Abstract
Introduction
Hormonal contraception (HC) use by HIV-infected women has been identified by the WHO as important strategy for reducing vertical HIV transmission. Little is known about factors associated with HC discontinuation among HIV-infected women.
Methods
We analyzed data from a prospective study of HC use among 231 HIV-infected oral contraceptive (OC) or injectable depot medroxyprogesterone acetate (DMPA) users in Uganda and Zimbabwe. We used Kaplan-Meier survival curves to estimate the median duration of OC and DMPA use and use of any highly effective contraceptive method. Cox proportional hazards models were used to investigate factors associated with HC discontinuation.
Results
Median duration was 36 months (95% CI 14, 61) for OC use and 19 months (95% CI 14, 24) for DMPA use. Median duration of any highly effective method was 36 months (95% CI 26, N/A) for OC users and 22 months (95% CI 14, 38) for DMPA users. In multivariable analyses, living in Zimbabwe (HR 0.39; 95% CI 0.18, 0.83), no partner (HR 7.18; 95% CI 3.05, 16.88) and cervical infection (HR 1.99; 95% CI 0.90, 4.41) were associated with OC discontinuation. No partner (HR 2.00; 95% CI 1.12, 3.58), nausea (HR 1.84; 95% CI 1.02, 3.34) and excessive night sweats (HR 1.80; 95% CI 0.95, 3.40) were associated with DMPA discontinuation.
Discussion
Long-term use of HC methods is acceptable to HIV-infected women. Women discontinue for a variety of reasons, primarily unrelated to HIV. Alternative methods and ongoing contraceptive counseling is essential to reduce unplanned pregnancies and vertical HIV transmission.
Keywords: vertical transmission, unplanned pregnancy, hormonal contraception, contraceptive discontinuation
Introduction
Over 16.8 million women are HIV-infected worldwide1. HIV-infected women face complex reproductive and sexual health choices involving managing their fertility and contraceptive choices2–4. The use of highly effective contraceptive methods, including hormonal contraception (HC), by HIV-infected women has been recognized by the WHO as an important strategy to prevent unintended pregnancies, reduce vertical transmission and increase a woman’s reproductive control5–7. However, little is known about long-term patterns of use or factors associated with HC discontinuation among HIV-infected women.
Expanding access to highly active antiretroviral therapy (HAART) likely influences the fertility intentions and contraceptive needs of HIV-infected women8–12. Changes in the WHO’s recommended CD4 threshold for HAART initiation has translated into over 8 million people on treatment worldwide7,13. Additionally, improved HAART regimens and better access to treatment have led to declines in pediatric HIV infections14. Currently, several countries in sub-Saharan Africa are considering changing national policies to place all pregnant HIV-infected women on lifelong HAART, regardless of CD4 count15,16. In this changing context, HIV-infected women need effective options for managing their fertility and achieving their reproductive goals17.
While HC is highly effective for preventing pregnancy, many healthcare providers emphasize condom use for HIV-infected women and put less emphasis on use of highly effective contraception. Healthcare providers may also be reluctant to put HIV-infected women on HC because of concerns about possible interactions between HC and antiretroviral drugs, which could theoretically reduce contraceptive or antiretroviral efficacy18. Concerns have also been raised about whether HC may increase HIV acquisition in women or transmission to men and/or accelerate disease progression19–21. However, several recent prospective studies and systematic reviews suggest that HC use is not associated with time to AIDS, death or HAART initiation, but further investigation is needed about whether DMPA increases the risk of HIV transmission22–27. In 2012, the WHO affirmed its recommendation that women living with HIV can continue to use all existing HC methods without restriction28.
The goal of this study was to examine factors associated with HC use among HIV-infected women by evaluating use of oral contraceptive pills (OC) and depot medroxygprogesterone acetate (DMPA) over an eight year period in a prospective cohort study of HIV-infected women in Uganda and Zimbabwe. The specific objectives of this analysis are (1) to describe the median duration of HC use among HIV-infected OC and DMPA users through a) first method-specific discontinuation and b) discontinuation of a highly effective method and (2) to examine factors associated with discontinuation of HC among HIV-infected OC and DMPA users.
Methods
We analyzed data from a prospective study of HC use among women who became HIV-infected while participating in the Hormonal Contraception and Risk of HIV Acquisition (HC-HIV) Study and a subsequent study of contraceptive use among HIV-infected women (Hormonal Contraception and HIV-1 Genital Shedding and Disease Progression (GS) Study) conducted from 2001 to 2009. Study procedures have been described previously29. Briefly, we recruited 86 Ugandan and 145 Zimbabwean (n=231) HIV-infected women ages 18 to 45 years who used either DMPA (150 mg administered quarterly), OCs (low-dose pills containing 30 mcg ethinyl estradiol and 150 mcg of levonorgestrel), or no hormonal method. Women were ineligible if they had an abortion or miscarriage in the 10 days prior to screening or if they had undergone hysterectomy. The institutional review boards of collaborating institutions in the United States, Uganda and Zimbabwe approved the study. All women provided written informed consent prior to study participation.
We conducted follow-up visits at 4, 8, and 12 weeks after enrollment and at 12-week intervals thereafter. At each study visit, women were interviewed about their contraceptive use, sexual behavior and reproductive health. We provided contraceptive, HIV risk reduction, and condom use counseling and free contraceptives and condoms. Each study visit also included physical examinations, pregnancy and reproductive tract infections (RTIs) testing as previously described30. We treated participants on-site for vaginal infections and recalled women diagnosed with asymptomatic chlamydia, gonorrhea, or syphilis for treatment. Beginning in 2003, we offered HAART and trimethoprim–sulfamethoxazole to women who developed severe symptoms of HIV infection (WHO clinical stage 4 or severe stage 3) or who within a six-month period had two CD4 counts ≤ 200 cells/mm3, in accordance with national ARV guidelines at the time.
For the analyses of contraceptive discontinuation and risk factors, we analyzed HIV-infected participants with at least one follow-up visit with valid contraceptive use data who (1) used either OCs or DMPA at the start of the GS study or (2) were not using HC at study enrollment but began using it during follow-up. For women using OCs or DMPA at enrollment, the contraceptive start date was the date of their first study visit. For women who began using OCs or DMPA during follow-up, the contraceptive start date was the first visit at which they reported OC or DMPA use. At each study visit, women were asked about contraceptive use since their last visit and reported start and stop dates for any method used. Time to discontinuation was calculated as the number of months from contraceptive start date to date of method discontinuation. For OC users, discontinuation was defined as any gap in the start and stop dates for OC use. For DMPA users, discontinuation was defined as not returning for an injection within 121 days of a woman’s last injection.
Our primary endpoint was the number of months from the contraceptive start date to either (1) method-specific contraceptive discontinuation or (2) the last study contact, whichever occurred earlier. A secondary endpoint was defined as the number of months from the contraceptive start date to (1) stopping use of a highly effective method (defined as OC, DMPA, IUD, implants or sterilization) or (2) the last study contact, whichever occurred earlier. For women who became pregnant during follow-up, if the estimated pregnancy date occurred prior to the date of method of discontinuation they were not counted as discontinuing their method and were right censored at the estimated pregnancy date. Women contributed person-time on a method prior to becoming pregnant, but were censored at their estimated pregnancy date because they would no longer eligible to discontinue contraception. Because HAART was not available throughout the entire study period, women who initiated HAART or who underwent hysterectomy were right-censored at the visit of the event.
We calculated the median number of months from contraceptive start date to method-specific discontinuation for all women who used HC during the study period. We compared the probability of discontinuation in OC and DMPA users over time using Kaplan–Meier survival analysis and log-rank tests. We conducted two sensitivity analyses. For the first, the contraceptive start date was calculated retrospectively going back before enrollment based on contraceptive calendar information obtained at the first study visit. The retrospective start date was not used as the primary contraceptive start date because covariate information between seroconversion and study entry was not available for all women. Because we were interested in understanding HC use after HIV-infection, person-time for women who continuously used OCs or DMPA before HIV infection were truncated at their estimated date of HIV infection. In the second, the analysis population was restricted to women on HC at their first study visit.
We evaluated factors related to OC or DMPA method-specific discontinuation (primary endpoint) using Cox proportional hazards models. Factors significant in bivariate analyses (at alpha ≤ 0.10) were included in full multivariable models and variables were eliminated using backwards selection with an alpha 0.10 cut-off. We decided to include a priori the following variables in all multivariable models: country, age and CD4 count. To estimate the effect of time-varying variables (e.g. sexual behaviors, condom use) on method discontinuation, a participant's time was divided into segments corresponding to the periods between study visits30. All analyses were conducted using SAS statistical software, version 9.3 (SAS Institute, Cary, NC).
Results
Of 231 enrolled women, 62 (27%) used OCs and 128 (55%) used DMPA at their first study visit. An additional 18 (8%) began OCs and 23 (10%) began DMPA during follow up. DMPA use was more popular than OCs, with 151 (65%) women either using DMPA at baseline or initiating use during follow up (Table 1). Women contributed a total of 1886 visit segments and were followed for a mean of 25 months and a median of 16 months. Over the follow-up period, 151 women discontinued their original method; 41 (18%) OC users and 110 (48%) DMPA users. Twenty (9%) women were censored due to pregnancy and 18 (8%) due to HAART initiation. The cumulative pregnancy rate was higher in Uganda (14.4 per100 person years) than Zimbabwe (7.7 per100 person years). The combined cumulative pregnancy rate was 10.1 per 100 person years31.
Table 1. Baseline characteristics for OC and DMPA HIV-infected users in Uganda and Zimbabwe.
Information on education was collected at the HC-HIV study baseline visit. Participant behavioral risk is defined as: multiple partners, new partner, commercial sex work or had sex with another man in the last 3 months. Missing data includes, primary partner spend any nights away from home in the last 30 day (n=4); current cervical infection (n=7) CD4 count (n=3). Column percentages calculated including missing values.
| Characteristic | OC N (%) N =80 (35.0) |
DMPA N (%) N =151 (65.0) |
Total N(%) N =231 |
|---|---|---|---|
| Sociodemographic characteristics | |||
| Uganda | 30 (37.5) | 56 (37.1) | 86 (37.2) |
| Zimbabwe | 50 (62.5) | 95 (62.9) | 145 (62.8) |
| Age at method start (years) | |||
| 19–24 | 27 (33.8) | 48 (31.8) | 75 (32.5) |
| 25–29 | 29 (36.3) | 56 (37.1) | 85 (36.8) |
| 30+ | 24 (30.0) | 47 (31.1) | 71 (30.7) |
| Education ≤9 years | 40 (50.0) | 70 (46.4) | 110 (47.6) |
| Reproductive and sexual health characteristics | |||
| Lifetime live births ≥2 | 54 (67.5) | 125 (82.8) | 179 (77.5) |
| Currently breastfeeding | 5 (6.3) | 15 (9.9) | 20 (8.7) |
| Coital Frequency (typical month in last 3 months) | |||
| 0–14 acts | 55 (68.8) | 111 (73.5) | 166 (71.9) |
| 15–29 acts | 21 (26.3) | 33 (21.9) | 54 (23.4) |
| 30+ acts | 4 (5.0) | 7 (4.6) | 11 (4.8) |
| Condom use (typical month in last 3 months) | |||
| Always | 22 (27.5) | 38 (25.2) | 60 (26.0) |
| Sometimes | 22 (27.5) | 44 (29.1) | 66 (28.6) |
| Never | 31 (38.8) | 58 (38.4) | 89 (38.5) |
| No sexual partner | 5 (6.3) | 11 (7.3) | 16 (6.9) |
| Behavioral Characteristics | |||
| Participant behavioral risk in last 3 months | 2 (2.5) | 10 (6.6) | 12 (5.2) |
| Primary partner spend any nights away from home in the last 30 day | 39 (48.8) | 75 (49.7) | 114 (49.4) |
| Reported symptoms and conditions | |||
| Nausea | 2 (2.5) | 10 (6.6) | 12 (5.2) |
| Current cervical infection (CT, GC) | 10 (12.5) | 13 (8.6) | 23 (10.0) |
| HIV-related symptoms and clinical characteristics | |||
| Excessive night sweats | 7 (8.8) | 9 (6.0) | 16 (6.9) |
| Loss of appetite or weight | 3 (3.8) | 10 (6.6) | 13 (5.6) |
| CD4 count | |||
| ≤200 cells/mm3 | 4 (1.3) | 4 (2.7) | 8 (3.5) |
| 201–499 cells/mm3 | 32 (40.0) | 63 (41.7) | 95 (41.1) |
| ≥500 cells/mm3 | 43 (53.8) | 82 (54.3) | 125 (54.1) |
Women in the study were approximately equally distributed among the age groups 19–24, 25–29 and 30+. The majority of women had at least two children (78%) and reported 0–14 sexual acts in a typical month (72%). Among women with partners, self-reported condom use was high but inconsistent; only 60 (26%) women reported always using a condom. Only 16 (7%) reported no condom use due to no partner (Table 1).
HIV-related side effects such as excessive night sweats and loss of appetite or weight were uncommon. Nausea was slightly higher among DMPA than OC users (7% vs 3%). Slightly more OC than DMPA users had a current cervical infection (CT or GC) at baseline (13% vs 9%). CD4 counts in the study population were relatively high at baseline; 125 (54%) had CD4 counts ≥500 cells/mm3 (Table 1).
Over the eight year study period, OC users continued on their method about twice as long as DMPA users. The median time to discontinuation for OC users was 35 months (95% CI 14, 61) and 19 months (95% CI 14, 24) for DMPA users (Figure 1). However, among women in Uganda the median times to discontinuation for each method were nearly identical; 21 months (95% CI 6, 36) for OC users and 21 months (95% CI 14, 31) for DMPA users. Conversely, in Zimbabwe median DMPA use was 17 months (95% CI 12, 24) while OC discontinuation never reached the median (50th) percentile (Figure 2). Similar trends were seen in sensitivity analyses when the duration of contraceptive use included time before enrollment (36 months for OCs; 21 months for DMPA) and likewise, when the analysis population was restricted to women on HC at study enrollment (36 months for OCs; 16 months for DMPA). Within the first year of use, discontinuation did not vary by method (36% of OC users and 37% of DMPA users).
Figure 1. Kaplan-Meier estimation of discontinuation probabilities by contraceptive groups.
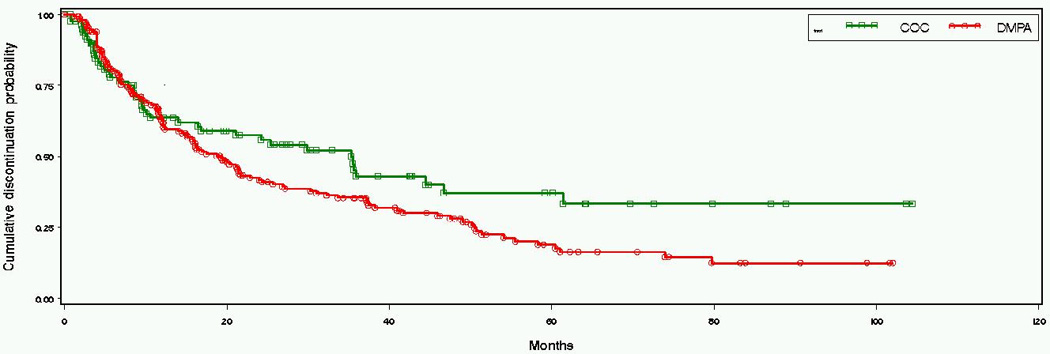
Log rank test for the distributions of time to discontinuation (p-value): 0.069.
Figure 2. Kaplan-Meier estimation of discontinuation probabilities by contraceptive groups and country.
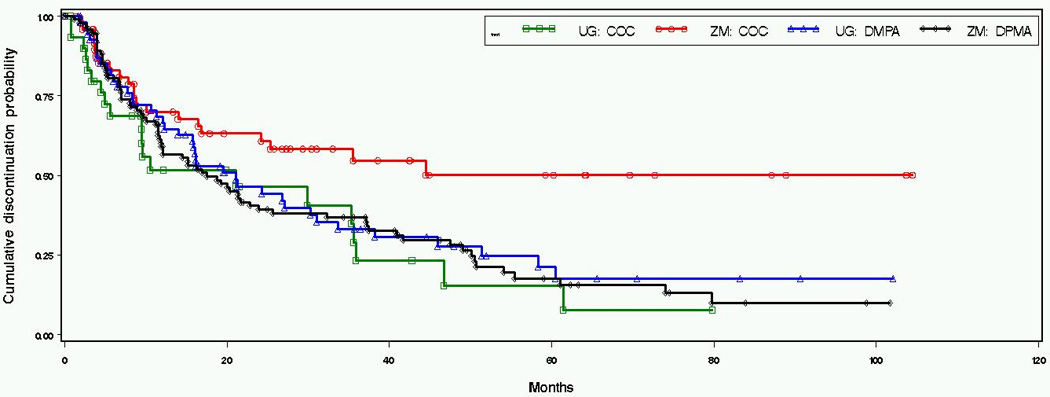
Log rank test for the distributions of time to discontinuation (p-value): 0.021.
At the time of discontinuation, women were asked the main reason for not continuing use of their current method. Of five possible answers, other non-medical reasons (such as no longer having a sexual partner) was the most common (32%), followed by bleeding problems (9%), other side-effects (8%), desires/desired pregnancy (3%) and unintended pregnancy (2%). Bleeding problems were more commonly cited as a reason for discontinuation among DMPA users (11% vs 2% among OC users), whereas ‘other’ side-effects was more commonly cited among OC users (12% vs 6% for DMPA users). Results were similar for reasons for discontinuation of any highly effective method; with a slightly higher proportion reporting desires/desired pregnancy (3%) and unintended pregnancy (3%).
The median time to discontinuation of any highly effective method was also 36 months (95% CI 26, NA) for OC users and 22 months (95% CI 14, 38) for DMPA users. Compared to the median time to method-specific discontinuation, median use of any highly effective method was shorter for DMPA users in Uganda (16 months; 95% CI 11, 38) and longer for DMPA users in Zimbabwe (32 months; 95% CI 12, 49). We found a significant country by method interaction for the hazard of discontinuation (p-value 0.01), and therefore constructed separate multivariable models for OC and DMPA users.
Among women who discontinued their original method but stayed on a highly effective method, all 12 originally on OCs switched to DMPA at their first visit after discontinuing OCs. Among 26 DMPA users who switched to another highly effective method, 25 (96%) switched to OCs and one woman had tubal ligation. At the visit a woman discontinued her original method, 28 (68%) of OC users and 87 (79%) of DMPA users reported having a primary partner.
In bivariable analyses among OC users, younger age (19–24), never using a condom, no sexual partner, participant behavioral risk (defined as multiple partners, new partner, commercial sex work or had sex with another man in the last 3 months) and having a current cervical infection were associated with an increased hazard of OC discontinuation. Increased coital frequency (15–29 acts) and living in Zimbabwe were protective against OC discontinuation. In multivariable analyses, living in Zimbabwe remained significantly protective against OC discontinuation (HR 0.39; 95% CI 0.18, 0.83), while not having a partner (HR 7.18; 95% CI 3.05, 16.88) remained associated with an increased hazard of discontinuation and current cervical infection (HR 1.99; 95% CI 0.90, 4.41) was marginally associated with increased OC discontinuation (Table 2).
Table 2. Factors associated with discontinuation of OCs among HIV-infected women in Uganda and Zimbabwe.
Additional factors investigated which were not significant in bivariable models include: living with a partner, education, parity, currently breastfeeding, primary partner’s HIV status, primary partner spent nights away, alcohol use, severe headaches, nausea, breast tenderness, bleeding between periods, abnormal vaginal discharge, current vaginal infection (TV, BV, Candida, unexplained fever, excessive night sweats, loss of appetite or weight and fatigue that interferes with daily living.
| Characteristic | Bivariable HR (95% CI) |
p- value |
Multivariable HR (95% CI) |
p- value |
|---|---|---|---|---|
| Time-invariant covariates | ||||
| Uganda | 1.00 | 1.00 | ||
| Zimbabwe | 0.48 (0.26, 0.84) | 0.01 | 0.39 (0.18, 0.83) | 0.01 |
| Age at method start (years) | ||||
| 30+ | 1.00 | 1.00 | ||
| 25–29 | 0.96 (0.44, 2.10) | 0.92 | 0.98 (0.45, 2.10) | 0.95 |
| 19–24 | 2.00 (0.96, 4.14) | 0.06 | 1.72 (0.74, 4.01) | 0.21 |
| Time-varying covariates | ||||
| Coital Frequency (typical month in last 3 months) | ||||
| 0–14 acts | 1.00 | N/A | ||
| 15–29 acts | 0.48 (0.20, 1.16) | 0.10 | N/A | |
| 30+ acts | 0.67 (0.15, 2.97) | 0.60 | N/A | |
| Condom use (typical month in last 3 months) | ||||
| Always | 1.00 | 1.00 | ||
| Sometimes | 1.32 (0.36, 4.83) | 0.67 | 1.03 (0.28, 3.79) | 0.97 |
| Never | 2.43 (1.01, 5.85) | 0.05 | 1.26 (0.51, 3.12) | 0.62 |
| No sexual partner | 7.02 (2.94, 16.78) | <0.01 | 7.18 (3.05, 16.88) | <0.01 |
| Participant behavioral risk in last 3 months | 5.81 (1.18, 28.53) | 0.03 | N/A | |
| Current cervical infection (GC or CT) | 2.01 (0.87, 4.66) | 0.10 | 1.99 (0.90, 4.41) | 0.09 |
| CD4 count | ||||
| ≤200 cells/mm3 | 1.00 | 1.00 | ||
| 201–499 cells/mm3 | 0.68 (0.21, 2.21) | 0.52 | 1.22 (0.30, 4.95) | 0.78 |
| ≥500 cells/mm3 | 1.45 (0.49, 4.29) | 0.50 | 1.54 (0.45, 5.33) | 0.49 |
Among DMPA users in bivariable analyses, no sexual partner, nausea, excessive night sweats and loss of appetite or weight were significantly associated with an increased hazard of discontinuation. Having a primary partner spend any nights away from home in the last month and baseline CD4 count 201–499 cells/mm3 were protective against discontinuation. In multivariable analyses, no sexual partner (HR 2.00; 95% CI 1.12, 3.58) and nausea (HR 1.84; 95% CI 1.02, 3.34) were significantly associated with an increased hazard of discontinuation and excessive night sweats (HR 1.80; 95% CI 0.95, 3.40) was marginally associated with increased DMPA discontinuation (Table 3).
Table 3. Factors associated with discontinuation of DMPA among HIV-infected women in Uganda and Zimbabwe.
Additional factors investigated which were not significant in bivariable models include: living with a partner, education, parity, participant’s behavioral risk, primary partner’s HIV status, alcohol use, coital frequency, severe headaches, breast tenderness, bleeding between periods, abnormal vaginal discharge, current vaginal infection (TV, BV, Candida), current cervical infection (CT or GC), unexplained fever and fatigue that interferes with daily living.
| Characteristic | Bivariable HR (95% CI) |
p-value | Multivariable HR (95% CI) |
p-value |
|---|---|---|---|---|
| Time-invariant covariates | ||||
| Uganda | 1.00 | 1.00 | ||
| Zimbabwe | 1.10 (0.75, 1.63) | 0.63 | 0.85 (0.54, 1.34) | 0.49 |
| Age at method start (years) | ||||
| 30+ | 1.00 | 1.00 | ||
| 25–29 | 1.08 (0.66, 1.75) | 0.77 | 1.17 (0.71, 1.92) | 0.53 |
| 19–24 | 1.05 (0.63, 1.75) | 0.85 | 1.03 (0.60, 1.75) | 0.93 |
| Time-varying covariates | ||||
| Condom use (typical month in last 3 months) | ||||
| Always | 1.00 | 1.00 | ||
| Sometimes | 0.89 (0.49, 1.63) | 0.71 | 0.86 (0.45, 1.65) | 0.65 |
| Never | 0.76 (0.46, 1.24) | 0.26 | 0.68 (0.38, 1.21) | 0.18 |
| No sexual partner | 2.24 (1.22, 4.11) | <0.01 | 2.00 (1.12, 3.58) | 0.02 |
| Primary partner spend any nights away from home in the last 30 | 0.72 (0.48, 1.07) | 0.10 | N/A | |
| Nausea | 1.94 (1.05, 3.59) | 0.04 | 1.84 (1.02, 3.34) | 0.04 |
| Excessive night sweats | 2.08 (1.11, 3.90) | 0.02 | 1.80 (0.95, 3.40) | 0.07 |
| Loss of appetite or weight | 1.89 (0.96, 3.71) | 0.06 | N/A | |
| CD4 count | ||||
| ≤200 cells/mm3 | 1.00 | 1.00 | ||
| 201–499 cells/mm3 | 0.52 (0.24, 1.11) | 0.09 | 0.58 (0.27, 1.24) | 0.16 |
| ≥500 cells/mm3 | 0.57 (0.26, 1.21) | 0.14 | 0.72 (0.33, 1.54) | 0.39 |
Discussion
In this prospective, multi-site cohort study of hormonal contraceptive use among HIV-infected women, overall median time to discontinuation within the eight year study period was relatively long; over 35 months for OC users and 19 months for DMPA users. In sensitivity analyses, median time to discontinuation did not change dramatically when time on HC between seroconversion and study enrollment was included or when the study population was restricted to women on HC at study enrollment. Many women were enrolled in the study soon after HIV seroconversion, therefore time on HC after seroconversion and before study entry was generally short. In a similar analysis of HC discontinuation among HIV-uninfected women, median duration of use from study entry was the same for DMPA users (19 months) but shorter for OC users (16 months), however participants were only followed for 15–24 months32. The median time to discontinuation of any highly effective method was similar; nearly 36 months for OC users and 22 months for DMPA users. Despite the extensive duration of HC use, 65% of women discontinued their original method at some point during follow-up.
While overall OC use was nearly twice as long as DMPA use, the length of time on a method varied by country. Living in Zimbabwe, compared to Uganda, was associated with over a 60% reduction in the hazard of discontinuing OCs. In contrast, among DMPA users, country was not an important factor for time to discontinuation. Historical country-specific programming may explain these observations. In Zimbabwe, family planning programs have traditionally emphasized OC use over DMPA and 41% of currently married Zimbabwean women use OCs33. In Uganda women tend to prefer injectables, however fewer women overall use modern contraception and only 14% use injectables34.
Little research on HC use by HIV-infected women has focused on contraceptive use over an extended time period or included time-varying factors related to contraceptive use35–37. By incorporating time-varying factors into our study, including side-effects related to HIV infection and HC use, as well as sexual behavior characteristics, we were able to assess how these factors influence HC discontinuation over time.
Throughout the follow-up period, few HIV-related side effects were associated with increased HC discontinuation. Symptoms suggesting opportunistic infections have previously been inversely associated with HC use38, however, in our study this was not the case. Consistent with previous work, higher CD4 counts were protective against DMPA discontinuation, although these results were not statistically significant39. The reverse was true for OC users; however, due to sparse data the estimates for the association between CD4 count and discontinuation among OC users are imprecise. Women in our analysis were censored at HAART initiation (HAART was not available throughout the study); therefore we cannot assess the impact of HAART use on HC discontinuation. However, our results suggest that pre-HAART initiation, HIV-related symptoms do not contribute significantly to HC discontinuation.
Few non-HIV related side-effects or health conditions were significant predictors of HC discontinuation in our study. Previous work has shown that side effects are often a primary reason for HC discontinuation37,40. In a similar analysis among HIV-uninfected women, side-effects including breast tenderness, nausea and bleeding problems (DMPA users) were significantly associated with HC discontinuation32. However, in our analysis only nausea was associated with DMPA discontinuation. Having a cervical infection was the only other health condition associated with OC discontinuation. The most commonly reported reason for discontinuation of original method was ‘other non-medical reasons’ (59% among OC users and 22% among DMPA users), suggesting that the decision to switch contraceptive methods may have more to do with preferences unrelated to side-effects.
Consistent with previous studies, not having a partner was strongly associated with discontinuation for both DMPA and OC users41–43. However, at the time of original method discontinuation, 86% of OC users and 79% of DMPA users reported having a primary partner. Such a large proportion of HIV-infected women with sexual partners highlights the importance of HC as an option for managing their reproductive choices.
Among HIV-uninfected women, younger age has been associated with HC discontinuation32,44. In our study, younger age (19–24 years) at method start was associated with discontinuation for OC users, although the results were not statistically significant. Age was not associated with DMPA discontinuation. Upon learning they are HIV-infected, many women choose to switch from combined oral contraceptive pills to an injectable contraceptive8,32,45. Our results suggest that injectables may be preferable in particular for younger HIV-infected women interested in long-term HC use46.
Discontinuation of a woman’s original method due to pregnancy, intended or unintended, was notably low in our analysis. Other studies have reported parity as being associated with HC use35,38. However, neither having two or more children at baseline or breastfeeding during follow-up (for DMPA users) were associated with HC discontinuation. This is likely explained by parity being relatively high among study participants, since most women already had at least two children. Further, while HIV-infected women may continue to desire children, they may choose to have fewer children overall, compared to HIV-uninfected women10–12.
Our study has several important strengths. We were able to collect extensive information on both clinical and behavioral factors that influence contraceptive use, and had the ability to analyze how these factors changed over an extended period of time. In addition, we evaluated how morbidity associated with HIV infection and disease progression influenced contraceptive use over time. A limitation of our study was the inability to account for multiple contraceptive method switches and only evaluate the median time to first method switch. In addition, because HAART was not available for the entire study women were censored at HAART initiation and therefore we could not evaluate contraceptive use after treatment initiation. Fertility preferences were not included in the multivariable analysis, however at the time of discontinuation only 5% of women reported discontinuing due to intended or unintended pregnancy, suggesting that fertility intention was not a primary reason for HC discontinuation.
Providing safe and effective contraceptive choices for HIV-infected women is critical to prevent unintended pregnancies and reduce vertical HIV transmission. As access to HAART expands and the risk of vertical transmission decreases, HIV-infected women will increasingly need effective contraception to attain their reproductive goals. Our results suggest that HC methods are acceptable and feasible for HIV-infected women to use over longer periods of time, but that women often chose to discontinue a method for a variety of reasons. Providing HIV-infected women with an expanded range of contraceptive choices, including longer-acting reversible methods (e.g., IUDs, implants), is urgently needed. Access to a range of contraceptive options and ongoing contraceptive counseling are essential components of improving HIV-infected women’s reproductive control and decreasing HIV transmission.
Acknowledgements
The authors would like to thank Ward Cates and Kavita Nanda for their helpful comments on an earlier version of this manuscript. This project has been funded with federal funds from the Eunice Shriver Kennedy National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH), Department of Health and Human Services through a contract with FHI 360 (Contract Number N01-HD-0-3310). Ms. Bengtson was supported by a fellowship through the University of North Carolina, Chapel Hill and FHI 360. The views expressed in this document are those of the authors and do not necessarily reflect those of FHI 360 or the funding agencies.
Sources of support: This project has been funded with federal funds from the Eunice Shriver Kennedy National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH), Department of Health and Human Services through a contract with FHI 360 (Contract Number N01-HD-0-3310). Ms. Bengtson was supported by a fellowship through the University of North Carolina, Chapel Hill and FHI360.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meetings presented at: Consortium of Universities for Global Health 4th Annual Conference
Conflicts of Interest: None declared
References
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS), World Health Organization. UNAIDS world AIDS day report. 2011
- 2.Agboghoroma CO. Contraception in the context of HIV/AIDS: A review. Afr J Reprod Health. 2011;15(3):15–23. [PubMed] [Google Scholar]
- 3.Coll O, Lopez M, Hernandez S. Fertility choices and management for HIV-positive women. Curr Opin HIV AIDS. 2008;3(2):186–192. doi: 10.1097/COH.0b013e3282f51219. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell HS, Stephens E. Contraception choice for HIV positive women. Sex Transm Infect. 2004;80(3):167–173. doi: 10.1136/sti.2003.008441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reynolds HW, Janowitz B, Homan R, Johnson L. The value of contraception to prevent perinatal HIV transmission. Sex Transm Dis. 2006;33(6):350–356. doi: 10.1097/01.olq.0000194602.01058.e1. [DOI] [PubMed] [Google Scholar]
- 6.Sweat MD, O'Reilly KR, Schmid GP, Denison J, de Zoysa I. Cost-effectiveness of nevirapine to prevent mother-to-child HIV transmission in eight African countries. AIDS. 2004;18(12):1661–1671. doi: 10.1097/01.aids.0000131353.06784.8f. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents. recommendations for a public health approach. 2010 [PubMed]
- 8.Hoffman IF, Martinson FE, Powers KA, et al. The year-long effect of HIV-positive test results on pregnancy intentions, contraceptive use, pregnancy incidence among Malawian women. J Acquir Immune Defic Syndr. 2008;47(4):477–483. doi: 10.1097/QAI.0b013e318165dc52. [DOI] [PubMed] [Google Scholar]
- 9.Kaida A, Laher F, Strathdee SA, et al. Childbearing intentions of HIV-positive women of reproductive age in Soweto, South Africa: The influence of expanding access to HAART in an HIV hyperendemic setting. Am J Public Health. 2011;101(2):350–358. doi: 10.2105/AJPH.2009.177469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.da Silveira Rossi A, Fonsechi-Carvasan GA, Makuch MY, Amaral E, Bahamondes L. Factors associated with reproductive options in HIV-infected women. Contraception. 2005;71(1):45–50. doi: 10.1016/j.contraception.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Ezeanolue EE, Stumpf PG, Soliman E, Fernandez G, Jack I. Contraception choices in a cohort of HIV+ women in the era of highly active antiretroviral therapy. Contraception. 2011;84(1):94–97. doi: 10.1016/j.contraception.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Cooper D, Harries J, Myer L, Orner P, Bracken H, Zweigenthal V. "Life is still going on": Reproductive intentions among HIV-positive women and men in South Africa. Soc Sci Med. 2007;65(2):274–283. doi: 10.1016/j.socscimed.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Joint United Nations Programme on HIV/AIDS (UNAIDS), World Health Organization. UNAIDS world AIDS day report. 2012
- 14.World Health Organization. Towards universal access: Scaling up priority HIV/AIDS interventions in the health sector. 2010
- 15.Schouten EJ, Jahn A, Midiani D, et al. Prevention of mother-to-child transmission of HIV and the health-related millennium development goals: Time for a public health approach. Lancet. 2011;378(9787):282–284. doi: 10.1016/S0140-6736(10)62303-3. [DOI] [PubMed] [Google Scholar]
- 16.WHO HIV/AIDS Programme. Programmatic update. use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. 2012
- 17.Wilcher R, Cates W. Reproductive choices for women with HIV. Bull World Health Organ. 2009;87(11):833–839. doi: 10.2471/BLT.08.059360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Department of Reproductive Health, World Health Organization. Medical eligibility criteria for contraceptive use. (4th ed) 2010
- 19.Lavreys L, Baeten JM, Kreiss JK, et al. Injectable contraceptive use and genital ulcer disease during the early phase of HIV-1 infection increase plasma virus load in women. J Infect Dis. 2004;189(2):303–311. doi: 10.1086/380974. [DOI] [PubMed] [Google Scholar]
- 20.Stringer EM, Kaseba C, Levy J, et al. A randomized trial of the intrauterine contraceptive device vs hormonal contraception in women who are infected with the human immunodeficiency virus. Am J Obstet Gynecol. 2007;197(2):144.e1–144.e8. doi: 10.1016/j.ajog.2007.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stringer EM, Levy J, Sinkala M, et al. HIV disease progression by hormonal contraceptive method: Secondary analysis of a randomized trial. AIDS. 2009;23(11):1377–1382. doi: 10.1097/QAD.0b013e32832cbca8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrison CS, Chen PL, Nankya I, et al. Hormonal contraceptive use and HIV disease progression among women in Uganda and Zimbabwe. J Acquir Immune Defic Syndr. 2011;57(2):157–164. doi: 10.1097/QAI.0b013e318214ba4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stringer EM, Giganti M, Carter RJ, et al. Hormonal contraception and HIV disease progression: A multicountry cohort analysis of the MTCT-plus initiative. AIDS. 2009;23(Suppl 1):69–77. doi: 10.1097/01.aids.0000363779.65827.e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polis CB, Wawer MJ, Kiwanuka N, et al. Effect of hormonal contraceptive use on HIV progression in female HIV seroconverters in Rakai, Uganda. AIDS. 2010;24(12):1937–1944. doi: 10.1097/QAD.0b013e32833b3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allen S, Stephenson R, Weiss H, et al. Pregnancy, hormonal contraceptive use, HIV-related death in Rwanda. J Womens Health (Larchmt) 2007;16(7):1017–1027. doi: 10.1089/jwh.2006.0151. [DOI] [PubMed] [Google Scholar]
- 26.Phillips SJ, Curtis KM, Polis CB. Effect of hormonal contraceptive methods on HIV disease progression: A systematic review. AIDS. 2012 doi: 10.1097/QAD.0b013e32835bb672. [DOI] [PubMed] [Google Scholar]
- 27.Polis CB, Phillips SJ, Curtis KM. Hormonal contraceptive use and female-to-male HIV transmission: A systematic review of the epidemiologic evidence. AIDS. 2013;27(4):493–505. doi: 10.1097/QAD.0b013e32835ad539. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. Hormonal contraception and HIV: Technical statement. 2012 [PubMed]
- 29.Morrison CS, Demers K, Kwok C, et al. Plasma and cervical viral loads among Ugandan and Zimbabwean women during acute and early HIV-1 infection. AIDS. 2010;24(4):573–582. doi: 10.1097/QAD.0b013e32833433df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrison CS, Richardson BA, Mmiro F, et al. Hormonal contraception and the risk of HIV acquisition. AIDS. 2007;21(1):85–95. doi: 10.1097/QAD.0b013e3280117c8b. [DOI] [PubMed] [Google Scholar]
- 31.Lancaster K. Pregnancy outcomes among HIV-infected women in Uganda and Zimbabwe. The 3rd International Workshop on HIV and Women. 2013 doi: 10.1016/j.ijgo.2015.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nanda K, Morrison CS, Kwok C, et al. Discontinuation of oral contraceptives and depot medroxyprogesterone acetate among women with and without HIV in Uganda, Zimbabwe and Thailand. Contraception. 2011;83(6):542–548. doi: 10.1016/j.contraception.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Zimbabwe National Statistics Agency (ZIMSTAT) and ICF International. Zimbabwe demographic and health survey 2010-11. 2012
- 34.Uganda Bureau of Statistics (UBOS) and ICF International. Uganda demographic and health survey 2011, preliminary report. 2012
- 35.Ali MM, Cleland J. Oral contraceptive discontinuation and its aftermath in 19 developing countries. . Contraception. 2010;81(1):22–29. doi: 10.1016/j.contraception.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Barden-O'Fallon J, Speizer IS, Calix J, Rodriguez F. Contraceptive discontinuation among Honduran women who use reversible methods. Stud Fam Plann. 2011;42(1):11–20. doi: 10.1111/j.1728-4465.2011.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blanc AK, Tsui AO, Croft TN, Trevitt JL. Patterns and trends in adolescents' contraceptive use and discontinuation in developing countries and comparisons with adult women. Int Perspect Sex Reprod Health. 2009;35(2):63–71. doi: 10.1363/ipsrh.35.063.09. [DOI] [PubMed] [Google Scholar]
- 38.Polis CB, Gray RH, Lutalo T, et al. Trends and correlates of hormonal contraceptive use among HIV-infected women in Rakai, Uganda, 1994–2006. Contraception. 2011;83(6):549–555. doi: 10.1016/j.contraception.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Massad LS, Evans CT, Wilson TE, et al. Contraceptive use among U.S. women with HIV. J Womens. Health (Larchmt) 2007;16(5):657–666. doi: 10.1089/jwh.2006.0204. [DOI] [PubMed] [Google Scholar]
- 40.Khan MA. Factors associated with oral contraceptive discontinuation in rural Bangladesh. Health. Policy Plan. 2003;18(1):101–108. doi: 10.1093/heapol/18.1.101. [DOI] [PubMed] [Google Scholar]
- 41.Beyeza-Kashesya J, Kaharuza F, Ekstrom AM, Neema S, Kulane A, Mirembe F. To use or not to use a condom: A prospective cohort study comparing contraceptive practices among HIV-infected and HIV-negative youth in Uganda. BMC Infect Dis. 2011;11:144-2334-11-144. doi: 10.1186/1471-2334-11-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frost JJ, Darroch JE. Factors associated with contraceptive choice and inconsistent method use, united states, 2004. Perspect Sex Reprod Health. 2008;40(2):94–104. doi: 10.1363/4009408. [DOI] [PubMed] [Google Scholar]
- 43.Yarnall KS, McBride CM, Lyna P, et al. Factors associated with condom use among at-risk women students and nonstudents seen in managed care. Prev Med. 2003;37(2):163–170. doi: 10.1016/s0091-7435(03)00109-9. [DOI] [PubMed] [Google Scholar]
- 44.Raine TR, Foster-Rosales A, Upadhyay UD, et al. One-year contraceptive continuation and pregnancy in adolescent girls and women initiating hormonal contraceptives. Obstet Gynecol. 2011;117(2 Pt 1):363–371. doi: 10.1097/AOG.0b013e31820563d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Magalhaes J, Amaral E, Giraldo PC, Simoes JA. HIV infection in women: Impact on contraception. . Contraception. 2002;66(2):87–91. doi: 10.1016/s0010-7824(02)00332-3. [DOI] [PubMed] [Google Scholar]
- 46.Mark KE, Meinzen-Derr J, Stephenson R, et al. Contraception among HIV concordant and discordant couples in Zambia: A randomized controlled trial. J Womens Health (Larchmt) 2007;16(8):1200–1210. doi: 10.1089/jwh.2006.0238. [DOI] [PubMed] [Google Scholar]


