Abstract
Purpose
Radiation-induced heart disease (RIHD) is a serious side effect of thoracic radiotherapy. The epidermal growth factor receptor (EGFR) pathway is essential for the function and survival of cardiomyocytes. Hence, agents that target the EGFR pathway are cardiotoxic. Tocotrienols protect from radiation injury, but may also enhance the therapeutic effects of EGFR pathway inhibitors in cancer treatment. This study investigates the effects of local irradiation on the EGFR pathway in the heart and tests whether tocotrienols may modify radiation-induced changes in this pathway.
Methods
Male Sprague-Dawley rats received image-guided localized heart irradiation with 21 Gy. Twenty four hours before irradiation, rats received a single dose of tocotrienol-enriched formulation or vehicle by oral gavage. At time points from 2 hours to 9 months after irradiation, left ventricular expression of EGFR pathway mediators was studied.
Results
Irradiation caused a decrease in the expression of epidermal growth factor (EGF) and neuregulin-1 (Nrg-1) mRNA from 6 hours up to 10 weeks, followed by an upregulation of these ligands and the receptor erythroblastic leukemia viral oncogene homolog (ErbB)4 at 6 months. In addition, the upregulation of Nrg-1 was statistically significant up to 9 months after irradiation. A long-term upregulation of ErbB2 protein did not coincide with changes in transcription or post-translational interaction with the chaperone heat shock protein 90 (HSP90). Pretreatment with tocotrienols prevented radiation-induced changes at 2 weeks.
Conclusions
Local heart irradiation causes long-term changes in the EGFR pathway. Studies have to address how radiation may interact with cardiotoxic effects of EGFR inhibitors.
Keywords: Radiation-induced heart disease, Epidermal Growth Factor Receptor pathway, Neuregulin-1, Tocotrienols
Introduction
Radiotherapy is an important treatment modality for cancer patients. Even though radiotherapy is targeted to kill cancer cells, it poses potential side effects to surrounding normal tissues. Novel improvements in cancer therapy have led to increased numbers of cancer survivors world wide. With cancer survivors living longer, the late side effects of cancer therapy are of great concern in patients treated with ionizing radiation (Gatta et al. 2009; Verdecchia et al. 2009). Radiation-induced heart disease (RIHD) is one of the late side effects of radiotherapy of thoracic and chest wall tumors, when all or part of the heart was exposed to ionizing radiation. It takes several years for patients to clinically present manifestations of RIHD such as, pericardial and myocardial fibrosis, accelerated atherosclerosis, cardiac valve injuries and conduction abnormalities (Adams et al. 2003; Heidenreich et al. 2005).
Work in our group is focused on the mechanisms by which local heart irradiation may cause myocardial degeneration and fibrosis. One of the main molecular systems in the heart that regulates cardiomyocyte survival and function is the epidermal growth factor receptor (EGFR) pathway. The EGFR pathway contains the tyrosine kinase receptors erythroblastic leukemia viral oncogene homolog (ErbB) 1/EGFR, ErbB2, ErbB3 and ErbB4, which upon activation by their ligands, stimulate cellular proliferation, differentiation, and survival (Bublil and Yarden 2007; Sanchez-Soria and Camenisch 2010). Neuregulin-1 (Nrg-1) and epidermal growth factor (EGF) are the two most common ligands of the EGFR pathway in the heart. ErbB2, ErbB4 or Nrg-1 knockout mice show failures in cardiac development and embryonic lethality (Gassmann et al. 1995; Lee et al. 1995; Meyer and Birchmeier 1995). Moreover, conditional inactivation of ErbB2 or ErbB4 leads to dilated cardiomyopathy and increased susceptibility to cardiac stress in the adult heart (Crone et al. 2002; Garcia-Rivello et al. 2005; Ozcelik et al. 2002). Even though the EGFR pathway plays an important role in cardiac function and disease, the role of the EGFR pathway in RIHD is unknown.
The EGFR pathway has been identified as a target for cancer therapy ever since the receptor tyrosine kinase ErbB2 was found to be overexpressed in 25% of breast cancer and was related to poor prognosis, increased metastasis and overall decreased survival (Slamon et al. 1987). In accordance with the role of the EGFR pathway in cardiac function and disease, Trastuzumab, a monoclonal antibody to ErbB2 that greatly improves cancer prognosis, also induces left ventricular dysfunction (Seidman et al. 2002). Prolonged treatments with inhibitors of the EGFR pathway, including Trastuzumab and tyrosine kinase inhibitors, after radiotherapy for intrathoracic cancers that involve exposure of the heart are becoming more common (Dienstmann et al. 2012; Pazo Cid and Anton 2012; Phillips et al. 2012). Nonetheless, the effects of cardiac radiation exposure on the myocardial toxicity of anti-EGFR pathway agents are not known. Because of the important role of the EGFR pathway in cardiac function and disease, and the increased use of inhibitors of the EGFR pathway in combination with radiotherapy in cancer treatment, we felt that it was important to study the effects of local irradiation on the EGFR pathway in the heart.
Tocotrienols are promising new agents that may reduce radiation toxicities. Tocopherols and tocotrienols are two classes of natural vitamin E, consisting of the four isoforms α-, β-, δ- and γ- tocopherol and α-, β-, δ- and γ- tocotrienol. Compared to tocopherols, tocotrienols are considered to have more potent antioxidant properties and accumulate in endothelial cells to 30–50 fold higher levels (Naito et al. 2005). In addition, γ- and δ-tocotrienols are the only isoforms that inhibit 3-hydroxy-3 methylglutaryl coenzyme A (HMG Co-A) reductase (Berbee et al. 2009; Pearce et al. 1992). Tocotrienols have shown to modulate the EGFR signaling pathway in pancreatic cancer cells (Shin-Kang et al. 2011), thus enhancing the therapeutic effects of anti-ErbB2 drugs such as Trastuzumab.
To provide first evidence for a potential role of the EGFR pathway in RIHD, the present study used a rat model of localized heart irradiation to investigate the effects of radiation on the EGFR pathway in the heart. Because of the interesting dual role of tocotrienols as radiation protectors and as enhancers of cancer treatment, we examined the effects of tocotrienols on radiation-induced changes in the EGFR pathway in the heart.
Materials and Methods
Animal model of local heart irradiation
All procedures in this study were approved by the Institutional Animal Care and Use Committee of the University of Arkansas for Medical Sciences. Male Sprague-Dawley rats were obtained from Harlan Laboratories (Indianapolis, IN, USA) and maintained in our Division of Laboratory Animal Medicine on a 12:12 light-to-dark cycle with free access to food and water. At a weight of 250–290 g the hearts of the rats were irradiated with the Small Animal Conformal Radiation Therapy Device (SACRTD) developed at our institution (Sharma et al. 2013). The SACRTD consists of a 225kVp X-ray source (GE Isovolt Titan 225, GE Sensing and Inspection Technologies, Lewistown, PA, USA) mounted on a custom made “gantry”, a stage mounted on a robotic arm positioning system (Viper™ s650 Adept Technology, Pleasanton, CA, USA), and a flat panel digital X-ray detector of 200 µm resolution (XRD 0820 CM3 Perkin Elmer, Fremont, CA, USA). For the purpose of local heart irradiation, a brass and aluminum collimating assembly was attached to the X-ray tube to produce a field of 19 mm diameter at the isocenter (Sharma et al. 2008).
Dosimetry was performed as described before (Sridharan et al. 2012). In short, the dose rate at the isocenter was measured using a pin-point ion chamber (PTW N301013, PTW, Freiburg, Germany; ADCL calibrated for 225 kV) following the TG-61 protocol of the American Association of Physicists in Medicine (Ma et al. 2001). In addition, dosimetry was performed with Gafchromic® EBT-2 films (Ashland Specialty Ingredients, Wayne, NJ, USA) that were calibrated with a Gamma Knife (Co-60) system (Elekta AB, Stockholm, Sweden) and analyzed as described before (Devic et al. 2005). To measure relative depth dose, 11 pieces of film were placed in between 11 slabs of solid water phantom each 5 mm thick. The film on the top of the phantom was kept at the isocenter, normal to the beam direction, and exposed to 5 Gy (225 kV, 13 mA)
For local heart irradiation, rats were anesthetized with 3% isoflurane and placed vertically in a cylindrical Plexiglas holder that was cut out such that no Plexiglas material was in between the radiation beam and the chest. The heart was exposed in three 19 mm-diameter fields (anterior-posterior and two lateral fields) of 7 Gy each. (225 kV, 13 mA, 0.5 mm Cu-filtration, resulting in 1.92 Gy/min at 1 cm tissue depth). The three doses of 7 Gy were given immediately after each other. Before each exposure, the location of the heart was verified with the X-ray detector (70 kV, 5 mA, <1 cGy) and, if necessary, the position of the rat was adjusted with the use of the robotic arm. Rats were sacrificed at 2 hours, 6 hours, 24 hours, 4 days, 2 weeks, 10 weeks, 6 months and 9 months after irradiation (n=5–6 at each time point) or sham treatment (n=5–6 at each time point), and left ventricular tissue samples were collected for analysis.
Administration of Tocomin SupraBio
Tocomin SupraBio® (TSB) and vehicle were kindly provided by Carotech (Perak, Malaysia). TSB is a tocotrienol/tocopherol enriched mixture that contains 17% tocotrienols (8% γ-tocotrienol, 5% α-tocotrienol, 3% δ-tocotrienol, 1% β-tocotrienol) and 5% α-tocopherol in a patented self-emulsifying delivery system designed for enhanced oral absorption. Rats were administered 500 µl of TSB (n=5–6), translating into 230 mg tocotrienols/kg body weight, or 500 µl vehicle (n=5–6), via oral gavage 24 hours before irradiation. These rats were sacrificed at 2 weeks after irradiation for analysis.
Rat Heart Endothelial Cell Culture
An immortalized cell line derived from rat heart microvascular endothelial cells (RHEC) was a kind gift from Dr. Van der Vusse (University Maastricht, The Netherlands) (Linssen et al. 1993). RHEC were cultured in Dulbecco’s Minimal Essential Medium (DMEM) containing 1 g/L D-glucose, supplemented with 10% Fetal Bovine Serum (FBS), 100 U/mL penicillin and 100 µg/mL streptavidin (all Life Technologies, Grand Island, NY, USA). Cells were maintained in cell culture incubators in a humidified atmosphere at 37°C and 5% CO2, under either 21% O2 (regular air) or 4% O2 as controlled by injection of appropriate amounts of medical grade N2. Cells were plated at a density of 4.8×103 cells/cm2 and treated 3 days later when still growing in logarithmic phase. Treatment with irradiation was performed in a Faxitron X-ray cabinet (Faxitron Bioptics, Tucson AZ, USA) at 150 kV, 6.6 mA, 0.8 mm Be filtration, at a dose rate of 1 Gy/min, to a total dose of 2 Gy, 10 Gy, or 21 Gy. Additional cultures were treated with angiotensin II at a final concentration of 0.01–1 µM or with phenylephrine at a final concentration of 1–100 µM (both Sigma Aldrich, St Louis, MO, USA), or vehicle. For this purpose, RHEC were serum starved for 24 hours before treatment and were cultured under 2% FBS or under 10% FBS. At different time points between 1 hour and 4 days after treatment, cells were harvested for RNA isolation and real-time polymerase chain reaction (Real-time PCR).
RNA isolation and real-time PCR
Gene expression was assessed with real-time PCR. Rats were anesthetized with 3% isoflurane, hearts were isolated and snap-frozen in liquid nitrogen. Frozen tissue samples from the left ventricle were homogenized in Ultraspec™ RNA reagent (Biotecx Laboratories, Houston, TX, USA). For endothelial cell cultures, after each time point media was removed from the plate and 1.5 mL of Ultraspec™ RNA reagent was added. Cells were scraped and collected in microfuge tube. RNA was isolated according to the manufacturer’s instructions. After treatment with RNA Qualified-DNAse I (RQ DNAse-1) (Promega, Madison, WI, USA) at 37°C for 30 min, followed by DNAse inactivation at 75°C for 10 min, cDNA was synthesized using the High Capacity cDNA Archive Kit™ (Life Technologies). Steady-state mRNA levels were measured with real-time quantitative PCR (TaqMan™) using the 7500 Fast Real-Time PCR System and the following pre-designed TaqMan Gene Expression Assays™ for rat: ErbB1 (EGFR) (Rn00580398_m1), ErbB2 (Rn00566561_m1), ErbB3 (Rn00568107_m1), ErbB4 receptor (Rn00572447_m1), EGF (Rn00563336_m1), Nrg-1 (Kn01482165_m1), (all Life Technologies). Relative mRNA levels were calculated with the delta delta threshold cycle (ΔΔCt) method, using 18S rRNA as normalizer.
Western-Blots
Left ventricular tissue was homogenized in radioimmunoprecipitation assay (RIPA) buffer with freshly added inhibitor cocktails of proteases (10 µL/mL) and phosphatases (10 µL/mL, both Sigma Aldrich), centrifuged at 20,000 g at 4°C for 15 minutes, and the supernatant was collected. Amounts of protein were determined with a bicinchoninic acid assay (BCA) (Sigma-Aldrich). A total of 50 µg protein was prepared in Laemmli sample buffer containing β-mercaptoethanol (1:20 vol/vol) and boiled for 2–3 minutes. Protein samples were separated either in Any kD™ Mini-Protean® polyacrylamide gels or 4% −20% gradient polyacrylamide gels (Bio-Rad, Hercules, CA, USA) at 100 Volts and transferred to polyvinylidene fluoride (PVDF) membranes at 20 Volts overnight at 4°C.
Non-specific antibody binding was reduced by Tris Buffered Saline (TBS) containing 0.05% Tween-20 and 5% Non fat dry milk. Membranes were then incubated overnight with rabbit anti-ErbB2 (1: 2,000, Santa Cruz, Santa Cruz, CA, USA), or rabbit anti-heat shock protein 90 (HSP90) (1:5,000), in TBS containing 5% non fat dry milk and 0.1% Tween-20, followed by horse radish peroxidase (HRP) conjugated mouse anti-rabbit at 1:10,000 for 1 hour (all Cell Signaling Technology, Danvers, MA, USA). Protein loading was corrected by incubating membranes in mouse anti-Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:20, 000, Santa Cruz) for 1 hour, followed by HRP-conjugated goat anti-mouse (1:20,000, Jackson ImmunoResearch, West Grove, PA, USA). Antibody binding was visualized with ECL™ Plus Western Blotting Detection reagent (GE Healthcare Life Sciences, Uppsala, Sweden) on CL-Xposure Film (Thermo Scientific, Waltham, MA, USA). Films were scanned using an AlphaImager® gel documentation system (Protein Simple, Santa Clara, CA, USA) and protein bands were quantified with the public domain software ImageJ.
Immunoprecipitation
Frozen left ventricle was rapidly homogenized in 500 µL buffer (50 mmol/L Tris-HCl, 150 mmol/L NaCl, 1 mmol/L ethylene glycol tetraacetic acid (EGTA), 1 mmol/L ethylenediaminetetraacetic acid (EDTA) and 1%Triton X 100). One milligram of protein was precleared with protein G-magnetic beads (Millipore, Carlsbad, CA, USA) and then incubated with 4 µg antibody (HSP90 or ErbB2) for 2 hours on a rotary shaker at 4°C. After incubation, protein G-magnetic beads were added for an additional hour, then washed with fresh buffer, and the immunoprecipitates were eluted with 1% sodium dodecyl sulfate (SDS) in phosphate buffered saline (PBS), boiled for 5 min in Laemmli SDS sample buffer, and frozen until used for Western blotting.
Statistical analysis
Data were evaluated with the software package NCSS 8 (NCSS, Kaysville, UT, USA). Data were analyzed with two-way Analysis of Variance (ANOVA), followed by Newman-Keuls individual comparisons. The criterion for significance was p<0.05.
Results
Effects of radiation on left ventricular mRNA levels in the EGFR pathway
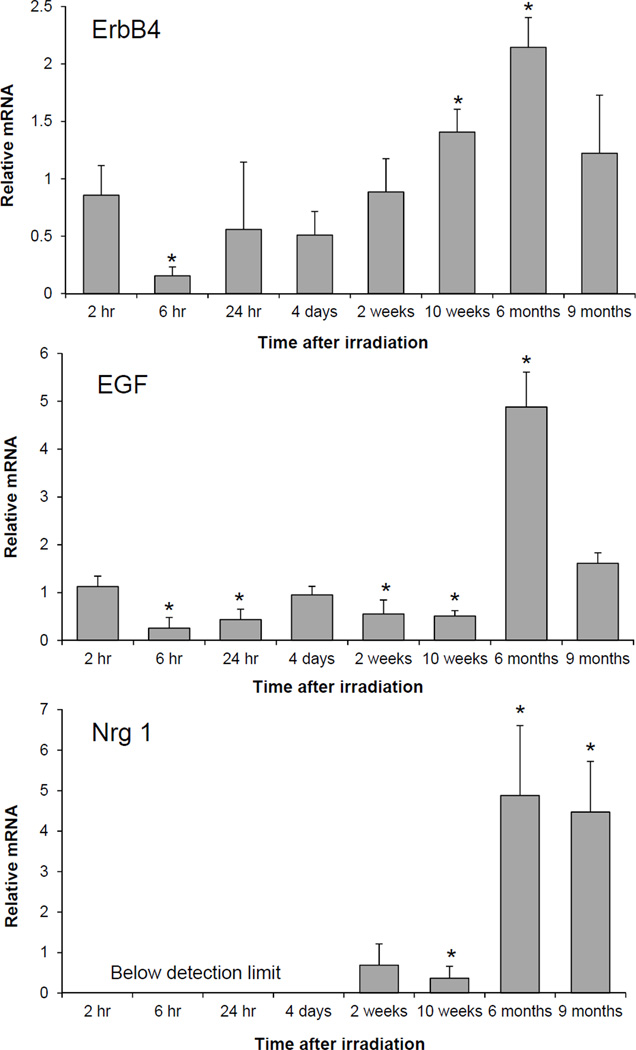
Figure 1 shows the time course of changes in the left ventricular mRNA expression of the receptor ErbB4 and the ligands EGF and Nrg-1. A prolonged downregulation of EGF gene expression was observed up to 10 weeks after irradiation, followed by a five fold increase at 6 months. Nrg-1 mRNA was undetectable up to 4 days after irradiation. As for EGF, we recorded a significant decrease in Nrg-1 mRNA at 10 weeks and a five-fold increase at 6 months and 9 months after irradiation. A reduction in the mRNA levels of the receptor ErbB4 was followed by a significant upregulation at 10 weeks and 6 months after radiation. The mRNA expression of the other receptors remained unchanged throughout the time course of the experiment (data not shown).
Figure 1.
Left ventricular relative mRNA levels of ErbB4, EGF and Nrg-1 from 2 hours to 9 months after local heart irradiation (21 Gy) as measured with real-time PCR. ErbB4 mRNA level was decreased at 6 hours after irradiation, which was followed by an increase at 10 weeks and 6 months. The prolonged down regulation of EGF and Nrg-1 mRNA levels up to 10 weeks was followed by a five-fold increase at 10 weeks and 6 months after local heart irradiation. Average ± standard deviation (SD), n=5. *p<0.05 when compared to time-matched sham-irradiated control.
Effects of radiation on the Nrg-1 mRNA expression in RHEC
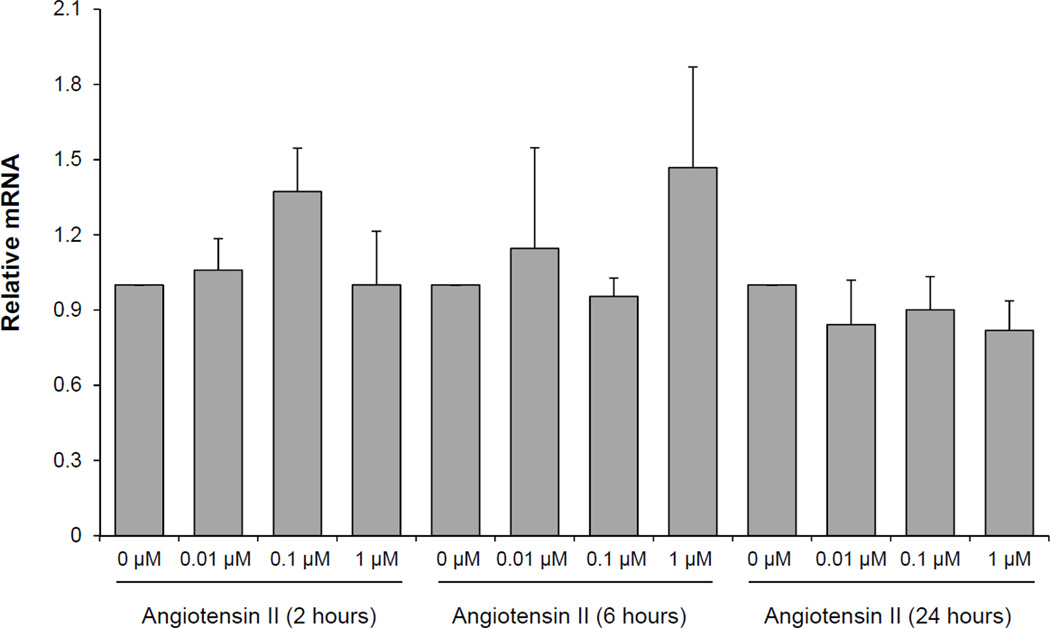
Next, we designed experiments to identify potential mechanisms by which local irradiation may cause such a dramatic down regulation of Nrg-1 in the heart. Microvascular endothelial cells are the main source of Nrg-1 in the heart (Cote et al. 2005). We therefore examined the effects of x-ray irradiation in cultures of the rat heart microvascular endothelial cell line RHEC. Doses of 2, 10 and 21 Gy did not alter the Nrg-1 mRNA at 1 hour, 3 hours, 6 hours, 24 hours or 4 days, whether cells were cultured and exposed under 4% oxygen or 21% oxygen (data not shown). Because irradiation did not directly alter endothelial Nrg-1 we hypothesized that Nrg-1 gene expression may be altered indirectly in response to another radiation-induced mediator. We treated RHEC with the known radiation–induced mediators angiotensin II and phenylephrine and examined the mRNA expression of Nrg-1 at different concentrations and time points. Treatment of RHEC with angiotensin II (Figure 2) and phenylephrine (data not shown) did not significantly alter the expression of Nrg-1 mRNA.
Figure 2.
Relative mRNA levels of Nrg-1 in RHEC exposed to Angiotensin II (0.01–1µM) as measured with real-time PCR. Treatment of RHEC with Angiotensin II did not alter the expression of Nrg-1 mRNA. Average ± SD, n=4.
Effects of radiation on the expression of ErbB2 protein in the rat heart
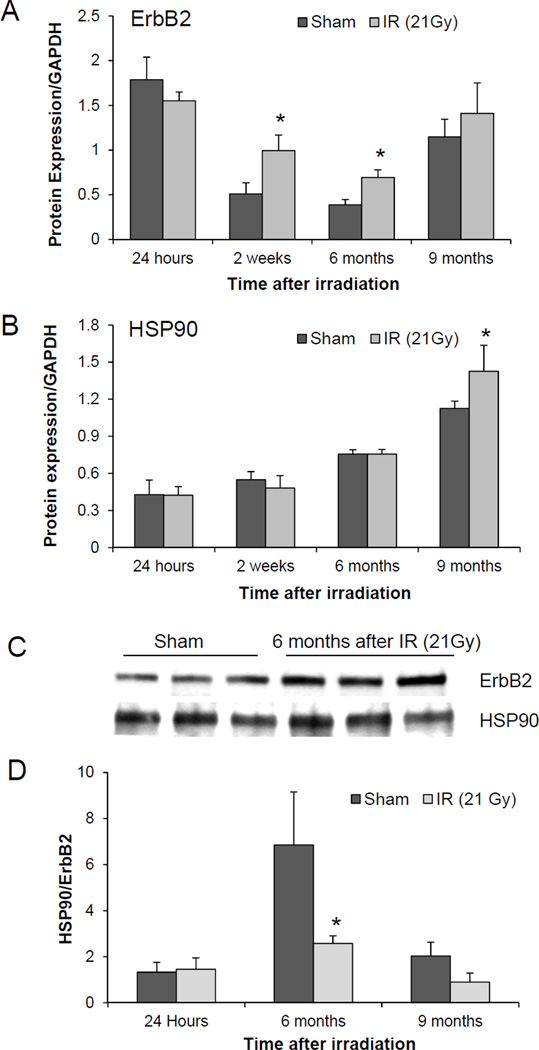
ErbB2 does not have a ligand binding domain but acts as a co-receptor for other members of the ErbB family (Cho et al. 2003; Garrett et al. 2003). Heterodimerization with ErbB2 increases ligand binding affinity and prolongs activation of the EGFR signaling pathway (Karunagaran et al. 1996). We detected an age-dependent decline in ErbB2 expression in sham-irradiated animals (Figure 3A).Even though left ventricular mRNA levels of ErbB2 did not change after irradiation, we observed a significant increase in ErbB2 protein expression at 2 weeks and 6 months after irradiation when compared to time and age-matched sham-irradiated controls (Figure 3A). These results led us to evaluate HSP90, which is a chaperone of the ErbB2 protein and is involved in its post-transcriptional stability. We did not observe a change in left ventricular protein levels of HSP90 up to 6 months after irradiation, but a significant increase was seen at 9 months post irradiation (Figure 3B). In addition, coimmunoprecipitation studies were performed to study the interaction of ErbB2 with HSP90. Increased levels of ErbB2 in irradiated compared to sham-irradiated hearts were consistent with western blot results (Figure 3C). However, the increased immunoprecipitation of ErbB2 did not coincide with an increased pull-down of HSP90, indicating that there was no enhanced binding of Erbb2 to HSP90 (Figure 3D).
Figure 3.
Effects of local heart irradiation (21 Gy) on left ventricular ErbB2 protein. A) Left ventricular ErbB2 expression after local heart irradiation. Densitometric analysis of Western-Blots showed an increase in ErbB2 protein levels at 2 weeks and 6 months after irradiation; B) Left ventricular HSP90 expression after local heart irradiation as measured with Western-Blots. HSP90 levels were increased at 9 months after irradiation only; C) Representative blot of coimmunoprecipitation of HSP90 with ErbB2 antibody in left ventricular lysates at 6 months after local heart irradiation; D) Densitometric analysis of coimmunoprecipitation did not show an increase in the interaction of HSP90 with ErbB2 in irradiated hearts. Average ± SD, n=3–5. *p<0.05 when compared to time-matched sham-irradiated control.
Effect of TSB on the EGF pathway after irradiation of rat hearts
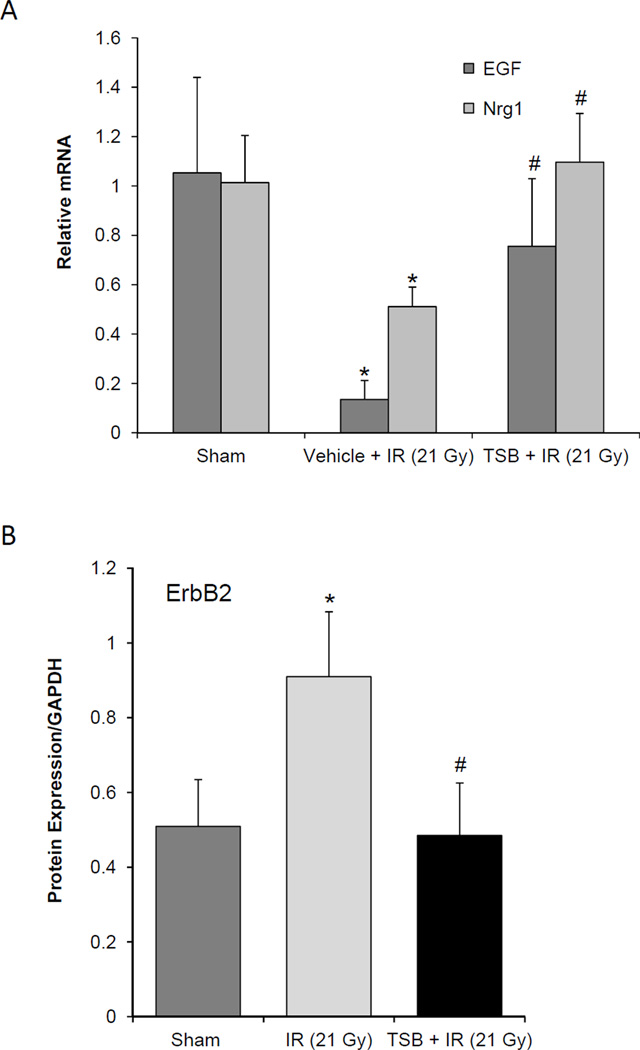
When administered 24 hours before irradiation, the tocotrienol-enriched oral formulation preserved the mRNA levels of EGF and Nrg-1 at 2 weeks after local heart irradiation (Figure 4A), but did not significantly alter ErbB2 or ErbB4 mRNA expression (data not shown). Further, TSB inhibited the effects of radiation on ErbB2 protein levels, while the expression of HSP90 did not significantly change in any of the groups (Figure 4B).
Figure 4.
Effects of TSB, given 24 hours before local heart irradiation (21 Gy), on left ventricular EGF, Nrg-1, and ErbB2. A) TSB enhanced left ventricular relative mRNA levels of EGF and Nrg-1 in irradiated hearts; B) TSB inhibited the effects of local heart irradiation on left ventricular protein levels of ErbB2. Average ± SD, n=5–6. *p<0.05 sham-irradiated controls. #p<0.05 TSB compared to vehicle-treated.
Discussion
This study investigated the effects of localized irradiation on the EGFR signaling pathway in the heart. The EGFR pathway is very important for the fetal development of the heart and the maintenance of the phenotype and function of adult cardiomyocytes (Crone et al. 2002; Garcia-Rivello et al. 2005; Gassmann et al. 1995; Lee et al. 1995; Meyer and Birchmeier 1995; Ozcelik et al. 2002). ErbB1, 2 and 4 are clearly expressed in the heart, while there is still some uncertainty with regard to the cardiac expression of ErbB3 (Camprecios et al. 2011). The ligand Nrg-1 and its receptors ErbB4 and ErbB2 play an important role in the EGFR signaling cascade in cardiomyocytes. Previous studies have shown that the survival of cardiomyocytes and the proliferation and regeneration of cardiomyocytes following myocardial injury is controlled by the Nrg-1/ErbB4/ErbB2 complex (Bersell et al. 2009; Fuller et al. 2008; Kuramochi et al. 2006). Cardiotoxicity associated with alterations in the EGFR signaling pathway has been demonstrated in cancer therapies with monoclonal antibodies directed against the EGFR pathway receptors. These side effects are even more significant when the EGFR pathway modifying agents are given in combination with anthracyclines, which are known to have cardiotoxic side effects of their own (Perez et al. 2008; Tan-Chiu et al. 2005).
We here show that ionizing radiation causes a prolonged downregulation of the two main ligands of the EGFR pathway in the heart, EGF and Nrg-1, followed by a significant upregulation at later time points. We have previously shown that myocardial degeneration and fibrosis start to become apparent at 10 weeks after local heart irradiation in rats and after that become progressively more severe. Hence, our results suggest that radiation-induced downregulations in the EGFR pathway may contribute to the onset of pathological changes, followed by upregulations at later time points as part of an attempt to regenerate the myocardium.
Studies with cancer cells have shown that clinical doses of ionizing radiation cause a change in proliferation rate with an increase in the phosphorylation of EGFR pathway receptors, irrespective of their expression levels. Hence, before and after radiotherapy a prolonged administration of EGFR pathway inhibitors may be required to inhibit the cancer cells (Kavanagh et al. 1995; Lammering et al. 2003; Schmidt-Ullrich et al. 1994; Schmidt-Ullrich et al. 2003). However, we here propose that prolonged use of these EGFR pathway inhibitors may possibly harm myocardial regeneration, thereby worsening manifestations of RIHD.
In the heart, microvascular endothelial cells are the main source of Nrg-1 (Kuramochi et al. 2004). Nrg-1 mRNA fell below detection in left ventricular homogenate at early time points after local heart irradiation and remained down regulated up to at least 10 weeks. Our observation led us to investigate the mechanism by which radiation may cause this down regulation of Nrg-1 mRNA. One of the transcription factors involved in the regulation of Nrg-1 gene expression is Specificity Protein 1 (Sp1) (Frensing et al. 2008).Since the DNA binding affinity of Sp1 is reduced by redox changes during oxidative stress (Ammendola et al. 1994; Sang et al. 2011; Wu et al. 1996), we hypothesized that ionizing radiation may directly inhibit Nrg-1 mRNA transcription. However, we did not find a change in the Nrg-1 mRNA levels in rat heart microvascular endothelial cells that were exposed to radiation in culture, leading us to consider the possibility of an indirect effect of irradiation as an alternative for the reduced levels of Nrg-1 mRNA. Earlier studies have demonstrated that localized heart irradiation causes an increase in angiotensin II (Wu and Zeng 2009), angiotensin converting enzyme and angiotensin type I receptors (Ferreira-Machado et al. 2010) and altered β-adrenergic signaling (Franken et al. 1992; Schultz-Hector et al. 1992). Previously, treatment of primary cultures of cardiac endothelial cells with angiotensin II and phenylephrine down regulated their Nrg-1 mRNA (Lemmens et al. 2006). In our present study, the expression of Nrg-1 mRNA in RHEC was not significantly altered by angiotensin II or phenylephrine. Although this difference in outcome may be due to the use of primary cells versus an immortal cell line, we could not provide evidence that radiation-induced down regulation of Nrg-1 occurs via angiotensin II of phenylephrine.
Earlier studies have shown that hypoxia plays a role in normal tissue radiation injury (Li et al. 2001; Vujaskovic et al. 2001). Macrovascular and microvascular changes are commonly found in animal models of RIHD (Adams et al. 2003; Heidenreich and Kapoor 2009; Schultz-Hector 1992) and myocardial perfusion defects are described in patients who have received thoracic radiotherapy (Marks et al. 2005). Therefore, hypoxia may also play a potential role in RIHD. The EGFR pathway in the heart is modified under hypoxic conditions (Munk et al. 2012). Hence, there may be a possible role for radiation-induced hypoxia in altering the EGFR pathway in the irradiated heart.
ErbB2 acts as a co-receptor for other receptors of the EGFR pathway; ErbB2 increases ligand binding affinity and enhances intracellular signaling (Cho et al. 2003; Garrett et al. 2003; Karunagaran et al. 1996). Local heart irradiation did not alter ErbB2 mRNA. However, a previous study by Gabrielson et al. has shown increased ErbB2 protein in the absence of a change in ErbB2 gene expression in the rat heart after doxorubicin treatment (Gabrielson et al. 2007). They concluded that enhanced binding of ErbB2 to HSP90, which as one of its main chaperones, may enhance ErbB2 expression. We therefore examined ErbB2 and HSP90 protein in the irradiated rat heart. In sham-irradiated animals, we detected an age-dependent decline in ErbB2 expression and an age-dependent increase in HSP90 expression. Previous studies have shown that the expression of ErbB2 and heat shock proteins may change with age (Calabrese et al. 2004; Tureaud et al. 1997). Hence, we included age and time matched sham-irradiated controls at every time point in our study to correct for any effects of age. We detected increased levels of ErbB2 protein in irradiated rat hearts when compared to age-matched sham-irradiated controls. On the other hand, local heart irradiation did not alter protein levels of HSP90. Further, our coimmunoprecipitation studies have demonstrated that the interaction between ErbB2 and HSP90 did not change, even though ErbB2 expression increased. Similar to our observation, there are reports which have shown that HSP90 levels do not change in dilated cardiomyopathy, ischemia (Knowlton et al. 1998), and heart failure after coronary heart ligation (Tanonaka et al. 2001). We suggest that the elevated levels of ErbB2 protein after irradiation are part of a survival response in the heart and that the ErbB2 chaperone HSP90 might not have a role in increasing ErbB2 stability. Rather, other mechanisms are likely involved in stabilizing ErbB2. For instance, studies have shown that ErbB2 is a target for deubiquitination and endosomal trafficking by the ubiquitin specific protease 8 (Meijer and van Leeuwen 2011). Further experiments to identify the specific role of the long term increase in ErbB2 expression are clearly warranted.
We evaluated the effects of a tocotrienol enriched oral formulation (Tocomin SupraBio) on the EGFR pathway in the irradiated heart. Tocotrienols possess antithrombotic, neuroprotective, cardioprotective, and anti-proliferative properties (Aggarwal et al. 2010; Theriault et al. 1999). Tocotrienols are of particular interest as radioprotective agents (Berbee and Hauer-Jensen 2012) and are currently undergoing advanced development for this indication. In addition to showing more potent antioxidant properties than tocopherols, tocotrienols also promote the degradation of HMG-CoA reductase and can accumulate in endothelial cells to 30–50 fold higher levels compared to tocopherols (Berbee et al. 2009; Naito et al. 2005; Pearce et al. 1992). By virtue of their HMG-CoA reductase inhibitory property, γ-tocotrienol has shown to ameliorate intestinal radiation injury, enhance recovery, decrease vascular oxidative stress and improve survival of animals exposed to total body irradiation (Berbee et al. 2009; Felemovicius et al. 1995). Moreover, radioprotective effects of γ-tocotrienol in hematopoietic tissue are associated with reduced oxidative stress and enhanced expression of granulocyte colony-stimulating factor (Kulkarni et al. 2010; Kulkarni et al. 2012), and δ-tocotrienol may exert radioprotection by stimulating mammalian target of rapamycin (mTOR) survival pathways in hematopoietic stem and progenitor cells (Li et al. 2010). Interestingly, tocotrienols enhance the therapeutic effects of EGFR pathway inhibitors on cancer cell proliferation (Shin-Kang et al. 2011). In this study, a single oral dose of TSB, 24 hours prior to local heart radiation preserved the mRNA levels of Nrg-1 and EGF and the protein expression of ErbB2. Administration of TSB prior to local heart irradiation might have led to the accumulation of tocotrienol in the vascular endothelial cells, thereby helping in the preservation of left ventricular Nrg-1 mRNA after exposure to irradiation. From our study it is evident that tocotrienols may potently inhibit adverse effects of radiation in the heart and should clearly be subject to further investigation.
To our knowledge, this is the first report showing that local heart irradiation can cause prolonged changes in the EGFR pathway. Our results suggest that radiation-induced downregulations in the EGFR pathway may contribute to the onset of myocardial degeneration and fibrosis, and that upregulations at later time points may be part of an attempt to regenerate the myocardium. Future studies have to address a) the dose-response effects of irradiation on the EGFR pathway, b) the mechanistic role of the EGFR pathway in RIHD, and c) potential interactions between radiotherapy for cancers of the thoracic region and other anti-cancer agents directed at the EGFR pathway in their cardiotoxic side effects.
Acknowledgements
The authors would like to thank Aaron Walters and Robin Mulkey for their excellent support in animal care.
This work was supported by the National Institutes of Health (CA148679, CA71382) and the American Cancer Society (RSG-10-125-01-CCE). The authors would also like to thank Carotech, Malaysia for providing TSB.
Footnotes
Declaration of Interest
The authors alone are responsible for the content and writing of the paper.
References
- Adams MJ, Hardenbergh PH, Constine LS, Lipshultz SE. Radiation-associated cardiovascular disease. Critical Reviews in Oncology Hematology. 2003;45:55–75. doi: 10.1016/s1040-8428(01)00227-x. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Sundaram C, Prasad S, Kannappan R. Tocotrienols, the vitamin E of the 21st century: its potential against cancer and other chronic diseases. Biochemical Pharmacology. 2010;80:1613–1631. doi: 10.1016/j.bcp.2010.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammendola R, Mesuraca M, Russo T, Cimino F. The DNA-binding efficiency of Sp1 is affected by redox changes. European Journal of Biochemistry. 1994;225:483–489. doi: 10.1111/j.1432-1033.1994.t01-1-00483.x. [DOI] [PubMed] [Google Scholar]
- Berbee M, Fu Q, Boerma M, Wang J, Kumar KS, Hauer-Jensen M. gamma-Tocotrienol ameliorates intestinal radiation injury and reduces vascular oxidative stress after total-body irradiation by an HMG-CoA reductase-dependent mechanism. Radiation Research. 2009;171:596–605. doi: 10.1667/RR1632.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbee M, Hauer-Jensen M. Novel drugs to ameliorate gastrointestinal normal tissue radiation toxicity in clinical practice: what is emerging from the laboratory? Current Opinion in Support and Palliative Care. 2012;6:54–59. doi: 10.1097/SPC.0b013e32834e3bd7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- Bublil EM, Yarden Y. The EGF receptor family: spearheading a merger of signaling and therapeutics. Current Opinion in Cell Biology. 2007;19:124–134. doi: 10.1016/j.ceb.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Scapagnini G, Ravagna A, Colombrita C, Spadaro F, Butterfield DA, Giuffrida Stella AM. Increased expression of heat shock proteins in rat brain during aging: relationship with mitochondrial function and glutathione redox state. Mech.Ageing Dev. 2004;125:325–335. doi: 10.1016/j.mad.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Camprecios G, Lorita J, Pardina E, Peinado-Onsurbe J, Soley M, Ramirez I. Expression, localization, and regulation of the neuregulin receptor ErbB3 in mouse heart. Journal of Cellular Physiology. 2011;226:450–455. doi: 10.1002/jcp.22354. [DOI] [PubMed] [Google Scholar]
- Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW, Jr, Leahy DJ. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421:756–760. doi: 10.1038/nature01392. [DOI] [PubMed] [Google Scholar]
- Cote GM, Miller TA, Lebrasseur NK, Kuramochi Y, Sawyer DB. Neuregulin-1alpha and beta isoform expression in cardiac microvascular endothelial cells and function in cardiac myocytes in vitro. Experimental Cell Research. 2005;311:135–146. doi: 10.1016/j.yexcr.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Crone SA, Zhao YY, Fan L, Gu Y, Minamisawa S, Liu Y, Peterson KL, Chen J, Kahn R, Condorelli G, Ross J, Jr, Chien KR, Lee KF. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nature Medicine. 2002;8:459–465. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- Devic S, Seuntjens J, Sham E, Podgorsak EB, Schmidtlein CR, Kirov AS, Soares CG. Precise radiochromic film dosimetry using a flat-bed document scanner. Medical Physics. 2005;32:2245–2253. doi: 10.1118/1.1929253. [DOI] [PubMed] [Google Scholar]
- Dienstmann R, Markman B, Tabernero J. Application of monoclonal antibodies as cancer therapy in solid tumors. Curr Clin Pharmacol. 2012;7:137–145. doi: 10.2174/157488412800228929. [DOI] [PubMed] [Google Scholar]
- Felemovicius I, Bonsack ME, Baptista ML, Delaney JP. Intestinal radioprotection by vitamin E (alpha-tocopherol) Annals of Surgery. 1995;222:504–508. doi: 10.1097/00000658-199522240-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira-Machado SC, Rocha NN, Mencalha AL, De Melo LD, Salata C, Ribeiro AF, Torres TS, Mandarim-De-Lacerda CA, Canary PC, Peregrino AA, Magalhaes LA, Cabral-Neto JB, Dealmeida CE. Up-regulation of angiotensin-converting enzyme and angiotensin II type 1 receptor in irradiated rats. International Journal of Radiation Biology. 2010;86:880–887. doi: 10.3109/09553002.2010.492489. [DOI] [PubMed] [Google Scholar]
- Franken NA, van der Laarse A, Bosker FJ, Reynart IW, Van Ravels FJ, Strootman E, Wondergem J. Time dependent changes in myocardial norepinephrine concentration and adrenergic receptor density following X-irradiation of the rat heart. International Journal of Radiation Oncology Biology Physics. 1992;24:721–727. doi: 10.1016/0360-3016(92)90720-3. [DOI] [PubMed] [Google Scholar]
- Frensing T, Kaltschmidt C, Schmitt-John T. Characterization of a neuregulin-1 gene promoter: positive regulation of type I isoforms by NF-kappaB. Biochimica et Biophysica Acta. 2008;1779:139–144. doi: 10.1016/j.bbagrm.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Fuller SJ, Sivarajah K, Sugden PH. ErbB receptors, their ligands, and the consequences of their activation and inhibition in the myocardium. Journal of Molecular and Cellular Cardiology. 2008;44:831–854. doi: 10.1016/j.yjmcc.2008.02.278. [DOI] [PubMed] [Google Scholar]
- Gabrielson K, Bedja D, Pin S, Tsao A, Gama L, Yuan B, Muratore N. Heat shock protein 90 and ErbB2 in the cardiac response to doxorubicin injury. Cancer Research. 2007;67:1436–1441. doi: 10.1158/0008-5472.CAN-06-3721. [DOI] [PubMed] [Google Scholar]
- Garcia-Rivello H, Taranda J, Said M, Cabeza-Meckert P, Vila-Petroff M, Scaglione J, Ghio S, Chen J, Lai C, Laguens RP, Lloyd KC, Hertig CM. Dilated cardiomyopathy in Erb-b4-deficient ventricular muscle. American Journal of Physiolology Heart and Circulatory Physiology. 2005;289:H1153–H1160. doi: 10.1152/ajpheart.00048.2005. [DOI] [PubMed] [Google Scholar]
- Garrett TP, McKern NM, Lou M, Elleman TC, Adams TE, Lovrecz GO, Kofler M, Jorissen RN, Nice EC, Burgess AW, Ward CW. The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Molecular Cell. 2003;11:495–505. doi: 10.1016/s1097-2765(03)00048-0. [DOI] [PubMed] [Google Scholar]
- Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, Lemke G. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378:390–394. doi: 10.1038/378390a0. [DOI] [PubMed] [Google Scholar]
- Gatta G, Zigon G, Capocaccia R, Coebergh JW, Desandes E, Kaatsch P, Pastore G, Peris-Bonet R, Stiller CA. Survival of European children and young adults with cancer diagnosed 1995–2002. European Journal of Cancer. 2009;45:992–1005. doi: 10.1016/j.ejca.2008.11.042. [DOI] [PubMed] [Google Scholar]
- Heidenreich PA, Hancock SL, Vagelos RH, Lee BK, Schnittger I. Diastolic dysfunction after mediastinal irradiation. American Heart Journal. 2005;150:977–982. doi: 10.1016/j.ahj.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Heidenreich PA, Kapoor JR. Radiation induced heart disease: systemic disorders in heart disease. Heart. 2009;95:252–258. doi: 10.1136/hrt.2008.149088. [DOI] [PubMed] [Google Scholar]
- Karunagaran D, Tzahar E, Beerli RR, Chen X, Graus-Porta D, Ratzkin BJ, Seger R, Hynes NE, Yarden Y. ErbB-2 is a common auxiliary subunit of NDF and EGF receptors: implications for breast cancer. EMBO Journal. 1996;15:254–264. [PMC free article] [PubMed] [Google Scholar]
- Kavanagh BD, Lin PS, Chen P, Schmidt-Ullrich RK. Radiation-induced enhanced proliferation of human squamous cancer cells in vitro: a release from inhibition by epidermal growth factor. Clin Cancer Res. 1995;1:1557–1562. [PubMed] [Google Scholar]
- Knowlton AA, Kapadia S, Torre-Amione G, Durand JB, Bies R, Young J, Mann DL. Differential expression of heat shock proteins in normal and failing human hearts. Journal o f Molecular and Cellular Cardiology. 1998;30:811–818. doi: 10.1006/jmcc.1998.0646. [DOI] [PubMed] [Google Scholar]
- Kulkarni S, Ghosh SP, Satyamitra M, Mog S, Hieber K, Romanyukha L, Gambles K, Toles R, Kao TC, Hauer-Jensen M, Kumar KS. Gamma-tocotrienol protects hematopoietic stem and progenitor cells in mice after total-body irradiation. Radiation Research. 2010;173:738–747. doi: 10.1667/RR1824.1. [DOI] [PubMed] [Google Scholar]
- Kulkarni SS, Cary LH, Gambles K, Hauer-Jensen M, Kumar KS, Ghosh SP. Gamma-tocotrienol, a radiation prophylaxis agent, induces high levels of granulocyte colony-stimulating factor. Int Immunopharmacol. 2012;14:495–503. doi: 10.1016/j.intimp.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Kuramochi Y, Cote GM, Guo X, Lebrasseur NK, Cui L, Liao R, Sawyer DB. Cardiac endothelial cells regulate reactive oxygen species-induced cardiomyocyte apoptosis through neuregulin-1beta/erbB4 signaling. Journal of Biological Chemistry. 2004;279:51141–51147. doi: 10.1074/jbc.M408662200. [DOI] [PubMed] [Google Scholar]
- Kuramochi Y, Guo X, Sawyer DB. Neuregulin activates erbB2-dependent src/FAK signaling and cytoskeletal remodeling in isolated adult rat cardiac myocytes. Journal of Molecular and Cellular Cardiology. 2006;41:228–235. doi: 10.1016/j.yjmcc.2006.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammering G, Hewit TH, Valerie K, Lin PS, Contessa JN, Schmidt-Ullrich RK. Anti-erbB receptor strategy as a gene therapeutic intervention to improve radiotherapy in malignant human tumours. Int J Radiat Biol. 2003;79:561–568. doi: 10.1080/0955300031000102632. [DOI] [PubMed] [Google Scholar]
- Lee KF, Simon H, Chen H, Bates B, Hung MC, Hauser C. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature. 1995;378:394–398. doi: 10.1038/378394a0. [DOI] [PubMed] [Google Scholar]
- Lemmens K, Segers VF, Demolder M, De Keulenaer GW. Role of neuregulin-1/ErbB2 signaling in endothelium-cardiomyocyte cross-talk. Journal of Biological Chemistry. 2006;281:19469–19477. doi: 10.1074/jbc.M600399200. [DOI] [PubMed] [Google Scholar]
- Li XH, Fu D, Latif NH, Mullaney CP, Ney PH, Mog SR, Whitnall MH, Srinivasan V, Xiao M. Delta-tocotrienol protects mouse and human hematopoietic progenitors from gamma-irradiation through extracellular signal-regulated kinase/mammalian target of rapamycin signaling. Haematologica. 2010;95:1996–2004. doi: 10.3324/haematol.2010.026492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YQ, Ballinger JR, Nordal RA, Su ZF, Wong CS. Hypoxia in radiation-induced blood-spinal cord barrier breakdown. Cancer Res. 2001;61:3348–3354. [PubMed] [Google Scholar]
- Linssen MC, van Nieuwenhoven FA, Duijvestijn AM, Glatz JF, van d, Vusse GJ. Continuous endothelial cells from adult rat heart. In Vitro Cellular and Developmental Biology-Animal. 1993;29A:611–613. doi: 10.1007/BF02634543. [DOI] [PubMed] [Google Scholar]
- Ma CM, Coffey CW, DeWerd LA, Liu C, Nath R, Seltzer SM, Seuntjens JP. AAPM protocol for 40–300 kV x-ray beam dosimetry in radiotherapy and radiobiology. Medical Physics. 2001;28:868–893. doi: 10.1118/1.1374247. [DOI] [PubMed] [Google Scholar]
- Marks LB, Yu X, Prosnitz RG, Zhou SM, Hardenbergh PH, Blazing M, Hollis D, Lind P, Tisch A, Wong TZ, Borges-Neto S. The incidence and functional consequences of RT-associated cardiac perfusion defects. Int.J.Radiat.Oncol.Biol.Phys. 2005;63:214–223. doi: 10.1016/j.ijrobp.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Meijer IM, van Leeuwen JE. ERBB2 is a target for USP8-mediated deubiquitination. Cellular Signaling. 2011;23:458–467. doi: 10.1016/j.cellsig.2010.10.023. [DOI] [PubMed] [Google Scholar]
- Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- Munk M, Memon AA, Goetze JP, Nielsen LB, Nexo E, Sorensen BS. Hypoxia changes the expression of the epidermal growth factor (EGF) system in human hearts and cultured cardiomyocytes. PLoS One. 2012;7:e40243. doi: 10.1371/journal.pone.0040243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y, Shimozawa M, Kuroda M, Nakabe N, Manabe H, Katada K, Kokura S, Ichikawa H, Yoshida N, Noguchi N, Yoshikawa T. Tocotrienols reduce 25-hydroxycholesterol-induced monocyte-endothelial cell interaction by inhibiting the surface expression of adhesion molecules. Atherosclerosis. 2005;180:19–25. doi: 10.1016/j.atherosclerosis.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Ozcelik C, Erdmann B, Pilz B, Wettschureck N, Britsch S, Hubner N, Chien KR, Birchmeier C, Garratt AN. Conditional mutation of the ErbB2 (HER2) receptor in cardiomyocytes leads to dilated cardiomyopathy. Proceedings of the National Academy of Sciences. 2002;99:8880–8885. doi: 10.1073/pnas.122249299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazo Cid RA, Anton A. Advanced HER2-positive gastric cancer: Current and future targeted therapies. Critical Reviews in Oncology Hematology. 2012 doi: 10.1016/j.critrevonc.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Pearce BC, Parker RA, Deason ME, Qureshi AA, Wright JJ. Hypocholesterolemic activity of synthetic and natural tocotrienols. Journal of Medicinal Chemistry. 1992;35:3595–3606. doi: 10.1021/jm00098a002. [DOI] [PubMed] [Google Scholar]
- Perez EA, Suman VJ, Davidson NE, Sledge GW, Kaufman PA, Hudis CA, Martino S, Gralow JR, Dakhil SR, Ingle JN, Winer EP, Gelmon KA, Gersh BJ, Jaffe AS, Rodeheffer RJ. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. Journal of Clinical Oncology. 2008;26:1231–1238. doi: 10.1200/JCO.2007.13.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips BE, Tubbs RR, Rice TW, Rybicki LA, Plesec T, Rodriguez CP, Videtic GM, Saxton JP, Ives DI, Adelstein DJ. Clinicopathologic features and treatment outcomes of patients with human epidermal growth factor receptor 2-positive adenocarcinoma of the esophagus and gastroesophageal junction. Diseases of the Esophagus. 2012 doi: 10.1111/j.1442-2050.2012.01369.x. in press. [DOI] [PubMed] [Google Scholar]
- Sanchez-Soria P, Camenisch TD. ErbB signaling in cardiac development and disease. Seminars in Cell and Developmental Biology. 2010;21:929–935. doi: 10.1016/j.semcdb.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang J, Yang K, Sun Y, Han Y, Cang H, Chen Y, Shi G, Wang K, Zhou J, Wang X, Yi J. SUMO2 and SUMO3 transcription is differentially regulated by oxidative stress in an Sp1-dependent manner. Biochemical Journal. 2011;435:489–498. doi: 10.1042/BJ20101474. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich RK, Contessa JN, Lammering G, Amorino G, Lin PS. ERBB receptor tyrosine kinases and cellular radiation responses. Oncogene. 2003;22:5855–5865. doi: 10.1038/sj.onc.1206698. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich RK, Valerie KC, Chan W, McWilliams D. Altered expression of epidermal growth factor receptor and estrogen receptor in MCF-7 cells after single and repeated radiation exposures. Int J Radiat Oncol Biol Phys. 1994;29:813–819. doi: 10.1016/0360-3016(94)90570-3. [DOI] [PubMed] [Google Scholar]
- Schultz-Hector S. Radiation-induced heart disease: review of experimental data on dose response and pathogenesis. International Journal of Radiation Biology. 1992;61:149–160. doi: 10.1080/09553009214550761. [DOI] [PubMed] [Google Scholar]
- Schultz-Hector S, Bohm M, Blochel A, Dominiak P, Erdmann E, Muller-Schauenburg W, Weber A. Radiation-induced heart disease: morphology, changes in catecholamine synthesis and content, beta-adrenoceptor density, and hemodynamic function in an experimental model. Radiation Research. 1992;129:281–289. [PubMed] [Google Scholar]
- Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, Murphy M, Stewart SJ, Keefe D. Cardiac dysfunction in the trastuzumab clinical trials experience. Journal of Clinical Oncology. 2002;20:1215–1221. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- Sharma S, Moros EG, Griffin RJ, Webber JS, Jia D, Corry PM. Demonstration of precise millimeter beam positioning of a conformal radiation therapy system for laboratory animal research; Radiation Research Society 54th Annual Scientific Meeting Abstract; 2008. [Google Scholar]
- Sharma S, Moros EG, Boerma M, Sridharan V, Han EY, Clarkson R, Hauer-Jensen M, Corry PM. A novel technique for image-guided local heart irradiation in the rat. Technology in Cancer Research and Treatment. 2013 doi: 10.7785/tcrtexpress.2013.600256. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin-Kang S, Ramsauer VP, Lightner J, Chakraborty K, Stone W, Campbell S, Reddy SA, Krishnan K. Tocotrienols inhibit AKT and ERK activation and suppress pancreatic cancer cell proliferation by suppressing the ErbB2 pathway. Free Radical Biology and Medicine. 2011;51:1164–1174. doi: 10.1016/j.freeradbiomed.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Sridharan V, Tripathi P, Sharma SK, Moros EG, Corry P, Lieblong BJ, Kaschina E, Unger T, Thoene-Reineke C, Hauer-Jensen M, Boerma M. Cardiac inflammation after local irradiation is influenced by the kallikrein-kinin system. Cancer Research. 2012;72:4984–4992. doi: 10.1158/0008-5472.CAN-12-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan-Chiu E, Yothers G, Romond E, Geyer CE, Jr, Ewer M, Keefe D, Shannon RP, Swain SM, Brown A, Fehrenbacher L, Vogel VG, Seay TE, Rastogi P, Mamounas EP, Wolmark N, Bryant J. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. Journal of Clinical Oncology. 2005;23:7811–7819. doi: 10.1200/JCO.2005.02.4091. [DOI] [PubMed] [Google Scholar]
- Tanonaka K, Yoshida H, Toga W, Furuhama K, Takeo S. Myocardial heat shock proteins during the development of heart failure. Biochemical and Biophysical Research Communications. 2001;283:520–525. doi: 10.1006/bbrc.2001.4801. [DOI] [PubMed] [Google Scholar]
- Theriault A, Chao JT, Wang Q, Gapor A, Adeli K. Tocotrienol: a review of its therapeutic potential. Clinical Biochemistry. 1999;32:309–319. doi: 10.1016/s0009-9120(99)00027-2. [DOI] [PubMed] [Google Scholar]
- Tureaud J, Sarkar FH, Fligiel SE, Kulkarni S, Jaszewski R, Reddy K, Yu Y, Majumdar AP. Increased expression of EGFR in gastric mucosa of aged rats. Am J Physiol. 1997;273:G389–G398. doi: 10.1152/ajpgi.1997.273.2.G389. [DOI] [PubMed] [Google Scholar]
- Verdecchia A, Guzzinati S, Francisci S, De AR, Bray F, Allemani C, Tavilla A, Santaquilani M, Sant M. Survival trends in European cancer patients diagnosed from 1988 to 1999. European Journal of Cancer. 2009;45:1042–1066. doi: 10.1016/j.ejca.2008.11.029. [DOI] [PubMed] [Google Scholar]
- Vujaskovic Z, Anscher MS, Feng QF, Rabbani ZN, Amin K, Samulski TS, Dewhirst MW, Haroon ZA. Radiation-induced hypoxia may perpetuate late normal tissue injury. Int J Radiat Oncol Biol Phys. 2001;50:851–855. doi: 10.1016/s0360-3016(01)01593-0. [DOI] [PubMed] [Google Scholar]
- Wu R, Zeng Y. Does angiotensin II-aldosterone have a role in radiationinduced heart disease? Medical Hypotheses. 2009;72:263–266. doi: 10.1016/j.mehy.2008.09.051. [DOI] [PubMed] [Google Scholar]
- Wu X, Bishopric NH, Discher DJ, Murphy BJ, Webster KA. Physical and functional sensitivity of zinc finger transcription factors to redox change. Molecular and Cellular Biology. 1996;16:1035–1046. doi: 10.1128/mcb.16.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]