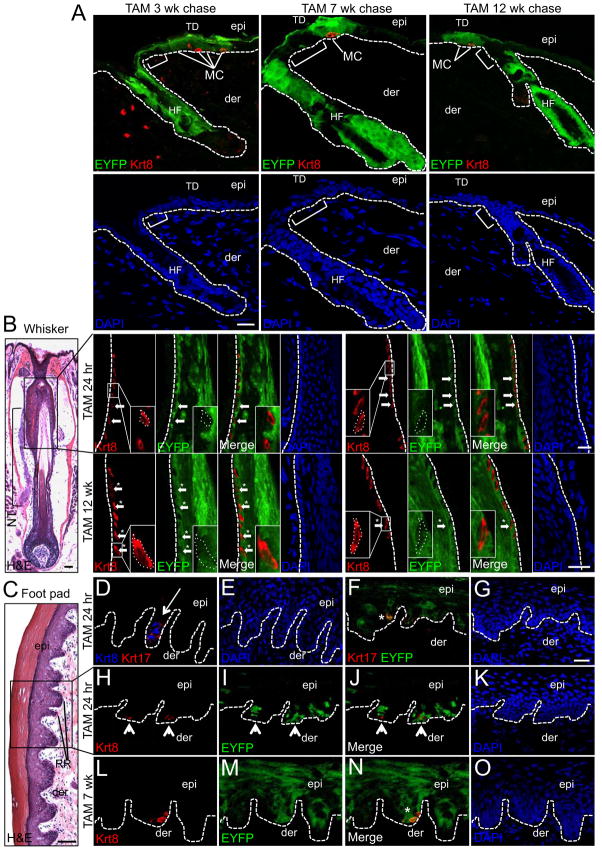
Consistent with the reported expression for Krt17 in the outer root sheath layer of the HF (Moll et al., 1982; McGowan and Coulombe, 1998), we also observed robust induction of EYFP in pelage HFs of K17CreERT2;R26REYFP mice 24 hr post TAM (Figures S3A, S3B and S3C) and EYFP expression was maintained out to 12 wk post TAM (Figure 2A). Although adult HF stem cells are not reported to contribute to epidermal homeostasis (Levy et al., 2005; Ito et al., 2005), their role in TD and Merkel cell homeostasis has not been directly addressed. We found that EYFP expression in HFs was restricted to the upper boundary of the infundibulum, and regions of the IFE between HFs and the TD were consistently devoid of EYFP at 3, 7 and 12 wk post TAM (Figure 2A), thereby excluding a role for Krt17+ HF stem cells in TD homeostasis. Collectively, these results provide the first genetic evidence that implicate Krt17+ TD keratinocytes as long-lived epidermal stem cells responsible for Merkel cell maintenance.
Fig. 2. Krt17+ keratinocytes maintain Merkel cells in pelage skin, vibrissae follicles and foot pad epidermis.
A, EYFP and Krt8 co-immunolabeling in K17CreERT2;R26REYFP dorsal skin sections at 3 wk (left), 7 wk (middle) and 12 wk (right) following TAM. Brackets designate epidermal region between the HF and TD that is EYFP-negative for each time point. DAPI-counterstained panels are provided to confirm the presence of a continuous nucleated basal layer between the HF outer root sheath and the epidermal TD. B, H&E-stained (left) or EYFP and Krt8 co-immunolabeling in whisker follicle sections from K17CreERT2;R26REYFP mice 24 hr (upper) and 12 wk (lower) post TAM. In H&E, box designates the upper permanent portion of the whisker that contains Merkel cells and is innervated by the nerve trunk (NT). In both rows (24 hr and 12 wk), the first and last 4 panels represent co-labeling of the same section derived from the left and right sides of the whisker follicle ORS, respectively. Inlays represent 3X magnification. C–O, H&E-stained (C) or co-immunolabeling with Krt8 and Krt17 (D–E); Krt17 and EYFP (F–G); or Krt8 and EYFP (H–O) in K17CreERT2;R26REYFP foot pad skin sections 24 hr (C–K) or 7 wk (L–O) post TAM. Where appropriate, arrowheads point to EYFP− and asterisks designate EYFP+ Merkel cells. Hashed lines demarcate the epidermal-dermal border (A, D–O) or the outer root sheath layer of the whisker follicle (B). Abbreviation: RR, rete ridge. Scale bars: H&E - 50 μm; Immunofluorescence - 20 μm. See also Figures S2 and S3.
Next, we examined other anatomical locations in K17CreERT2;R26REYFP mice that house Merkel cells, including the upper permanent portion of vibrissae follicles (Figure 2B) and in the epidermal rete ridges of foot pad skin (Moll et al., 1996) (Figure 2C), for the presence of additional Krt17+ niches that may contribute to Merkel cell homeostasis. In vibrissae follicles, EYFP was detected throughout the outer root sheath of the upper follicle at 24 hr post TAM; however, most Krt8+ Merkel cells in this region were EYFP− at this early chase time point (Figure 2B). However, at 12 wk post TAM, EYFP+ Merkel cells were routinely detected in the outer root sheath of vibrissae follicles (Figure 2B) confirming their derivation from Krt17+ follicular keratinocytes.
In the glabrous skin of the foot pad, Krt17 expression was observed in cells confined to the tips of epidermal rete ridges (Figures 2D and 2E) and Krt17+ cells were tightly apposed to Krt8+ Merkel cells (Figure 2D) in a similar fashion to the localization of Krt17+ stem cells and Krt8+ Merkel cells in the TD (Figure 1). We confirmed the presence of EYFP in Krt17+ keratinocytes localized to the tips of rete ridges in K17CreERT2;R26REYFP mice at 24 hr post TAM (Figures 2F and 2G). Similar to vibrissae follicles, no EYFP+ Merkel cells were detected at 24 hr post TAM in these areas (Figures 2H, 2I, 2J and 2K); however, at 7 wk post TAM EYFP+ Merkel cells were present in the tips of the rete ridges (Figures 2L, 2M, 2N and 2O). Interestingly, EYFP+ cells were also abundant in the suprabasal, postmitotic keratinocyte layers of paw epidermis indicating that Krt17+ keratinocyte stem cells in glabrous mouse skin maintain Merkel and squamous lineages during homeostasis. A better understanding of the comparative anatomical relationship between Krt17+ stem cells and Merkel cells in the TD, whisker follicle and foot pad will be the focus of future investigation.
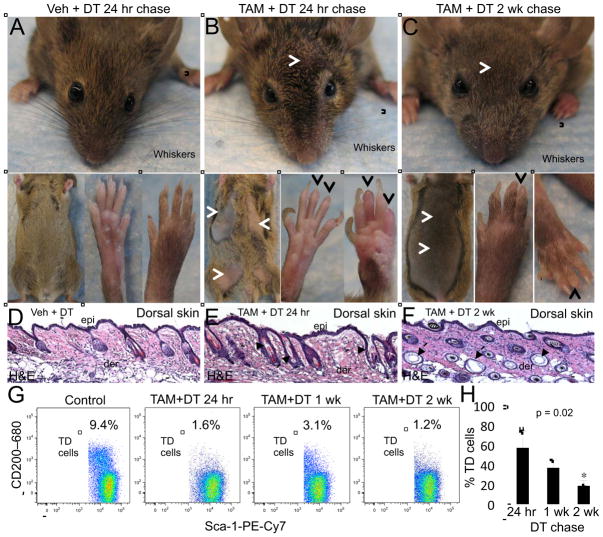
To further probe the cellular dynamics of the TD niche, we next performed selective genetic ablation of TD stem cells using K17CreERT2 mice that were crossed with R26RiDTR mutant mice, which express a Cre-inducible Diphtheria Toxin Receptor (iDTR) (Buch et al., 2005) to generate K17CreERT2;R26RiDTR bigenic mice. K17CreERT2;R26RiDTR mice were treated with TAM as described above, which was directly followed by treatment with Diphtheria Toxin A (DT) (100 ng twice daily for 7 days) as previously described (Buch et al., 2005) (Figure S4A). K17CreERT2;R26RiDTR control mice were administered oil instead of TAM followed by DT treatment. Gross inspection of K17CreERT2;R26RiDTR mice treated with TAM + DT revealed substantial, albeit incomplete, loss of pelage hairs on the ventral and dorsal skin and whisker follicles at 24 hr after the last DT administration (Figure 3B and data not shown); whereas, the hairs of control mice appeared normal (Figure 3A). We also observed a rapid loss of nails in TAM + DT mice at only 24 hr post DT (Figure 3B), a finding that supports the reported role for Krt17 in proper nail differentiation (McGowan and Coulombe, 2000). At 2 wk post DT, the loss of pelage and whisker hairs was not restored but, in fact, continued to progress (Figure 3C) indicating that bulge stem cells in these appendages were effectively targeted by DT, which was validated by FACS analysis (Figure S4B). The nails did regenerate by 2 wk post DT; however, new nails appeared abnormal with a wider and flattened shape (Figure 3C) compared to control nails (Figure 3A). The skin of TAM + DT mice appeared normal by gross inspection (Figures 3B and 3C). Histopathological analysis of TAM + DT skin showed HFs containing structural defects; however, the Krt17− epidermis was left intact (Figures 3D, 3E and 3F). These findings validate K17CreERT2;R26RiDTR mice as an effective tool to selectively ablate the Krt17-expressing lineages in the skin.
Fig. 3. Genetic depletion of TDKCs in K17CreERT2;R26RiDTR bigenic mice.
A–C, Representative gross images of whiskers, pelage (A–C) and foot pad (glabrous) skin in Veh− (A) and TAM-treated (B–C) K17CreERT2;R26RiDTR bigenic mice at 24 hr (A–B) and 2 wk (C). Brackets designate the breadth of whiskers. White arrowheads point out areas of hair thinning on the head in pelage skin (B, C). Black arrowheads indicate nail loss. D–F, Representative H&E of dorsal skin for each condition. Arrows point to perturbed HFs (E–F). Scale bar: 50 μm. G, Representative CD200 (y-axis) and Sca-1 (x-axis) FACS dot plots showing the percentage of TDKCs in the touch dome at 2 wk for control and at each TAM + DT chase time point. H, Bar graph depicting the average percentage of TD keratinocytes in TAM + DT mice at each chase time point compared to Veh + DT mice. Error bars show s.d. See also Figures S3 and S4.
Next, we examined the levels of TD stem cells in TAM + DT-treated K17CreERT2;R26RiDTR mice by FACS analysis. TD cells were gated as α6 integrin+CD34−Sca-1+CD200+ cells as described above and quantified as the percentage of the live cell population from K17CreERT2;R26RiDTR skin harvested at 24 hr, 1 and 2 wk post DT (Figure 3G). A substantial loss of the TD keratinocyte population was observed at 24 hr post DT (42.8% ± 16.5) (Figure 3H) indicating that the TD population was effectively targeted in K17CreERT2;R26RiDTR mice. The loss of TD cells was not restored but, strikingly, was further diminished at 1 wk (63.2% ± 8.2) and 2 wk (81.6% ± 5.5) post DT (Figures 3G and 3H), which is a sufficient time for epidermal regeneration and homeostasis to be reinstated following full-thickness wounding (Lau et al., 2009). These findings indicate that the loss of TD stem cells is not rescued by other niches in the IFE and suggest that TD stem cells represent an autonomous niche. To confirm this idea and exclude the possibility that the loss of TD stem cells may be recovered at later time points following DT administration, K17CreERT2;R26RiDTR mice were chased for 4 weeks following administration of higher doses of DT (Figure S4A). A 4 wk time frame is sufficient for surgically implanted dissociated TD cells to fully regenerate TDs including mature Merkel cells that are innervated by host-derived sensory afferents (Woo et al., 2010). At 4 wk following DT, the TD pool was still profoundly diminished (29.3% of control levels) compared to vehicle-treated mice (86.9% of control levels) (Figure S4C). Collectively, the restricted cellular input of TD stem cells during homeostasis (Figures 1 and 2) and the lack of recovery of TD ablation by other epidermal niches (Figure 3) suggest that the TD represents an autonomous niche that does not overlap with stem or progenitor pools in the remainder of the epidermis.
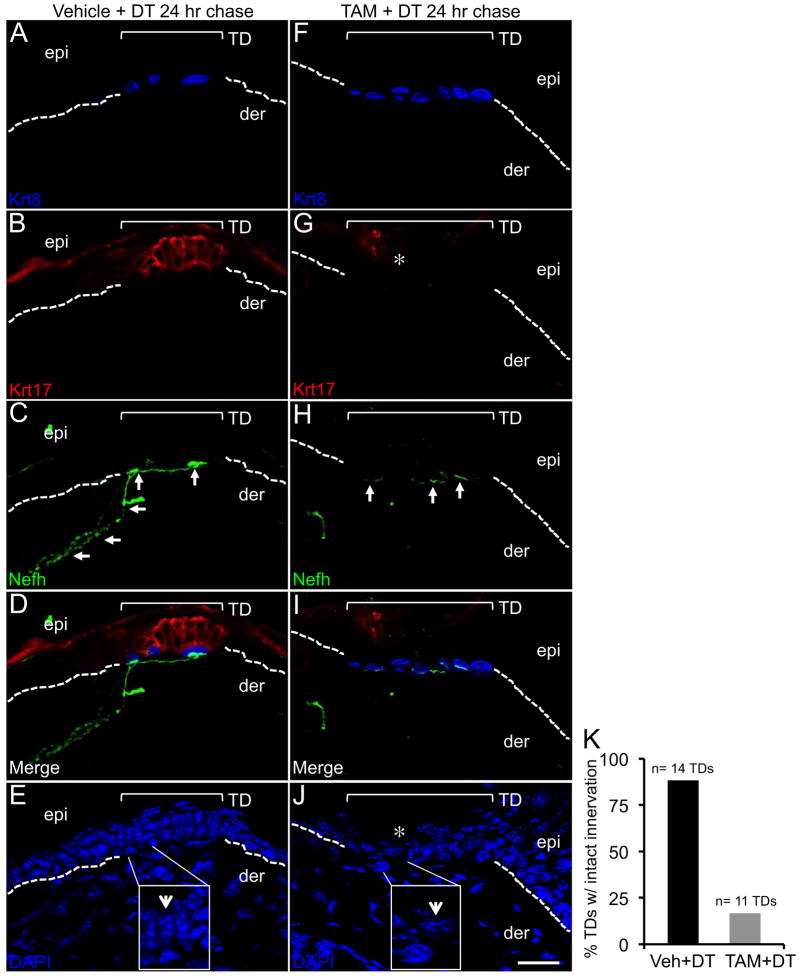
The loss of TD stem cells quantified by FACS analysis (Figures 3G and 3H) was further confirmed by immunolabeling skin sections with the TD markers Krt17 and Krt8. At 24 hr post DT, Merkel cells were still detected in Veh- (Figure 4A) and TAM-treated K17CreERT2;R26RiDTR skin (Figure 4F). However, in TAM + DT mice Krt17 labeling was absent (Figure 4G) compared to Veh + DT mice (Figure 4B). Labeling of the nuclei with DAPI confirmed that the loss of Krt17 immunolabeling was due the ablation of the columnar-shaped keratinocytes of the TD in TAM + DT mice (Figure 4J), which were still present the TDs of Veh + DT mice (Figure 4E).
Fig. 4. Ablation of TD keratinocytes leads to loss of innervation by Nefh+ sensory afferents.
A–J, Krt8, Krt17 and Nefh co-immunolabeling in thick (20 μm) Vehicle + DT (A–E) and TAM + DT treated mouse skin sections (F–J) at 24 hr post DT. Asterisks denote lost Krt17-expressing cells (G) and the loss of columnar shaped keratinocytes in the TD with DAPI staining (J) as detailed in the inlays (4X) and indicated by white arrowheads (E, J). White arrows mark normal innervation Nefh+ afferents in Veh-treated skin (C) and residual Nefh staining localized only to Merkel cells (H). Note the lack of immunoreactivity in the dermis (H–I). Brackets frame the TD area in each panel. Hashed lines demarcate the epidermal-dermal border. Scale bar: 20 μm. K, Histogram illustrating the percentages of TDs with intact innervation in Vehicle + DT versus TAM + DT treated mice. See also Figure S4.
It has been previously shown that Atoh1 conditional null mice lack Merkel cells and are not responsive to light touch stimuli (Maricich et al., 2009). Interestingly, TDs are still present in the skin of Atoh1 mutant mice and remain highly innervated by sensory neurons (Maricich et al., 2009). While these findings underscore the importance of Merkel cell neuroendocrine function in touch sensation, they suggest that other cells besides Merkel cells may regulate the innervation of epidermal TDs by sensory afferents. To address this idea, we examined TDs in thick sections of Veh + DT and TAM + DT mouse skin for any potential defects in the innervation of Merkel cells by sensory afferents as detected by Neurofilament heavy (Nefh) expression (Maricich et al., 2009, Woo et al., 2010). Intact innervation was defined as the presence of Nefh+ afferents in the dermis that extended up to and made contact with Krt8+ Merkel cells. In Veh + DT K17CreERT2;R26RiDTR skin, the majority of the TDs demonstrated intact innervation (88.2%) by Nefh+ neurons (Figures 4C and 4D) (n= 17 sections from 14 TDs examined). However, in TAM + DT K17CreERT2;R26RiDTR skin only 16.7% of the TDs demonstrated intact innervation (Figures 4H and 4I and 4K) (n = 18 sections from 11 TDs examined). In TAM + DT K17CreERT2;R26RiDTR skin, Nefh immunoreactivity could still be observed in direct proximity to Merkel cells, presumably at the synapse, but the remainder of sensory afferent fibers below the synapse were completely deteriorated (Figures 4H and 4I). Intact innervation could be observed in TDs sustaining only partial ablation of Krt17+ stem cells in TAM + DT K17CreERT2;R26RiDTR skin (data not shown); however, our findings cannot address the possibility of whether TD innervation is maintained by a subset of K17+ TD keratinocytes, which would implicate some degree of heterogeneity within the TD niche. These findings uncover a previously unexpected role for TD keratinocyte stem cells, while not serving as progenitors for dorsal root ganglion (DRG) neurons, in maintaining appropriate innervation of the TD by DRG-derived sensory afferents.
Experimental Procedures
Generation of K17CreERT2 BAC transgenic mice
An internal ribosome entry sequence (IRES) was ligated together with the CreERT2 sequence (Feil et al., 1997) and sub-cloned into the pL451 recombineering plasmid (Copeland et al., 2001; Liu et al., 2003) (NCI) to generate a modified pL451-IRES-CreERT2 vector. Insertion of an intact IRES-CreERT2 cassette was confirmed by direct sequencing (data not shown). BAC clone RP24–357E17 (CHORI) containing the keratin Krt17 locus was electroporated into SW105 cells (NCI). The IRES-CreERT2 and Frt-Neo-Frt-loxP cassettes were PCR amplified from the pL451-IRES-CreERT2 vector using oligonucleotide primers containing upstream arms homologous to the Krt17 3′ UTR (Table S3). The IRES-CreERT2-Frt-Neo-Frt-loxP amplicon was recombined into the Krt17 3′ UTR in SW105 cells (NCI) as previously described (Copeland et al., 2001; Liu et al., 2003) and the insertion site was 121 bp downstream of the Krt17 stop codon (Figure S2B). Following Flpase excision of the Neo cassette, the modified BAC DNA was extracted and appropriate insertion of the IRES-CreERT2 cassette into the Krt17 locus was confirmed in BAC DNA by PCR and direct sequencing analyses (data not shown). The Krt17-IRES-CreERT2 BAC was microinjected into fertilized oocytes and two positive founder mice (1F3, 1F4) were identified by PCR analysis (Figure S2C) using oligonucleotide primers targeting the CreERT2 sequence (Table S3). Krt17-IRES-CreERT2 BAC transgenic mice, herein referred to as K17CreERT2 mice, are viable and appear grossly normal (data not shown). All animals were housed according to Columbia University Institute of Comparative Medicine guidelines and experiments were conducted under approved IACUC protocols.
Lineage analysis
For genetic pulse chase studies, K17CreERT2 mice were crossed with R26REYFP reporter mice (Srinivas et al., 2001) (B6.129X1-Gt(ROSA)26Sortm1(EYFP)Cos/J) (Jackson Laboratories), which express EYFP following Cre excision of a loxP-flanked cassette, to generate K17CreERT2;R26REYFP bigenic mice (Figure S2D). To induce EYFP expression, K17CreERT2;R26REYFP mice received daily intraperitoneal (i.p.) injections of 2 mg of tamoxifen (TAM) (Sigma) or sunflower oil alone (vehicle) for four days. Dorsal skin specimens (n = 3 mice per time point) were harvested at the following time after TAM administration: 24 hr and 1, 3, 7 and 12 wk (Figure S2D).
In vivo genetic ablation studies
K17CreERT2 mice were crossed with R26RiDTR mice (Buch et al., 2005) (C57BL/6-Gt(ROSA)26Sortm1(HBEGF)Awai/J) (Jackson Laboratories), which express a Cre-inducible Diphtheria Toxin receptor (iDTR), to generate K17CreERT2;R26RiDTR bigenic mice. Six to seven-week old K17CreERT2;R26RiDTR mice were treated with TAM or vehicle as outlined above followed by twice daily injections of 100 ng of Diphtheria toxin (DT) (Sigma) for 7 days (Buch et al., 2005) (Figure S4A). In some cases, mice received twice daily injections of 200 ng DT for 4 days followed by once daily injections of 1 μg DT for 3 days (Figure S4A). Dorsal skin specimens were harvested for control (Veh + DT) and experimental (TAM + DT) K17CreERT2;R26RiDTR mice at 24 hr, 1 and 2 wk or 4 wk following the last DT injection.
Tissue harvesting and histology
Belly and dorsal mouse skin, whisker and foot pad specimens were surgically excised from wild-type, K17CreERT2;R26REYFP and K17CreERT2;R26RiDTR mice between 8 and 13 weeks of age. All harvested tissues were fixed in 4% paraformaldehyde (PFA), processed in 30% sucrose and embedded in O.C.T. medium and cryopreserved. In some cases, dorsal skin sections were harvested from wild-type adult mice, fixed in formalin and paraffin embedded. For histopathological analysis, 7 μm sections were stained with hematoxylin and eosin and bright field images were captured using a Zeiss Axiophot 2 microscope.
Tissue immunolabeling
For immunofluorescence labeling in dorsal skin sections, thin (7 μm) histological sections from K17CreERT2;R26REYFP, K17CreERT2;R26RiDTR or wild-type mice were stained with the following primary antibodies overnight at 4°C: cytokeratin Krt8 (Troma-I; Developmental Studies Hybridoma Bank), GFP-FITC (600–102–215; Rockland Immunochemicals) and cytokeratin Krt17 (EP1623; Epitomics) with the exception that thick (20 μm) histological skin sections were utilized for Neurofilament-heavy (Nefh) (NFH; Aves Labs) antibody labeling. Thick (20 μm) histological sections were utilized for Krt8, Krt17 and EYFP immunolabeling in whisker follicle and foot pad labeling. Species-specific Alexa Fluor 488−, 546− and 680-conjugated secondary antibodies (Life Technologies) were used to detect primary antibody labeling. Confocal stacks were captured using a Zeiss LSM 5 Exciter confocal microscope and reconstructed using NIH ImageJ software. For genetic pulse chase studies, the total of Merkel cells and EYFP+ Merkel cells were counted in reconstructed images (Table S2). For immunohistochemistry, 7 μm sections of paraffin-embedded dorsal skin from wild-type mice were probed with Krt17 antibodies and detected using a VectaStain ABC kit (Vector Labs) using DAB as a chromagen as previously described (Bachelor et al., 2011).
Flow cytometry
Primary adult epidermal keratinocytes were isolated from the dorsal skin of K17CreERT2;R26REYFP, K17CreERT2;R26RiDTR and wild-type mice as previously described (Jensen et al., 2008; Woo et al., 2010). Single cell suspensions of epidermal keratinocytes were labeled with antibodies to the following surface proteins: α6 integrin (PE-conjugated GoH3 clone), CD34 (APC-conjugated RAM34 clone), Sca-1 (PE-Cy7-conjugated D7 clone) and CD200 (OX-90 clone) (BD Biosciences) detected with anti-Rat Alexa Fluor 680 antibodies (Life Technologies). Cells were stained with 4′,6-diaminido-2-phenylindole (DAPI) and subjected to FACS analysis using a LSRII Cell Analyzer and FACSDiva version 6.1.1 software (BD Biosciences). After eliminating DAPI-positive dead cells, FITC, PE, APC, PE-Cy7 and Alexa 680 signals were collected through 530/30, 575/26, 660/20 nm, 780/60 nm and 710/20 nm band pass filters, respectively. Touch dome keratinocytes (TDKCs) were gated as the α6+CD34−Sca-1+CD200+ population as previously described (Woo et al., 2010) (Figures S3D, S3E and S3F). The percentage of TDKCs that were EYFP+ or EYFP− (K17CreERT2;R26REYFP mice) or the total percentage of TDKCs (K17CreERT2;R26RiDTR) were calculated using FlowJo software (Tree Star).
Supplementary Material
Highlights.
Epidermal Merkel cells are genetic descendants of Krt17+ touch dome stem cells
Touch dome Merkel cells are turned over every two months in adult skin
The touch dome is an autonomous stem cell niche
Touch dome stem cells also play a role in skin innervation by sensory afferents
Acknowledgments
Pierre Chambon (Institut de Génétique et de Biologie Moléculaire et Cellulaire, Illkirch-Graffenstaden, France) provided the pCreERT2 expressing vector. K17CreERT2 BAC transgenic mice were generated by microinjection at the Columbia University Transgenic Mouse Core Facility. FACS experiments were conducted at the Columbia University Flow Cytometry Core Facility. Histological services were provided by Yan Lu at the Department of Dermatology Skin Diseases Research Center (NIH grant AR044535). This work was supported by NIH grant ES020060 (D.M.O.).
Footnotes
Publisher's Disclaimer: This is a PDF le of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its nal citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bachelor MA, Lu Y, Owens DM. L-3-Phosphoserine Phosphatase (PSPH) regulates cutaneous squamous cell carcinoma proliferation independent of L-serine biosynthesis. J Dermatol Sci. 2011;63:164–172. doi: 10.1016/j.jdermsci.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr MM, Sternberg PW. A polycystic kidney-disease gene homologue required for male mating behaviour in C. elegans. Nature. 1999;401:386–389. doi: 10.1038/43913. [DOI] [PubMed] [Google Scholar]
- Bergman E, Ulfhake B, Fundin BT. Regulation of NGF-family ligands and receptors in adulthood and senescence: correlation to degenerative and regenerative changes in cutaneous innervation. Eur J Neurosci. 2000;12:2694–2706. doi: 10.1046/j.1460-9568.2000.00149.x. [DOI] [PubMed] [Google Scholar]
- Buch T, et al. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat Methods. 2005;2:419–426. doi: 10.1038/nmeth762. [DOI] [PubMed] [Google Scholar]
- Copeland NG, Jenkins NA, Court DL. Court Recombineering: a powerful new tool for mouse functional genomics. Nat Rev Genetics. 2001;2:769–779. doi: 10.1038/35093556. [DOI] [PubMed] [Google Scholar]
- Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Comm. 1997;237:752–757. doi: 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- Halata Z, Grim M, Bauman KI. Friedrich Sigmund Merkel and his “Merkel cell”, morphology, development, and physiology: review and new results. Anat Rec A Discov Mol Cell Evol Biol. 2003;271:225–239. doi: 10.1002/ar.a.10029. [DOI] [PubMed] [Google Scholar]
- Ito M, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- Jensen UB, et al. A distinct population of clonogenic and multipotent murine follicular keratinocytes residing in the upper isthmus. J Cell Sci. 2008;121:609–617. doi: 10.1242/jcs.025502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KO, Yoshioka T, Vega-Bermudez F. Tactile functions of mechanoreceptive afferents innervating the hand. J Clin Neurophysiol. 2000;17:539–558. doi: 10.1097/00004691-200011000-00002. [DOI] [PubMed] [Google Scholar]
- Johnson KO. The roles and functions of cutaneous mechanoreceptors. Curr Opin Neurobiol. 2001;11:455–461. doi: 10.1016/s0959-4388(00)00234-8. [DOI] [PubMed] [Google Scholar]
- Kaffman A, Meaney MJ. Neurodevelopmental sequelae of postnatal maternal care in rodents: clinical and research implications of molecular insights. J Child Psychol Psychiatry. 2007;48:224–244. doi: 10.1111/j.1469-7610.2007.01730.x. [DOI] [PubMed] [Google Scholar]
- Lau K, et al. Exploring the role of stem cell in cutaneous wound healing. Exp Dermatol. 2009;11:921–933. doi: 10.1111/j.1600-0625.2009.00942.x. [DOI] [PubMed] [Google Scholar]
- Levy V, Lindon C, Harfe BD, Morgan BA. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev Cell. 2005;9:855–861. doi: 10.1016/j.devcel.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Liu P, Jenkins NA, Copeland NG. A highly efficient recombineering-based method for generating conditional knockout mutation. Genome Res. 2003;13:476–484. doi: 10.1101/gr.749203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumpkin EA, Marshall KL, Nelson AM. The cell biology of touch. J Cell Biol. 2010;191:237–248. doi: 10.1083/jcb.201006074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maricich SM, et al. Merkel cells are essential for light touch responses. Science. 2009;324:1580–1582. doi: 10.1126/science.1172890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan KM, Coulombe PA. Onset of keratin 17 expression coincides with the definition of major epithelial lineages during skin development. J Cell Biol. 1998;143:469–486. doi: 10.1083/jcb.143.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan KM, Coulombe PA. Keratin 17 expression in the hard epithelial context of the hair and nail, and its relevance for the Pachyonychia Congenita phenotype. J Invest Dermatol. 2000;114:1101–1107. doi: 10.1046/j.1523-1747.2000.00986.x. [DOI] [PubMed] [Google Scholar]
- Moll I, Paus R, Moll R. Merkel cells in mouse skin: intermediate filament pattern, localization, and hair cycle-dependent density. J Invest Dermatol. 1996;106:281–286. doi: 10.1111/1523-1747.ep12340714. [DOI] [PubMed] [Google Scholar]
- Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Morrison KM, Miesegaes GR, Lumpkin EA, Maricich SM. Merkel cells are descended from the epidermal lineage. Dev Biol. 2009;336:76–83. doi: 10.1016/j.ydbio.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkus F. Uber einen bisher unbekannten Nebenapparat am Haarsystem des Menschen: Haarscheiben. Dermatologische Zeitschrift. 1902;9:465–499. [Google Scholar]
- Reinisch CM, Tschachler E. The touch dome in human skin is supplied by different types of nerve fibers. Ann Neurol. 2005;58:88–95. doi: 10.1002/ana.20527. [DOI] [PubMed] [Google Scholar]
- Selden ST. Tickle. J Am Acad Dermatol. 2004;50:93–97. doi: 10.1016/s0190-9622(03)02737-3. [DOI] [PubMed] [Google Scholar]
- Smith KR. The Haarscheibe. J Invest Dermatol. 1977;69:68–74. doi: 10.1111/1523-1747.ep12497883. [DOI] [PubMed] [Google Scholar]
- Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeder V, Grim M, Halata Z, Sieber-Blum M. Neural crest origin of mammalian Merkel cells. Dev Biol. 2003;253:258–263. doi: 10.1016/s0012-1606(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Van Keymeulen A, et al. Epidermal progenitors give rise to Merkel cells during embryonic development and adult homeostasis. J Cell Biol. 2009;187:91–100. doi: 10.1083/jcb.200907080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SH, Stumpfova M, Jensen UB, Lumpkin EA, Owens DM. Identification of epidermal progenitors for the Merkel cell lineage. Development. 2010;137:3965–3971. doi: 10.1242/dev.055970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Owens DM. The skin: a home to multiple classes of epithelial progenitor cells. Stem Cell Rev. 2008;4:113. doi: 10.1007/s12015-008-9022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.