Letter to the Editor
Glucagon-like peptide-1 (GLP-1) is released in response to food intake and acts through both peripheral and central mechanisms to regulate energy homeostasis and feeding behavior. Exendin-4 (Ex-4) is an analog of GLP-1 with a significantly longer half-life and greater potency (1) and is marketed synthetically as exenatide (Byetta™ and Bydureon™) as a treatment for type 2 diabetes mellitus. We show that administration of exendin-4 attenuates the rewarding effects of cocaine in mice, thus highlighting the therapeutic potential of GLP-1 receptor (GLP-1R) agonists for the treatment of psychostimulant addiction.
Emerging data suggest that hormones and peptides that play a role in feeding behavior, such as insulin, GLP-1, leptin, orexin, and ghrelin, may also be involved in drug reward (2, 3) and could thereby be targeted as therapies for the treatment of addiction. GLP1-Rs are expressed within the brain, including in the ventral tegmental area (VTA) and the nucleus accumbens (NAc) (4, 5). These brain regions are part of the mesolimbic reward circuit considered to play a major role in the rewarding properties of drugs, including the cocaine (6). Activation of mesolimbic GLP-1Rs decreases the intake of highly palatable foods, suggesting that GLP-1R activation contributes to the hedonic components of food intake (4). Drug reward and feeding behavior utilize overlapping brain circuitry and mechanisms, thus we hypothesized that the GLP-1R agonist Ex-4 would reduce the rewarding effects of cocaine.
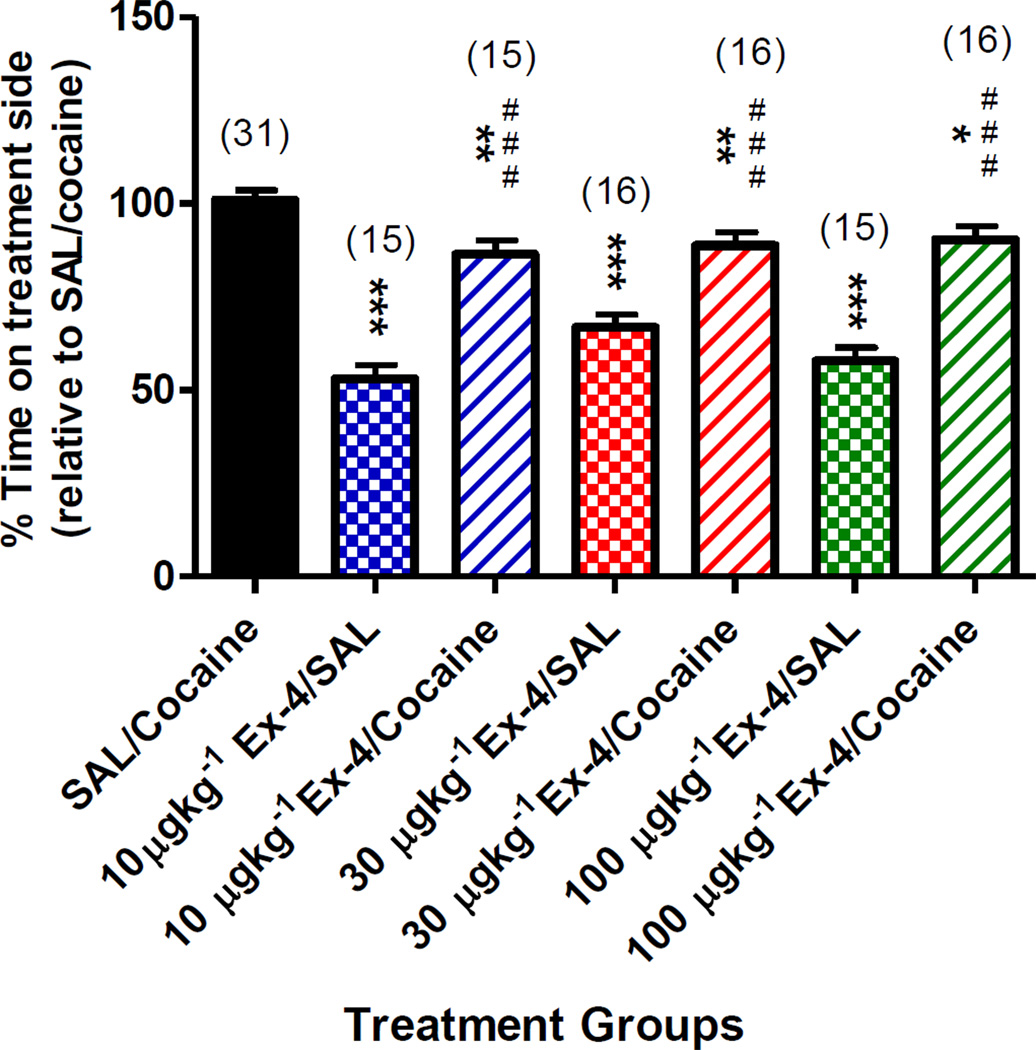
In a test of conditioned place preference (CPP), a significant cocaine-induced CPP was achieved (SAL/Cocaine group, Fig. 1). However, the rewarding effects of cocaine were attenuated in mice pretreated with Ex-4 (Ex-4/Cocaine group, regardless of pretreatment dose), suggesting that Ex-4 reduces the hedonic effects of cocaine. This response was not influenced by Ex-4-induced hypoactivity, as Ex-4 pretreatment did not significantly lessen cocaine’s locomotor-stimulating properties (Supplementary Fig. 1C). As hypothesized, Ex-4-pretreated mice spent more time in the treatment chamber during the test phase following cocaine treatment but not to the same extent as cocaine-treated mice without the Ex-4 pretreatment, and this finding was not contingent upon the Ex-4 dose used. Notably, Ex-4 treatment alone at any of the doses investigated did not condition an aversion (or a preference) to the treatment-associated chamber as there was no significant change in the amount or percentage of time spent in the treatment chamber (Fig. 1, all Ex-4/SAL groups).
Figure 1.
Percent time spent on the treatment-affiliated compartment during the CPP test phase. A main effect of Treatment was found (F(6,117)=31.66, p<0.0001). Data are normalized against the SAL/cocaine group. Sample size for each group is indicated in parentheses above each bar. **p < 0.01, ***p < 0.001 vs. SAL/cocaine group; ###p < 0.001 vs. respective Ex-4/cocaine-treated group.
How Ex-4 exerts this decrease in cocaine reward is unknown. Ex-4 readily crosses the blood brain barrier (7), GLP-1Rs are present in brain reward circuitry (4, 5), and local administration of Ex-4 into the mesolimbic dopamine system alters hedonic responses to food (4). It is thus likely that Ex-4 blunts cocaine CPP by targeting GLP-1Rs within the NAc or ventral midbrain. A previous study has shown that GLP-1Rs can specifically interact with the G-protein coupled receptor sorting protein (GASP-1) (8), and genetic knockout loss of GASP-1, in turn, alters cocaine-induced behavioral responses (9). Therefore, GLP-1Rs in the hypothalamus may mediate homeostatic regulation of food intake (10), whereas GLP-1Rs in the VTA and NAc might instead represent hedonic value, perhaps through alterations in dopamine transporter and/or receptor function (9, 11). However, other mechanisms, including a role of peripheral GLP-1Rs, cannot be ruled out at this time.
Our study extends the potential therapeutic value of GLP-1R stimulation beyond metabolic disorders and identifies a new molecular target for the treatment of psychostimulant abuse. Moreover, even the lowest dose of Ex-4 used (10 µg/kg) produced a blunted cocaine CPP response, suggesting a GLP-1R-dependent mechanism involved in drug reward in addition to those already known involving neurotransmitters (e.g., dopamine, serotonin, norepinephrine, glutamate, and the opioids). Importantly, these data and that of others (5) indicate that Ex-4 treatment alone appears to be neither aversive nor pleasurable, suggesting that patient compliance due to negative side-effects or the potential for addiction to Ex-4, respectively, is of little concern. Ex-4, marketed in its synthetic form as exenatide, is already approved for human use as a treatment for type 2 diabetes. Whether these findings extend to other psychostimulants (e.g. amphetamines) and other drug classes has yet to be determined. Additional studies will also be required to examine full doseresponse functions, as it appears that even the 10 µg/kg dose was sufficient to attenuate cocaine-induced CPP; the duration of effectiveness; and the blockade or reversal of the cellular and molecular neuroadaptations that accompany chronic drug use and addiction. Self-administration studies will also be needed to fully explore the ability of GLP-1 signaling to regulate addictive processes. Finally, it is also worth noting that, although effective, even the highest dose of Ex-4 (100 µg/kg) did not completely eliminate cocaine-induced CPP. Thus our data also demonstrate the presence of a GLP-1 receptor-independent component to cocaine reward, suggesting exenatide treatment as a possible complementary therapy of drug abuse.
Supplementary Material
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Young AA, Gedulin BR, Bhavsar S, Bodkin N, Jodka C, Hansen B, et al. Diabetes. 1999 May;48(5):1026–1034. doi: 10.2337/diabetes.48.5.1026. [DOI] [PubMed] [Google Scholar]
- 2.Kenny PJ. Nat Rev Neurosci. 2011 Nov;12(11):638–651. doi: 10.1038/nrn3105. [DOI] [PubMed] [Google Scholar]
- 3.Erreger K, Davis AR, Poe AM, Greig NH, Stanwood GD, Galli A. Physiol Behav. 2012 Jun 25;106(4):574–578. doi: 10.1016/j.physbeh.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dossat AM, Lilly N, Kay K, Williams DL. J Neurosci. 2011 Oct 12;31(41):14453–14457. doi: 10.1523/JNEUROSCI.3262-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alhadeff AL, Rupprecht LE, Hayes MR. Endocrinology. 2012 Feb;153(2):647–658. doi: 10.1210/en.2011-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koob GF, Volkow ND. Neuropsychopharmacology. 2010 Jan;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. Endocrinology. 2011 Aug;152(8):3103–3112. doi: 10.1210/en.2011-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heydorn A, Sondergaard BP, Ersboll B, Holst B, Nielsen FC, Haft CR, et al. J Biol Chem. 2004 Dec 24;279(52):54291–54303. doi: 10.1074/jbc.M406169200. [DOI] [PubMed] [Google Scholar]
- 9.Thompson D, Martini L, Whistler JL. PLoS One. 2010;5(6):e11038. doi: 10.1371/journal.pone.0011038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, et al. Nature. 1996 Jan 4;379(6560):69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 11.Owens WA, Sevak RJ, Galici R, Chang X, Javors MA, Galli A, et al. J Neurochem. 2005 Sep;94(5):1402–1410. doi: 10.1111/j.1471-4159.2005.03289.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.