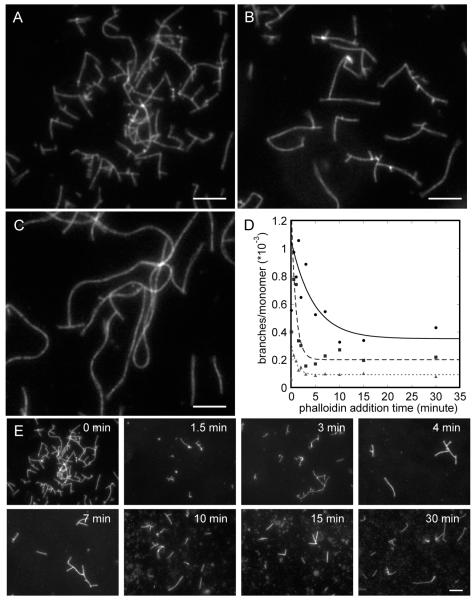
Figure 5.
Fluorescence micrographs of actin filaments stained with rhodamine-phalloidin. Actin monomers at concentrations of 1, 2, or 4 μM were polymerized with 10 nM Arp2/3 complex and 300 nM GST-hWASp-VCA in buffer containing 50 mM KCl, 1 mM EGTA, 1 mM MgCl2, and 10 mM imidazole, pH 7.0. (A–C) Reactions with equimolar rhodamine-phalloidin present during the polymerization reaction. Images are shown for (A) 1 μM actin, (B) 2 μM actin, and (C) 4 μM actin. (D, E) Rhodamine-phalloidin equimolar to actin was added at times ranging from 0 to 30 min after initiating the reaction. (D) Counts of the number of branches per subunit of filament in the micrograph. Exponential fits through the data (excluding the points at zero time) yield half-times of 4.6 ± 2.5 min with 1 μM actin (filled circles), 0.9 ± 0.3 min with 2 μM actin (filled squares), and 1.1 ± 0.2 min with 4 μM actin (triangles). (E) Micrographs from an experiment with phalloidin added from 0 to 30 min to a reaction with 1 μM actin, 10 nM Arp2/3 complex, and 300 nM GST-hWASp-VCA.