Abstract
Aim of the study
We investigated the effects of ischemic postconditioning (IPC) with and without cardioprotective vasodilatory therapy (CVT) at the initiation of cardiopulmonary resuscitation (CPR) on cardio-cerebral function and 48-hour survival.
Methods
Prospective randomized animal study. Following 15 minutes of ventricular fibrillation, 42 Yorkshire farm pigs weighing an average of 34±2 kg were randomized to receive standard CPR (SCPR, n=12), SCPR+IPC (n=10), SCPR+IPC+CVT (n=10), or SCPR+CVT (n=10). IPC was delivered during the first 3 minutes of CPR with 4 cycles of 20 seconds of chest compressions followed by 20-second pauses. CVT consisted of intravenous sodium nitroprusside (2 mg) and adenosine (24 mg) during the first minute of CPR. Epinephrine was given in all groups per standard protocol. A transthoracic echocardiogram was obtained on all survivors 1 and 4 hours post-ROSC. The brains were extracted after euthanasia at least 24 hours later to assess ischemic injury in 7 regions. Ischemic injury was graded on a 0–4 scale with (0=no injury to 4= >50% neural injury). The sum of the regional scores was reported as cerebral histological score (CHS). 48 hours survival was reported.
Results
Post-resuscitation left ventricular ejection (LVEF) fraction improved in SCPR+CVT, SCPR+IPC+CVT and SCPR+IPC groups compared to SCPR (59%±9%, 52%±14%, 52%±14% vs. 35%±11%, respectively, p<0.05). Only SCPR+IPC and SCPR+IPC+CVT, but not SCPR+CVT, had lower mean CHS compared to SCPR (5.8±2.6, 2.8±1.8 vs. 10±2.1, respectively, p<0.01). The 48-hour survival among SCPR+IPC, SCPR+CVT, SCPR+IPC+CVT and SCPR was 6/10, 3/10, 5/10 and 1/12, respectively (Cox regression p<0.01).
Conclusions
IPC and CVT during standard CPR improved post-resuscitation LVEF but only IPC was independently neuroprotective and improved 48-hour survival after 15min of untreated cardiac arrest in pigs.
Introduction
An estimated 350,000 patients suffer from out-of-hospital cardiac arrest (OHCA) each year in the United States.1 Even with the best clinically documented methods of cardiopulmonary resuscitation (CPR), more than 85–90% of OHCA patients die or have severe neurological deficits.2 Cerebral and cardiac dysfunction following successful resuscitation from cardiac arrest is the major cause of death and long-term morbidity.3, 4
We recently have shown that after 15 minutes of untreated cardiac arrest due to ventricular fibrillation, ischemic postconditioning (IPC) at the initiation of standard CPR, can improve neurological intact survival.5 IPC with four, 20 seconds pauses during the first 3 minutes of CPR have been shown to be synergistic with sodium nitroprusside “enhanced” CPR (SNPeCPR) which utilizes active compression and decompression CPR with an inspiratory impedance threshold device and abdominal binding.6
In this investigation we try to build upon our previous published studies and evaluate the effects of cardioprotective vasodilator therapy (CVT) alone and in combination with IPC in a model of standard CPR (SCPR). We sought to provide evidence of reduced global reperfusion injury after prolonged ischemia with histological and biomarker based endpoints in addition to the clinical endpoints.
We hypothesized that by using a simple CPR strategy designed to control the initial reintroduction of blood flow during Basic Life Support (BLS), we could protect vital organs from injury and substantially improve outcomes after 15 minutes of untreated ventricular fibrillation. Further, we hypothesized that the addition of cardioprotective vasodilatory agents would act synergistically with IPC.
Materials and Methods
All studies were performed on Yorkshire farm pigs weighing an average of 34±2 kg. A certified and licensed veterinarian provided a blinded neurologic assessment at 24 and 48 hours. The Institutional Animal Care Committee of the Minneapolis Medical Research Foundation approved the protocol (number 11-05, approved on 5/10/2011). 7
Preparatory phase
The anesthesia, surgical preparation, data monitoring, and recording procedures used in this study have been described previously in detail and the study protocol was used unaltered from Segal et al.5 After endotracheal intubation, inhaled isoflurane at a dose of 0.8% to 1.2% was used for anesthesia up until ventricular fibrillation (VF) induction. Anesthesia was restarted after return of spontaneous circulation (ROSC). The animal’s bladder temperature was maintained at 37.5±0.5°C with a warming blanket (Bair Hugger, Augustine Medical, Eden Prairie, MN). Central aortic and right atrial pressures were recorded continuously with micromanometer-tipped catheters (Mikro-Tip Transducer, Millar Instruments, Houston, TX). The left internal carotid artery was surgically exposed and an ultrasound flow probe (Transonic 420 series multichannel, Transonic Systems, Ithaca, NY) placed to quantify blood flow (mL/min). Compression force, rate and depth, were continuously recorded throughout all experiments and controlled during CPR to assure all groups received identical CPR quality.
Experimental protocol
After the surgical preparation was complete, oxygen saturation on room air was >95%, and ETCO2 was stable between 35 and 42 mmHg for 5 minutes, VF was induced by delivering direct intracardiac current. Standard chest compression cardiopulmonary resuscitation was performed with a pneumatically driven automatic piston device (Pneumatic Compression Controller, Ambu International, Glostrup, Denmark) as previously described.8 During SCPR, we delivered uninterrupted chest compressions at a rate of 100 compressions/min, with a 50% duty cycle and a compression depth of 25% of the anteroposterior chest diameter. Asynchronous positive-pressure ventilations were delivered with room air (FIO2 of 0.21) with a manual resuscitator bag. The tidal volume was maintained at ~10 mL/kg and the respiratory rate was 10 breaths/min. The investigators were blinded to hemodynamics during CPR. If ROSC was not achieved, defibrillation was delivered every 2 minutes thereafter during CPR. Resuscitation efforts were continued until ROSC was achieved or for a total of 15 minutes.
Protocol
We tested two interventions in this study, independently, and in combination during SCPR. These interventions included, a) IPC, that was delivered with four cycles of 20-second pauses for the first 3 minutes of the resuscitation effort 5 and b) administration of cardioprotective vasodilator therapy (CVT). CVT consisted of sodium nitroprusside (SNP) and adenosine. SNP was given as a 2mg bolus at minute 1 and a second 1mg bolus at minute 3 of CPR.6 Adenosine was given as a single 24mg bolus after the first SNP bolus (after preliminary studies demonstrated superiority of this dose in improving post-resuscitation left ventricular (LV) dysfunction.9 Epinephrine was administered in all groups in a 0.5mg (~15 μg/kg) bolus at minute 4 of CPR, 60 seconds before first defibrillation.
Following 15 minutes of untreated VF, 42 pigs were randomized prospectively using a computer-generated program into four groups:
SCPR group as controls: Received only SCPR and epinephrine (12 animals).
IPC group: Received four 20-second pauses during the first 3 minutes of SCPR.
CVT group: Received SNP and adenosine as described above while performing SCPR.
IPC+CVT group: Received both four 20-second pauses of CPR (IPC) and CVT, as described above. Groups II–IV had 10 animals each.
Post-ROSC Care
The protocol for post resuscitation care has been described in detail by Segal et al.5 Supplemental oxygen was added only if arterial saturation was lower than 90%. Animals were observed under general anesthesia with isoflurane until hemodynamically stable. Hemodynamic stability was defined as a mean aortic pressure >55mmHg without pharmacologic support for 10 minutes as well as normalization of ETCO2 and acidosis. Animals that were hypotensive post-ROSC received increments of 0.1 mg intravenous epinephrine every 5 minutes until mean arterial pressure rose above 50mmHg. If pH was lower than 7.20, 50–100mEq of NaHCO3 were given intravenously.
All groups received post-resuscitation therapeutic hypothermia as recommended by the American Heart Association for comatose patients resuscitated from VF to simulate best practice and optimize the chances of the control group for neurological recovery.10 Target temperature was set at 34°C and was maintained at that level with the use of a cutaneous cooling device (Arctic Sun, Medivance Inc., Louisville, CO). Central temperature was measured at the bladder of the animals. Total hypothermic time was 12 hours.10
Survivors were given intramuscular injections of nonsteroidal analgesics.11 Animals were returned to their runs and were observed every 2 hours for the first 6 hours for signs of distress or accelerated deterioration of their function If animals met predetermined criteria of an adverse outcome such as status epilepticus, severe cardio-respiratory distress or deep coma after 24 hours based on the judgment of a veterinarian, blinded to the intervention, they were euthanized per protocol.
Neurologic assessment
Twenty-four and 48 hours after ROSC, a certified veterinarian, blinded to the intervention, assessed the pigs’ neurologic function based on a modified cerebral performance category (CPC) scoring system for pigs. That has been described in detail by our group.5 The following scoring system was used: 1 = normal; 2 = slightly disabled; 3 = severely disabled but conscious; 4 = vegetative state; and 5 was given to animals that died in the lab due to unachievable ROSC or died in the run following ROSC.
Echocardiographic evaluation of the left ventricle
A transthoracic echocardiogram was obtained on all survivors 1 and 4 hours post-ROSC. Parasternal long and short axis views were obtained at each time point. Ejection fraction (EF) was assessed by visual estimation from two independent clinical echocardiographers blinded to the treatments and if there was more than 10 % difference between the two readings, the EF was calculated by using Simpson’s method of volumetric analysis.12
Neurohistology
Animals that were found dead without direct observation were excluded from histological analysis due to unknown time of death. At 48 hours (or if the veterinarian decided that the animals needed to be sacrificed after the 24 hour evaluation) animals underwent sedation, were intubated, and anesthetized as described previously. Surgical exposure and cannulation of both common carotid arteries were performed under full anesthesia protocol.
An IV bolus of heparin (3000 units) was administered. Animals were then sacrificed by an injection of 10 mEq potassium chloride via direct cardiac stick. Immediately upon sacrifice, normal saline was infused through the carotid sheaths via a peristaltic pump (Cole Parmer, Vernon Hills, IL). The head was removed from the body, and 10% buffered formalin pumped through the carotid sheaths. The brain was allowed to fix for 1–3 hours prior to removal and then the whole brain was removed and placed in 10% buffered formalin solution.
After fixation with formalin, brains were stained with hematoxilin and eosin (H&E). Assessment was modeled after a previously published method.13.
Each pig brain was sectioned coronally into 5 slices (excluding brainstem and cerebellum) with the first cut at the level of the mammillary bodies for consistency. Within the 5 brain slices, sections sampled included; 1) Frontal cortex, including watershed zones and underlying structures at the level of the head of the caudate. 2) Deep gray nuclei (caudate putamen and globus pallidus), temporal cortex and hippocampus at the level of the mammillary bodies. 3) Hippocampus and temporal cortex at the level of thalamus (including thalamus), 4) midbrain, 5) pons, 6) medulla and 7) cerebellum (1 cm from the midline including watershed zones). When regions were represented at more than one level, scoring was based on the section where damage was more extensive and notations made if significant differences are noted at two levels (i.e. head and body of caudate or anterior and posterior hippocampus). Light microscopic evaluations were made using an Olympus microscope. A board certified human neuropathologist blinded to the group treatments performed the histological evaluations at the core laboratories of the University of Minnesota.
We used a semi-quantitative scale for ischemic injury. Seven specific brain regions were graded on a 0–4 scale (0: no injury, 1 to 4, mild to severe). A score of 0 indicated that less than 1% ischemic neurons were seen in the region, 1 (minimal) was used if fewer than 10% of neurons appeared ischemic, 2 (mild) was used if 10–25% of neurons were ischemic, 3 (moderate) was used for 26–50% ischemia, and 4 (marked) for more than 50% ischemic neurons. The sum of categorical scores for regional prevalence throughout the brain describes the overall prevalence of ischemic neurons and is described here as total Cerebral Histological Score (CHS)
Cardiac biomarkers
A 3-ml sample of arterial blood was obtained from all survivors 4 hours after ROSC. Cardiac-specific troponin-I and creatinine phosphokinase-MB (CK-MB) were quantified via a two-site sandwich assay (Stratus CS Acute Care, Siemens, Tarrytown, NY). Personnel performing the analyses were blinded to treatment.
Statistical analysis
Values are expressed as mean±SD. The primary end point was the incidence of major adverse outcomes at 48 hours. The adverse outcomes were defined by the protocol as death or euthanasia after the veterinarian’s recommendation due to status epilepticus, severe cardiorespiratory distress with evidence of agonal breathing with cyanosis or pulmonary edema and coma at 24 hours with the inability to respond to painful stimuli. Secondary endpoints were a) 4-hour left ventricular ejection fraction (LVEF) and b) the cerebral histological score. A single-factor ANOVA was used to determine statistical significance of differences in means of continuous variables between groups. Pairwise comparison of subgroups was performed with the Student-Newman-Keuls test. Significance was set at a value of p<0.05. A Kaplan Maier curve was plotted to show freedom from major adverse outcomes (death or euthanasia after 24 hours due to coma, refractory seizures and cardio-respiratory distress) in the intervention and control groups. Cox regression analysis was used to assess the effect of IPC, and CVT on major adverse outcomes up to 48 hours. The computer generated randomization process allocated the numbers of the animals in each group prospectively.
Results
All animals were included in the analysis. No adverse event occurs during the experiment. There were no significant baseline differences between treatment groups. (Table-1 and Table-2).
Table 1.
The hemodynamics, ROSC and survival during CPR, ROSC, post-ROSC and 48 hours.
| CPR Method | Measurements | Baseline | 2min CPR | 4min CPR | 1hr ROSC | 4hr ROSC | Number of Shocks | Post-ROSC Epi | ROSC |
|---|---|---|---|---|---|---|---|---|---|
| SCPR | SBP | 104±18 | 44±14 | 68±12 | 107±4 | 101±10 | 6±1 | 2±1.2 | 9/12 |
| DBP | 73±13 | 14±7 | 29±7 | 67±15 | 67±9 | ||||
| RA | 4±2 | 3±3 | 5±3 | 1±2 | 4±2 | ||||
| CPP | 69±15 | 11±4 | 24±6 | 66±11 | 63±4 | ||||
| CBF | 201±47 | 26±5 | 21±6 | 113±26 | 137±16 | ||||
| LVEF | N/A | N/A | N/A | 36±5 | 35±11 | ||||
| SCPR+IPC | SBP | 94±30 | 73±31 | 75±15 | 95±19 | 83±8 | 2±1* | 0.8±0.2* | 10/10 |
| DBP | 66±22 | 22±14 | 35±11 | 68±12 | 59±9 | ||||
| RA | 5±2 | 2±1 | 6±3 | 9±3 | 8±1 | ||||
| CPP | 61±27 | 20±15* | 29±15 | 58±14 | 51±11 | ||||
| CBF | 182±83 | 39±3* | 56±27* | 214±49* | 227±86* | ||||
| LVEF | N/A | N/A | N/A | 56±14* | 52±14* | ||||
| SCPR+CVT | SBP | 91±13 | 41±15 | 72±9 | 92±9 | 73±10 | 4±3 | 1±0.2* | 10/10 |
| DBP | 65±8 | 15±6 | 35±9 | 69±9 | 55±8 | ||||
| RA | 5±2 | 3±3 | 8±3 | 8±3 | 7±2 | ||||
| CPP | 60±8 | 12±8 | 27±13 | 61±9 | 48±17 | ||||
| CBF | 178±43 | 48±19* | 45±19* | 264±72* | 167±66 | ||||
| LVEF | N/A | N/A | N/A | 57±12* | 59±9* | ||||
| SCPR+IPC+CVT | SBP | 98±11 | 57±21 | 72±15 | 87±13 | 88±9 | 6±2 | 1.4±0.5 | 9/10 |
| DBP | 69±7 | 18±12 | 32±12 | 64±9 | 58±7 | ||||
| RA | 5±2 | 6±3 | 4±4 | 4±1 | 6±2 | ||||
| CPP | 64±9 | 12±5 | 28±18 | 60±11 | 52±12 | ||||
| CBF | 168±35 | 67±30* | 49±17* | 263±66* | 218±53* | ||||
| LVEF | N/A | N/A | N/A | 54±18* | 52±7* |
Values are shown as mean ±SD. CPR was performed with either SCPR, SCPR + IPC, SCPR+CVT or SCPR+IPC+CVT. All pressures are in mm Hg and all flows in mL/min. CBF: carotid blood flow, CPP: coronary perfusion pressure, DBP: diastolic blood pressure, EPI: Epinephrine, RA: right atrial pressure, SBP: systolic blood pressure, LVEF: left ventricular ejection fraction (%). The dose of EPI is in milligrams.
means statistically significant difference compared to SCPR group with a p value of <0.05.
Table 2.
Arterial blood gasses during cardiopulmonary resuscitation and after return of spontaneous circulation.
| CPR Method | Measurement | Baseline | 5-min CPR | 30min ROSC | 4hr ROSC |
|---|---|---|---|---|---|
| SCPR | pH | 7.44±0.03 | 7.28±0.2 | 7.26±0.08 | 7.39±0.06 |
| PCO2 | 42±2 | 49±13 | 47±10 | 41±3 | |
| PO2 | 96±14 | 100±34 | 98±28 | 80±25 | |
| HCO3 − | 28±2 | 22±3 | 20±2 | 24±3 | |
| ETCO2 | 40±2 | 43±14 | 39±5 | 41±5 | |
| SCPR+IPC | pH | 7.45±0.04 | 7.26±0.08 | 7.32±0.03 | 7.43±0.06 |
| PCO2 | 40±5 | 47±4 | 38±4* | 41±2 | |
| PO2 | 90±20 | 89±14 | 83±26 | 75±20 | |
| HCO3 − | 28±1 | 21±3 | 21±2 | 27±3 | |
| ETCO2 | 39±3 | 45±7 | 35±3 | 35±2 | |
| SCPR+CVT | pH | 7.45±0.03 | 7.29±0.06 | 7.34±0.03* | 7.40±0.07 |
| PCO2 | 43±2 | 51±5 | 43±6 | 42±3 | |
| PO2 | 83±19 | 97±25 | 86±26 | 76±18 | |
| HCO3 − | 29±1 | 25±5 | 23±2 | 26±4 | |
| ETCO2 | 40±1 | 44±8 | 37±3 | 35±4 | |
| SCPR+IPC+CVT | pH | 7.46±0.04 | 7.25±0.08 | 7.35±0.04* | 7.46±0.06 |
| PCO2 | 40±3 | 49±9 | 40±2* | 39±5 | |
| PO2 | 83±21 | 104±21 | 78±19 | 89±16 | |
| HCO3 − | 29±2 | 22±4 | 22±2 | 28±2 | |
| ETCO2 | 38±3 | 44±5 | 34±4 | 34±5 |
Mean ± SD. Arterial blood gas measurements at baseline and after ROSC. Partial pressures in torr. SaO2: percent oxygen saturation; HCO3: bicarbonate; ETCO2: end-tidal CO2.
means statistically significant difference compared to SCPR group with a p value of <0.05.
Hemodynamics and arterial blood gasses
During the first 3 minutes of CPR all animals had similar hemodynamics except, as expected, during the periods where pauses of compression and ventilations were introduced. (Table 1) CVT therapy did not significantly affect coronary perfusion pressures. Arterial blood gases during CPR and at recovery are shown in Table 2.
ROSC and 48-hr incidence of major adverse outcomes
There were no significant differences in ROSC between groups (Table 1). IPC treated animals had a significant decrease in the combination of death and pre-specified major adverse outcomes (coma at 24 hours, refractory seizures and cardio-respiratory distress leading to euthanasia) during the 48 hours of observation. IPC was independently and strongly associated with a decrease in the risk of death and major adverse outcomes [HR=0.13, 95% CI=0.035, 0.53, p=0.004] and there was no observed synergy between IPC and CVT (Figure 1, Table 3). In the same fashion, IPC (p=0.01) and IPC+CVT (p=0.024) were superior to SCPR. CVT alone was not superior to SCPR.
Figure 1.
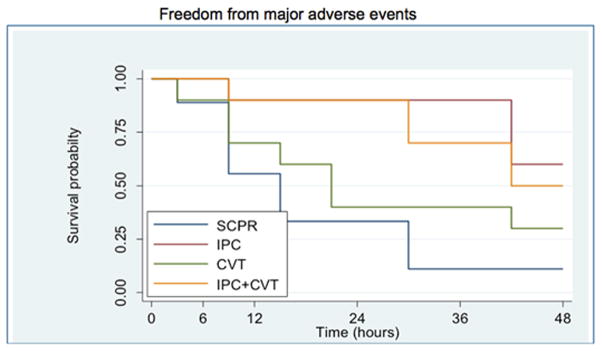
Kaplan-Meier curve of all 4 groups including only animals that achieved ROSC. The data demonstrate a significantly lower incidence of major adverse outcomes at 48 hours post-ROSC with IPC and IPC + CVT interventions compared with SCPR (p = 0.0097) (Major adverse outcomes are defined as death or coma, refractory seizures and cardio-respiratory distress leading to euthanasia). SCPR: Standard CPR, IPC: Ischemic post-conditioning, CVT: cardioprotective vasodilatory therapy
Table 3.
Effects of IPC, and CVT on major adverse outcomes up to 48 hours (Cox regression analysis). SCPR is the reference.
| Treatment | HR ± SE | P value | CI |
|---|---|---|---|
| SCPR | Ref | Ref | Ref |
| IPC | 0.2±0.1 | 0.01 | 0.05–0.7 |
| CVT | 0.55±0.3 | 0.26 | 0.19–1.5 |
| IPC+CVT | 0.3±0.2 | 0.02 | 0.08–0.8 |
CI=Confidence interval, HR=Hazard ratio, Ref=reference
Left Ventricular Function
Echocardiographic evaluation at 1 hour revealed that animals receiving CVT, CVT+IPC or IPC had a significantly higher LVEF than those treated with SCPR (57±12%, 54±18%, 56±14% vs. 36±5%, respectively, p<0.01 for all compared to SCPR). These results were sustained up to 4 hrs after ROSC (59±9%, 52±7%, 52±14% vs. 35±11%, respectively, p<0.05). There was no difference in the LVEF among CVT, IPC-CVT or IPC alone groups, either at 1 or 4 hours. (Table 1)
Cardiac Biomarkers
Plasma was obtained at 4 hours in all animals. IPC, CVT+IPC and CVT alone resulted in significantly lower CK-MB and Troponin-I levels (all in μg/L) at 4 hours compared to SCPR controls. The Troponin-I level was 31±34 at 4 hours in SCPR as compared to 8.5±7, 7±7, 5±5 for IPC, CVT, IPC+CVT, respectively (p<0.05). CK-MB was measured at 37±24 in SCPR group compared to 13±10, 18±13, 11±9 for IPC, CVT and IPC+CVT groups, respectively (p<0.05). There was no difference the cardiac biomarkers among the intervention groups.
Neurologic Function
Blinded assessment of cerebral performance category (CPC) at 24 hours on the live animals showed improvement in the CVT, CVT-IPC and IPC groups compared to SCPR group (2.6±0.9, 2.25±1, 2.75±0.4 vs. 3.5±0.5, respectively, p<0.05 for all). At 48 hours, there was only one animal that survived in the SCPR group and had CPC score of 3 (severe deficit). Surviving animals treated with CVT, CVT+IPC, and IPC alone at 48 hours had a mean CPC score of 2.3±1.6, 1.8±0.8, 2.2±0.9 with no difference between the groups, but with improvement in all intervention groups at 48 hours compared with 24 hours.
Neurohistopathology
The mean time for brain harvest was shorter in the SCPR group since more animals died earlier or had major adverse outcomes requiring euthanasia. The mean harvest time for SCPR was 20±12 hours compared to 39.0±12.4, 38.4±13 and 42.0±12.0 hours for IPC, CVT+IPC, CVT, respectively. Despite later evaluation of histopathological samples, IPC and IPC+CVT resulted in significantly lower total cerebral histological score (CHS) compared to SCPR group (5.8±2.6, 2.8±1.8 vs. 10±2.1, respectively, p<0.01). (Figure 2) One animal in the IPC group and two animals in the IPC+CVT group showed no ischemic damage at 48 hours (≤2 total CHS). CVT alone did not improve CHS (6.6±3.7) compared to SCPR group (p=0.1).
Figure 2.
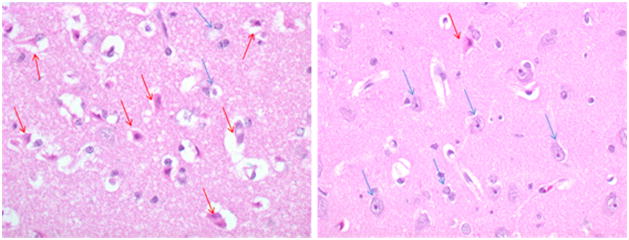
Left panel: Severe ischemic Brain Injury (SCPR), Right panel: Minimal Ischemic Brain Injury (IPC) Representative Samples of Severe versus Minimal Histologic Ischemic Brain Injury. Purple condensed nuclei are a marker of ischemic insult (red arrow). Healthy neurons are shown with blue arrow.
Discussion
Results from this investigation demonstrate that cardiac and cerebral function can be preserved after prolonged global ischemic insult of 15 minutes of untreated ventricular fibrillation cardiac arrest by early application of ischemic post-conditioning and use of cardioprotective vasodilators during CPR. These findings provide, for the first time, strong support for a simple BLS strategy that includes four controlled pauses of compressions during the first three minutes of CPR improves post-resuscitation LV function. This strategy also decreases the levels of cardiac biomarkers of injury at 4 hours and leads to a significant decrease of the ischemic histological injury of the brain leading to better neurological outcomes at 24 and 48 hours compared to standard CPR.
In this study, ROSC was not a predictor of neurological and cardiac function post-resuscitation. This finding that is consistent with large human studies showing dissociation between ROSC rates and improved survival with good neurological function.14, 15
Our data suggest that IPC is the most important factor leading to a significant improvement in cardiac function and survival with good neurological function. IPC decreased that risk of death and major adverse events by almost 80% compared to SCPR. It was also associated with significant improvement in cerebral histological injury; a combination of improved survival and cerebral preservation is currently considered the ultimate outcome for any intervention evaluated during cardiac arrest.
Our study is also in concordance with the published study by Wang et al that showed that IPC was effective in protecting the brains of rats from a global 10-minute ischemic insult that were not in cardiac arrest.16 This new observation should cause reassessment of the notion that cerebral recovery is not feasible after 10–12 minutes of cardiac arrest.17 In a recent series of papers, Allen et al have shown that controlled reperfusion of the brain with the use of bypass and a special reperfusion solution that mitigates reperfusion injury can result in the absence of ischemic changes in the brain even after 30 minutes of isolated global cerebral ischemia.18, 19 Based on similar principles, we have provided a simple method of IPC (with four 20-sec pauses in compressions and ventilation during the first 3 minutes of CPR) that could be easily applied in the clinical setting and translated to patients receiving CPR for treatment of cardiac arrest.
We used IPC during CPR because of the described mechanisms of protection from reperfusion injury which currently is thought to be modulation of the opening of mitochondrial permeability transition pores (mPTP) and KATP channels.20–26 Our results support the contention that the previously described protective mechanisms can also be observed during the global ischemia and reperfusion of cardiac arrest, decreasing vital organ injury as documented, in this study, with the histological evaluation of the brain and functional and biomarker assessment of the surviving animals.
CVT with sodium nitroprusside and adenosine did not improve neurological outcomes but led to an independent improvement in post-resuscitation cardiac function and lower levels of biomarkers of cardiac injury. This is consistent with published evidence supporting the protective properties of these two medications after cardiac ischemia.27 CVT therapy did not show synergy with IPC in clinical outcomes but there was an improvement in cerebral histological score and the IPC+CVT group provided animals with the lowest scores recorded in histopathology analysis of the brain, effectively showing no ischemic changes in any of the regions examined.
By contrast, CVT alone has been shown to be very effective in improving outcomes when it is applied on CPR strategies that provide superior hemodynamics such as active compression decompression CPR and the impedance threshold device in combination with abdominal binding.28 In this setting, the CPR method can increase vital organ perfusion pressures and blood flow and lead to improved outcomes compared to SCPR.9 It became evident in our present study, which used SCPR, that CVT does not offer any significant survival advantage, although it did improve cardiac function after ROSC and there were no detectable side effects.
Our study has limitations. We did not perform a dosing study so we cannot comment on the optimal IPC strategy, or the optimal CVT dosing. Dosing studies are difficult in a large animal laboratory due to the large number of animals needed. We did not evaluate mechanism in this study. We did, nevertheless, establish that our observed improved clinical outcomes were associated with evidence of cardiac and cerebral protection from ischemic injury in this global ischemic model of cardiac arrest. The histological assessment was performed in different times between the standard and the intervention groups. It is well established that histological evidence of ischemia becomes more pronounced after 24 hours and progressively gets worse up to 48-hours.29 The observed differences in total cerebral histological score therefore are very likely to be an underestimation since there was significantly more time in the intervention groups to develop the observed ischemic changes.
Conclusion
A simple IPC strategy applied at the initiation of CPR significantly improved clinical, histopathological and biomarker-related cardiocerebral outcomes in a porcine model of cardiac arrest of prolonged, untreated ventricular fibrillation. CVT offered a significant and independent improvement in post-resuscitation cardiac function and decreased levels of cardiac biomarkers at 4 hours post-ROSC but it did not show any independent or synergistic effects with IPC effects on neurological and survival outcomes.
Acknowledgments
Funding Sources
The study was funded by an Institutional, Division of Cardiology grant at the University of Minnesota and an R01 HL108926-01 NIH grant to Dr. Yannopoulos.
Footnotes
Statement of Authorship
All authors have participated to the conception, design and writing of this manuscript. This manuscript represents valid work and that neither this manuscript nor one with substantially similar content under our authorship has been published or is being considered for publication elsewhere.
Our current manuscript, including related data, figures and tables, has not been published previously and that the manuscript is not under consideration elsewhere. As described above the experimental design is the same with our previously published paper in Resuscitation by Segal et al. but we describe in this manuscript a whole new series of experiments.
Ethical Adherence
The study was approved by the Institutional Animal Care Committee of the Minneapolis Medical Research Foundation of Hennepin County Medical Center, and all animals received treatment in compliance with the National Research Council’s 1996 Guide for the Care and Use of Laboratory Animals.
Prior Publication
none
Copyright Constraints
none
Conflict of Interest
Demetris Yannopoulos MD, is the Medical Director of the Minnesota Resuscitation Consortium, a state wide initiative to improve survival in the state of MN from cardiac arrest. This initiative is sponsored by the Medtronic Foundation and is part of the Heart Rescue Program. There are no conflicts related to this investigation. Dr. Aufderheide has grants from NIHBI for the Resuscitation Outcomes Consortium, the ResQTrial, and the Immediate Trial; a grant from NINDS for the Neurological Emergency Treatment Trials (NETT) Network; he completed a paid consultancy on an acute myocardial infarction study with Medtronic in November, 2011; he volunteers on the Board of Directors for Take Heart America, President, Citizen CPR Foundation and is a volunteer for the National American Heart Association on the Basic Life Support Subcommittee and Research Working Group. He has no conflicts related to this investigation.
The rest of the authors have no conflicts related to the study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nichol G, Soar J. Regional cardiac resuscitation systems of care. Curr Opin Crit Care. 2010;16:223–30. doi: 10.1097/MCC.0b013e32833985b5. [DOI] [PubMed] [Google Scholar]
- 2.Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300:1423–31. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grubb NR. Managing out-of-hospital cardiac arrest survivors: 2. Cardiological perspective. Heart. 2001;85:123–4. doi: 10.1136/heart.85.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grubb NR. Managing out-of-hospital cardiac arrest survivors: 1. Neurological perspective. Heart. 2001;85:6–8. doi: 10.1136/heart.85.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Segal N, Matsuura T, Caldwell E, Sarraf M, McKnite S, Zviman M, et al. Ischemic postconditioning at the initiation of cardiopulmonary resuscitation facilitates functional cardiac and cerebral recovery after prolonged untreated ventricular fibrillation. Resuscitation. 2012;83:1397–403. doi: 10.1016/j.resuscitation.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yannopoulos D, Segal N, McKnite S, Aufderheide T, Lurie KG. Controlled Pauses at the Initiation of Sodium Nitroprusside Enhanced CPR Facilitate Neurological and Cardiac Recovery after 15 minutes of Untreated Ventricular Fibrillation. Crit Care Med. 2012;40:1562–9. doi: 10.1097/CCM.0b013e31823e9f78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS biology. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shultz JJ, Coffeen P, Sweeney M, Detloff B, Kehler C, Pineda E, et al. Evaluation of standard and active compression-decompression CPR in an acute human model of ventricular fibrillation. Circulation. 1994;89:684–93. doi: 10.1161/01.cir.89.2.684. [DOI] [PubMed] [Google Scholar]
- 9.Yannopoulos D, Segal N, McKnite S, Aufderheide TP, Lurie KG. Controlled pauses at the initiation of sodium nitroprusside-enhanced cardiopulmonary resuscitation facilitate neurological and cardiac recovery after 15 mins of untreated ventricular fibrillation. Crit Care Med. 2012;40:1562–9. doi: 10.1097/CCM.0b013e31823e9f78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–63. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 11.Schultz J, Segal N, Kolbeck J, Caldwell E, Thorsgard M, McKnite S, et al. Sodium nitroprusside enhanced cardiopulmonary resuscitation prevents post- resuscitation left ventricular dysfunction and improves 24-hour survival and neurological function in a porcine model of prolonged untreated ventricular fibrillation. Resuscitation. 2011;82S:S35–S40. doi: 10.1016/S0300-9572(11)70149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quinones MA, Waggoner AD, Reduto LA, Nelson JG, Young JB, Winters WL, Jr, et al. A new, simplified and accurate method for determining ejection fraction with two-dimensional echocardiography. Circulation. 1981;64:744–53. doi: 10.1161/01.cir.64.4.744. [DOI] [PubMed] [Google Scholar]
- 13.Högler S, Sterz F, Sipos W, Schratter A, Weihs W, Holzer M, et al. Distribution of neuropathological lesions in pig brains after different durations of cardiac arrest. Resuscitation. 2010;81:1577–83. doi: 10.1016/j.resuscitation.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Hagihara A, Hasegawa M, Abe T, Nagata T, Wakata Y, Miyazaki S. Prehospital epinephrine use and survival among patients with out-of-hospital cardiac arrest. JAMA. 2012;307:1161–8. doi: 10.1001/jama.2012.294. [DOI] [PubMed] [Google Scholar]
- 15.Aufderheide TP, Frascone RJ, Wayne MA, Mahoney BD, Swor RA, Domeier RM, et al. Standard cardiopulmonary resuscitation versus active compression-decompression cardiopulmonary resuscitation with augmentation of negative intrathoracic pressure for out-of-hospital cardiac arrest: a randomised trial. Lancet. 2011;377:301–11. doi: 10.1016/S0140-6736(10)62103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang JY, Shen J, Gao Q, Ye ZG, Yang SY, Liang HW, et al. Ischemic postconditioning protects against global cerebral ischemia/reperfusion-induced injury in rats. Stroke. 2008;39:983–90. doi: 10.1161/STROKEAHA.107.499079. [DOI] [PubMed] [Google Scholar]
- 17.Shaffner DH, Eleff SM, Brambrink AM, Sugimoto H, Izuta M, Koehler RC, et al. Effect of arrest time and cerebral perfusion pressure during cardiopulmonary resuscitation on cerebral blood flow, metabolism, adenosine triphosphate recovery, and pH in dogs. Crit Care Med. 1999;27:1335–42. doi: 10.1097/00003246-199907000-00026. [DOI] [PubMed] [Google Scholar]
- 18.Allen BS, Ko Y, Buckberg GD, Tan Z. Studies of isolated global brain ischaemia: III. Influence of pulsatile flow during cerebral perfusion and its link to consistent full neurological recovery with controlled reperfusion following 30 min of global brain ischaemia. Eur J Cardiothorac Surg. 2012;41:1155–63. doi: 10.1093/ejcts/ezr318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen BS, Ko Y, Buckberg GD, Tan Z. Studies of isolated global brain ischaemia: II. Controlled reperfusion provides complete neurologic recovery following 30 min of warm ischaemia - the importance of perfusion pressure. Eur J Cardiothorac Surg. 2012;41:1147–54. doi: 10.1093/ejcts/ezr317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao ZQ. Postconditioning in reperfusion injury: a status report. Cardiovasc Drugs Ther. 2010;24:265–79. doi: 10.1007/s10557-010-6240-1. [DOI] [PubMed] [Google Scholar]
- 21.Argaud L, Gateau-Roesch O, Raisky O, Loufouat J, Robert D, Ovize M. Postconditioning inhibits mitochondrial permeability transition. Circulation. 2005;111:194–7. doi: 10.1161/01.CIR.0000151290.04952.3B. [DOI] [PubMed] [Google Scholar]
- 22.Cour M, Gomez L, Mewton N, Ovize M, Argaud L. Postconditioning: from the bench to bedside. J Cardiovasc Pharmacol Ther. 2011;16:117–30. doi: 10.1177/1074248410383174. [DOI] [PubMed] [Google Scholar]
- 23.Eldaif SM, Deneve JA, Wang NP, Jiang R, Mosunjac M, Mutrie CJ, et al. Attenuation of renal ischemia-reperfusion injury by postconditioning involves adenosine receptor and protein kinase C activation. Transpl Int. 2010;23:217–26. doi: 10.1111/j.1432-2277.2009.00949.x. [DOI] [PubMed] [Google Scholar]
- 24.Kin H, Zatta AJ, Lofye MT, Amerson BS, Halkos ME, Kerendi F, et al. Postconditioning reduces infarct size via adenosine receptor activation by endogenous adenosine. Cardiovasc Res. 2005;67:124–33. doi: 10.1016/j.cardiores.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 25.Mykytenko J, Reeves JG, Kin H, Wang NP, Zatta AJ, Jiang R, et al. Persistent beneficial effect of postconditioning against infarct size: role of mitochondrial K(ATP) channels during reperfusion. Basic Res Cardiol. 2008;103:472–84. doi: 10.1007/s00395-008-0731-2. [DOI] [PubMed] [Google Scholar]
- 26.Ovize M, Baxter GF, Di Lisa F, Ferdinandy P, Garcia-Dorado D, Hausenloy DJ, et al. Postconditioning and protection from reperfusion injury: where do we stand? Position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res. 2010;87:406–23. doi: 10.1093/cvr/cvq129. [DOI] [PubMed] [Google Scholar]
- 27.Hillegass WB, Dean NA, Liao L, Rhinehart RG, Myers PR. Treatment of no-reflow and impaired flow with the nitric oxide donor nitroprusside following percutaneous coronary interventions: initial human clinical experience. J Am Coll Cardiol. 2001;37:1335–43. doi: 10.1016/s0735-1097(01)01138-x. [DOI] [PubMed] [Google Scholar]
- 28.Yannopoulos D, Matsuura T, Schultz J, Rudser K, Halperin HR, Lurie KG. Sodium nitroprusside enhanced cardiopulmonary resuscitation improves survival with good neurological function in a porcine model of prolonged cardiac arrest. Crit Care Med. 2011;39:1269–74. doi: 10.1097/CCM.0b013e31820ed8a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Back T, Schuler OG. The natural course of lesion development in brain ischemia. Acta Neurochir Suppl. 2004;89:55–61. doi: 10.1007/978-3-7091-0603-7_7. [DOI] [PubMed] [Google Scholar]


