Abstract
Tumor heterogeneity presents a substantial barrier to increasing clinical responses mediated by targeted therapies. Broadening the immune response elicited by treatments that target a single antigen is necessary for the elimination of tumor variants that fail to express the targeted antigen. In this study, it is shown that adoptive transfer of T cells bearing a chimeric antigen receptor (CAR) inhibited the growth of target-expressing and –deficient tumor cells within ovarian and lymphoma tumors. Mice bearing the ID8 ovarian or RMA lymphoma tumors were treated with T cells transduced with a NKG2D-based CAR (chNKG2D). NKG2D CAR T cell therapy protected mice from heterogeneous RMA tumors. Moreover, adoptive transfer of chNKG2D T cells mediated tumor protection against highly heterogeneous ovarian tumors in which 50%, 20%, or only 7% of tumor cells expressed significant amounts of NKG2D ligands. CAR T cells did not mediate an in vivo response against tumor cells that did not express sufficient amounts of NKG2D ligands, and the number of ligand-expressing tumor cells correlated with therapeutic efficacy. In addition, tumor-free surviving mice were protected against a tumor re-challenge with NKG2D ligand-negative ovarian tumor cells. These data indicate that NKG2D CAR T cell treatment can be an effective therapy against heterogeneous tumors and induce tumor-specific immunity against ligand-deficient tumor cells.
Keywords: chNKG2D, adoptive T cell therapy, immunotherapy, chimeric antigen receptors, CD8 T cells, epitope spreading
Introduction
Tumor heterogeneity and acquired resistance present two significant obstacles to the clinical success of anti-cancer treatments. Tumor cells within the same neoplasm often express heterogeneous antigens on their cell surface and selectively lose expression of a target antigen following any treatment targeting a specific molecule 1–3. This diversity in antigen expression contributes to tumor persistence and incomplete responses in some clinical trials employing adoptive T cell transfer 4, 5. However, some adoptive T cell therapies have been shown to induce objective responses and decrease morbidity and mortality in some scenarios 6–9. The ability of any targeted therapy to mediate long-term clinical remission is dependent on the elimination of tumor variants that lose expression of the targeted antigens.
Harnessing endogenous lymphocyte immunity is one method of enhancing the efficacy of therapies targeting a single molecule. Although host lymphocytes infiltrate tumors, they are often unable to reduce tumor growth and may persist in a suppressed state due to tumor-mediated immune regulation. However, therapies that modify the tumor microenvironment are capable of relieving immunosuppression and activating host lymphocytes to promote tumor destruction 10–14. In fact, anti-cancer treatments targeting a single molecule have been shown to activate an endogenous response against non-targeted tumor antigens 15–20. Since host T cells can express a broad receptor repertoire that recognizes many tumor antigens and once activated are capable of responding against tumors, these host T cells may enhance the efficacy of anti-cancer treatments by controlling the outgrowth of tumor variants.
Methods of re-directing T cell specificity to MHC unrestricted tumor antigens have been developed. Chimeric antigen receptor (CAR) transduced T cells have been engineered to recognize CD19, Her2/neu, NKG2D ligands, and a variety of other targets 21, 22. CAR expressing cells signal through CD3ζ and other co-stimulatory molecules to activate T cell effector function and induce tumor elimination following engagement with target-positive tumor cells 22. Treatment of tumor-bearing mice with NKG2D CAR T cells induces long-term tumor-free survival in several tumor models, including the ID8 ovarian cancer model 23–25. NKG2D CAR T cells activate endogenous tumor-specific CD8+ and CD4+ T cell responses that are required for optimal elimination of the tumor 24, 26, 27. However, CAR T cells target a single antigen, so heterogeneity in target antigen expression within the tumor may impair CAR T cell-mediated tumor destruction. This study demonstrates that NKG2D CAR T cell treatment inhibits the growth of heterogeneous tumors consisting of NKG2D ligand-expressing and ligand-deficient tumor cells. Furthermore, tumor-free mice were protected from a challenge with NKG2D ligand-deficient tumor cells. These data demonstrate the ability of NKG2D CAR T cells to treat ligand heterogeneous tumors and prevent tumor variant outgrowth. In addition, these data highlight the potential for CAR expressing T cells to attack tumor cells and shape the tumor microenvironment to promote host immunity to eliminate tumors.
Results and Discussion
CAR T cell therapy treats heterogeneous lymphomas and ovarian tumors
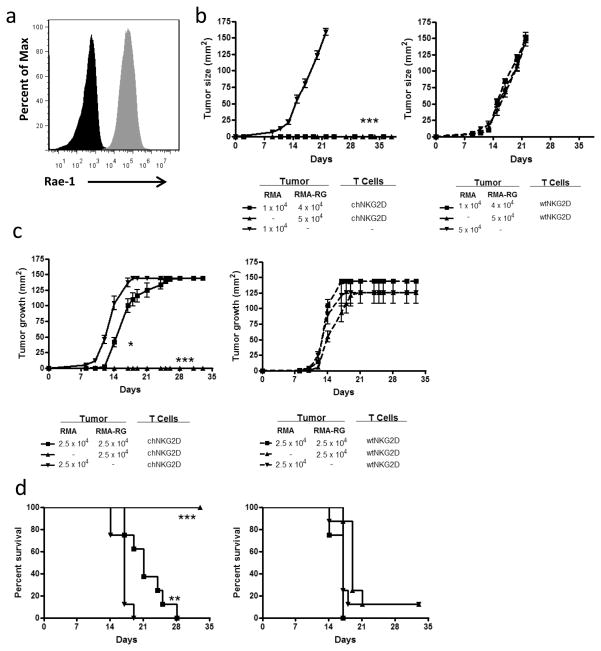
Tumor antigen expression is often heterogeneous within the tumor 28, 29. When a single targeting agent is used, it may lead to the survival and outgrowth of tumor cells that have lost or reduced expression of the targeted molecule. Because adoptively transferred effector T cells have the ability to directly attack tumors and activate host anti-tumor immunity, it is possible for this type of immunotherapy to result in a host immune response against tumor antigens other than the target antigen, a phenomenon referred to as epitope spreading. To determine whether adoptive T cell therapy with NKG2D CAR T cells induced a response that inhibited the growth of NKG2D ligand-deficient tumor cells within a heterogeneous tumor, mice were inoculated with mixtures of ligand+ and ligand- tumor cells along with chNKG2D T cells or wtNKG2D control T cells. RMA-RG tumor cells express high amounts of the NKG2D ligand Rae-1 (Fig. 1A). Mice received 5 × 104 or 1 × 104 RMA lymphoma cells (no ligands), 5 × 104 RMA-RG tumor cells (ligand+), or a mixture of 1 × 104 RMA and 4 × 104 RMA-RG tumor cells (20% ligand− cells). In the same inoculation, mice were treated with chNKG2D or wtNKG2D T cells. ChNKG2D T cell treatment resulted in no tumor growth, while mice treated with wtNKG2D T cells failed to control the growth of heterogeneous lymphoma tumors (Fig 1B). In a second set of experiments, mice received 2.5 × 104 RMA lymphoma cells (ligand−), 2.5 × 104 RMA-RG tumor cells (ligand+), or a mixture of 2.5 × 104 RMA and 2.5 × 104 RMA-RG tumor cells (50% ligand−). The mice receiving the 1:1 tumor mixture received an equal number (2.5 × 104 cells) of each tumor type as the single tumor control groups, and this was double the total tumor cell inoculums of the controls. NKG2D CAR T cell treatment impaired tumor growth and enhanced the survival of mice given heterogeneous lymphoma tumors in which half of the tumor cells lacked expression of NKG2D ligands, and resulted in no tumor growth in mice given NKG2D ligand expressing tumor cells (Fig. 1C and 1D). Mice given ligand-deficient RMA tumor cells and chNKG2D T cells did not have altered tumor growth compared to control mice with RMA tumor cells. These data demonstrate that NKG2D CAR T cell therapy impairs the growth of NKG2D ligand-negative tumor cells within ligand heterogeneous lymphomas.
Figure 1. CAR T cell therapy treats heterogeneous lymphomas.
(a) Rae-1 expression on RMA (black) and RMA-RG (gray) tumor cells was assessed by flow cytometry. (b) RMA tumor cells (1 × 104 or 5 × 104), RMA-RG cells (5 × 104) or a 4:1 mixture of both tumor cells (RMA-RG:RMA) were injected s.c. into mice (n = 8). At the same time as tumor inoculation, wtNKG2D or chNKG2D T cells were injected together with the tumor cells s.c.. Tumor area was measured and data are presented in mm2. (***, p < 0.001 vs. wtNKG2D). RMA tumor cells (2.5 × 104), RMA-RG cells (2.5 × 104) or a 1:1 mixture of both tumor cells (2.5 × 104 RMA-RG: 2.5 × 104 RMA) were injected s.c. into mice (n = 8). At the same time, wtNKG2D T cells or chNKG2D T cells were injected together with the tumor cells s.c.. (c) Tumor area and (d) survival were measured (n = 8). Data are presented in mm2. (*, p < 0.05; **, p < 0.01; ***, p < 0.001 vs. RMA + chNKG2D). Differences between groups in a and b were analyzed by ANOVA and Newman-Keuls post test, and by log-rank test in c. SD is shown.
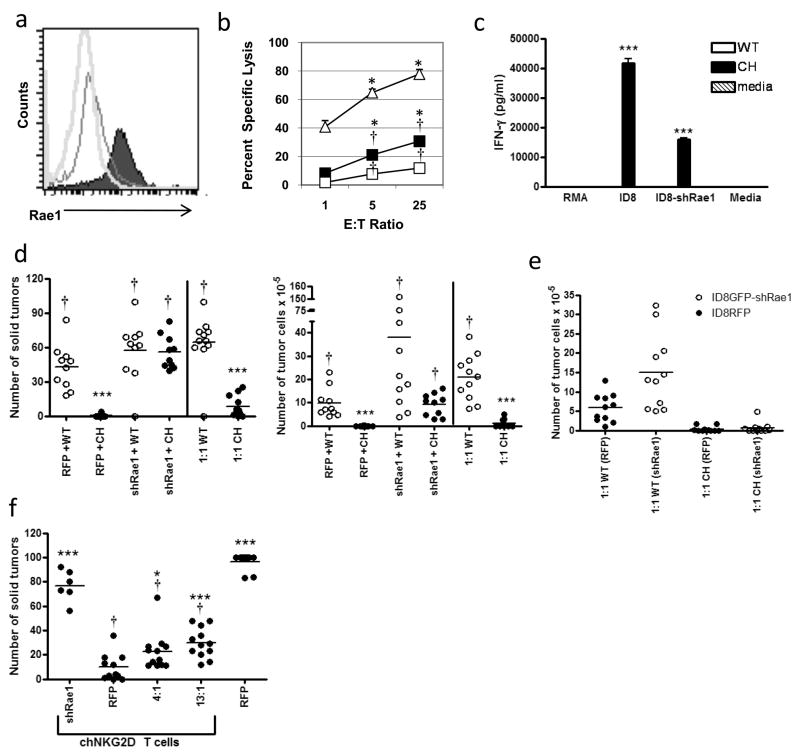
It was previously shown that T cell effector cytokines secreted by adoptively transferred T cells recruit host leukocytes and transform the tumor milieu from immunosuppressive to immunostimulatory in ID8 ovarian carcinoma 24, 26. The CAR T cell treatment-induced pro-inflammatory changes to the tumor microenvironment likely enhance the efficacy of NKG2D CAR T cell therapy by promoting destruction of any ligand-negative tumor cells. To determine the extent that chNKG2D T cell therapy inhibited the growth of NKG2D ligand-negative tumor cells, ID8 tumor cells were engineered to reduce surface expression of NKG2D ligands using an shRNA against all Rae1 isoforms (Fig. 2A). ID8 ovarian cancer cells do not express the NKG2D ligands Mult1 or H-60, so reduction in Rae-1 expression results in no NKG2D ligand expression 30. The expression of Rae1 was reduced 90% compared to wildtype ID8 tumor cells (Fig. 2A), and the tumor cells grew at a similar rate in vitro regardless of Rae1 expression. Knockdown of Rae1 significantly impaired chNKG2D T cell lysis of ID8/GFP-shRae1 tumor cells compared to ID8/GFP tumor cells, but there was more lysis of ID8/GFP-shRae1 tumor cells when compared to wtNKG2D T cell lysis (Fig. 2B). ChNKG2D T cells also produced significantly less IFN-γ in response to stimulation with ID8/GFP-shRae1 cells compared to ID8/GFP cells (Fig. 2C). Importantly, the expression of Rae1was reduced to the point that treatment with chNKG2D T cells was ineffective at inhibiting the growth of ID8/GFP-shRae1 cells in vivo (Fig 2D).
Figure 2. CAR T cell therapy treats heterogeneous ovarian tumors.
(a) Expression of the NKG2D ligand Rae1 on sorted ID8/GFP-shRae1 cells (black unfilled; MFI 17), ID8/RFP cells (black filled; MFI 89), and isotype control cells (gray unfilled; MFI 10). (b) Lysis of target ID8/GFP cells (unfilled triangle) or ID8/GFP-shRae1 cells (filled square) by effector chNKG2D T cells or lysis of ID8/GFP cells by wtNKG2D T cells (unfilled square) is shown for three different effector to target ratios. (*, p < 0.05 vs. wtNKG2D + ID8/GFP; †, p < 0.05 vs. chNKG2D + ID8/GFP-shRae1). (c) IFN-γ production by wtNKG2D or chNKG2D T cells stimulated with media or irradiated RMA, ID8, or ID8/GFP-shRae1 tumor cells was measured. Differences between groups were analyzed by Student’s t-test (***, p < 0.001 vs. media). (d) Mice (10–12 per group) were injected with 106 ID8/GFP-shRae1 cells, ID8/RFP cells, or a 1:1 mixture of both. The mice receiving the 1:1 tumor mixture received the same number (106 cells) of each tumor type as each single tumor control, which was double the total tumor cell inoculum. On weeks 5, 7, and 9 tumor-bearing mice were treated with 5 × 106 chNKG2D or wtNKG2D T cells. The number of solid tumors and tumor cells in the ascites was assessed on week 12 (***, p < 0.001 vs. RFP+WT; †, p < 0.001 vs. RFP+CH). (e) The number of RFP (Rae1+) and GFP (Rae1−) tumor cells in the ascites of mice bearing 1:1 mixed tumors was assessed. (f) Mice (6–12 per group) were injected with 2 × 106 ID8/GFP-shRae1 cells, ID8/RFP cells, or a 4:1 or 13:1 (ID8/GFP-shRae1: ID8/RFP) mixture of both. Mice were treated with 5 × 106 chNKG2D or wtNKG2D T cells on weeks 5, 7, and 9. Mice were sacrificed twelve weeks post-tumor inoculation and the number of solid tumors was assessed. Differences between groups were analyzed by ANOVA and Newman-Keuls post test. (***, p < 0.001; *, p < 0.05 vs. ID8/RFP (+ chNKG2D T cells); †, p < 0.001 vs. shID8/GFP (+ chNKG2D T cells) and ID8/RFP).
To determine how tumor heterogeneity affected chNKG2D T cell inhibition of ovarian tumor growth, ID8/RFP (ligand expressing) or ID8/GFP-shRae1 (ligand-deficient) tumor cells were injected into mice. Mice were treated with chNKG2D or wtNKG2D T cells 5, 7, and 9 weeks post-tumor injection, a time when solid tumors are known to be present in the peritoneum 26. The chNKG2D T cell treatment inhibited tumor growth in mice bearing ID8/RFP (ligand+), but not in mice bearing ID8/GFP-shRae1 tumors (ligand−) alone, where tumor cells lacked expression of Rae1 (Fig. 2D). Thus, although the expression of Rae1 was reduced but not eliminated by shRNA knockdown, the remaining Rae1 expression did not lead to a significant anti-tumor response by the chNKG2D T cells in vivo. However, chNKG2D T cells reduced tumor burden in mice bearing a 1:1 mixture of ID8/RFP and ID8/GFP-shRae1 tumors compared to wtNKG2D T cell treated mice. The reduction not only occurred due to a decrease in the number of NKG2D ligand+ tumor cells, but also in the number of NKG2D ligand-deficient tumor cells (Fig. 2E). In a second set of experiments, mice received 2 × 106 ID8/RFP (ligand+) cells, ID8/GFP-shRae1 (ligand−) cells, or mixtures of 1:4 RFP:GFP cells (80% ligand− cells) or 1:13 RFP:GFP cells (93% ligand− cells). There was a significant decrease in overall tumor burden in mice that had 80% or even 93% of ligand- tumor cells compared to control treated mice (Fig. 2F).
The elimination of ligand-deficient tumor cells following CAR T cell transfer may be due to the recruitment and activation of host leukocytes. ChNKG2D T cell therapy recruits activated host antigen-specific CD4+ and CD8+ T cells, and tumor elimination mediated by this therapy is impaired in Rag −/− hosts 24, 26, 27. These activated host lymphocytes may respond and lyse ligand-negative tumor cells. In addition, chNKG2D T cell therapy activates endogenous macrophages in an IFN-γ-dependent manner 26, 31. The activated macrophages at the tumor site have been shown to kill ID8 tumor cells, and their depletion with clodronate-encapsulated liposomes demonstrated their requirement for optimal tumor elimination following chNKG2D T cell transfer 31. Activated macrophages may be one mechanism to remove the NKG2D ligand-deficient tumor cells. Moreover, CAR T cell-derived cytokines change the tumor microenvironment to make it unfavorable for tumor growth. After chNKG2D T cell transfer, macrophages and leukocytes at the tumor site produce less VEGF, IL-6, IL-10 and other regulatory molecules that promote tumor growth 31. Taken together, tumor-variants not recognized by the CAR-bearing T cells may be eliminated by activated host myeloid cells and host lymphocytes at the tumor site.
Previous studies demonstrated that lymphodepletion with cyclophosphamide did not improve or worsen the therapeutic efficacy of NKG2D CAR T cell therapy 23. We suspect the reason is that although lymphodepletion creates space and promotes increased survival of the infused T cells, it also eliminates host leukocytes that are activated by CAR T cell-derived cytokines and prevents their anti-tumor activities. Thus, the benefits of lymphodepletion were cancelled out by the adverse effects on host leukocytes, the end result being similar survival.
In addition, these results demonstrate a correlation with the amount of target antigen heterogeneity and the therapeutic efficacy of NKG2D CAR T cell therapy. The outgrowth of tumors in mice challenged with 1:1 lymphoma tumors (50% ligand−), but not in mice challenged with 4:1 tumors (20% ligand−), may be due to fewer targeted tumor cells within the tumor or the inability of the CAR T cells to stimulate host immunity fast enough to eliminate this rapid growing lymphoma. Fewer tumor cells recognized by the CAR T cells may result in the secretion of lower amounts of effector cytokines, which have been shown to modify the tumor microenvironment and activate host immunity 24, 26. The greater numbers of ligand-negative tumor cells in the 1:1 lymphomas may escape tumor control mediated by CAR therapy prior to the initiation of a robust host anti-tumor immune response. The more robust inflammatory response initiated by NKG2D CAR T cells in mice bearing 4:1 lymphomas may disrupt the tumor microenvironment and activate sufficient host immunity to destroy the remaining ligand-negative tumor cells before they escape immune control.
ChNKG2D T cell therapy confers protection against a NKG2D ligand-negative tumor cell re-challenge
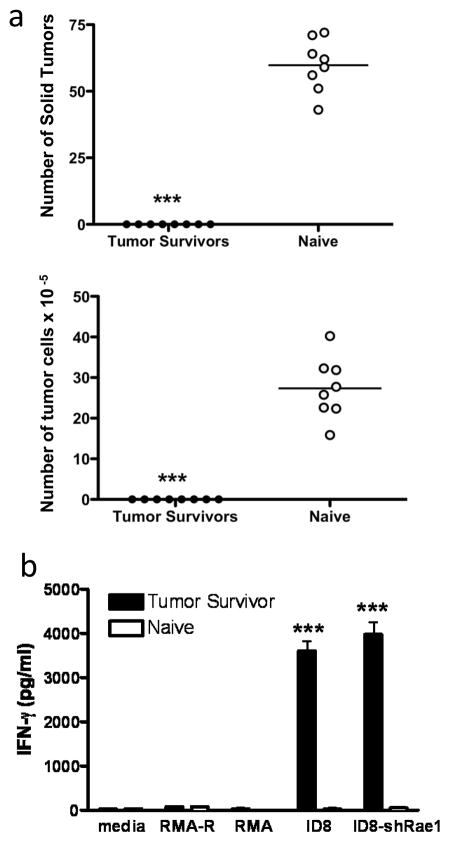
It has been shown that chNKG2D T cells induce host immunity against tumor antigens in several murine tumor models, and that the host immune response plays a key role in NKG2D CAR T cell mediated long-term protection and increased survival 23–26, 32, 33. Long-term surviving mice are resistant to specific tumor challenge and generate specific anti-tumor responses, and the evidence indicates that there are no chNKG2D T cells remaining in these mice 23, 33. Moreover, mice-bearing RMA-RG tumors (Rae1+) and treated with chNKG2D T cells are protected from a secondary tumor challenge with parental RMA tumor cells (Rae1−) 25, 32. To test whether host protection induced by chNKG2D T cell therapy also conferred protection against a re-challenge with NKG2D ligand-deficient ovarian tumor cells, ID8/GFP-shRae1 cells were injected i.p. into tumor-free surviving mice or naïve mice and tumor burden was evaluated in these mice after eight weeks. Both the formation of solid tumors and growth of tumor cells in ascites were inhibited compared to naïve mice (Fig. 3A). Thus, mice that had been successfully treated with NKG2D CAR T cells were protected against a re-challenge with ID8/GFP-shRae1 tumor cells, whereas naïve mice were not protected from tumor growth.
Figure 3. NKG2D CAR T cell therapy confers protection against a NKG2D ligand-negative tumor cell re-challenge.
Mice injected with ID8/GFP tumor cells received three doses of chNKG2D T cells 5, 7, and 9 weeks post-tumor inoculation, and these mice became long-term tumor-free survivors. (a) Tumor-surviving mice were re-challenged with 2 × 106 ID8/GFP-shRae1 cells i.p. 250 days after ID8/GFP injection. After 8 weeks, the number of solid tumors and tumor cells in ascites were assessed (***, p < 0.001 vs. naïve). (b) Splenocytes from tumor-surviving mice who did not receive a tumor re-challenge were isolated (at 250 days) and tested for production of IFN-γ in response to ID8, ID8/GFP-shRae1, RMA, and RMA-Rae1 (RMA-R) cells (***, p < 0.001 vs. naïve).
In contrast to naïve mice, T cells from tumor surviving mice responded to ID8/GFP-shRae1 tumor cells as evidenced by IFN-γ production when spleen cells were cultured with tumor cells in vitro (Fig. 3B). It has been shown previously that this IFN-γ response was due to host T cell production of IFN-γ 24, 33. However, culture of the same splenocytes with RMA or RMA-Rae1 lymphoma cells did not induce IFN-γ production, indicating the activity was not related to the recognition of Rae1 but to specific recognition of ID8 tumor cells.
In this study, we provide evidence that NKG2D CAR T cell treatment can inhibit the growth of antigen heterogeneous tumors in which a many of the tumor cells lack expression of the targeted tumor molecule. The ability of CAR T cells to attack heterogeneous tumors and induce endogenous immunity against various tumor antigens likely enhances immune surveillance and thus prevents tumor variant survival and tumor escape. In conclusion, CAR-bearing T cells not only directly attack tumor cells, but they modulate the host microenvironment to promote anti-tumor immunity and eliminate tumor variants leading to increased overall survival. These data have implications for the efficacy of CAR directed immunotherapies and for host conditioning regimens that deplete leukocytes prior to adoptive T cell transfer.
Methods
Mice
Female C57BL/6 mice were purchased from the National Cancer Institute (Frederic, MD) and were older than seven weeks before use. All animal work was performed in the Center for Comparative Medicine and Research in accordance with institutional guidelines.
Cell lines
Murine ovarian cancer cell lines (ID8, ID8/GFP, ID8/RFP and ID8/GFP-shRae1) cells were grown in the Dulbecco modified Eagle medium (DMEM) and the murine lymphoma cell lines (RMA and RMA-RG) were grown in RPMI medium.
Generation of ID8-GFP-shRae1 cells
A shRNA sequence specific for Rae-1 was designed using the BLOCK-iT RNAi Designer (Invitrogen). The shRNA sequence 5′-GATCCGGACACTCACAAGAC AATGGTTCAAGAGACCATTGGTCTTGTGAGTGTCCTTTTTG-3′, and its complementary sequence, 5′-AATTCAAAAAAGGACACTCACAAGACCAATGGTCATTTGAACCATTGGTCTGTGAGTGTCCG-3′, were annealed at 95°C for 30 seconds, 72°C for 2 minutes, 37°C for 2 minutes, and 25°C for two minutes. The product was inserted into a linearized retroviral pSIREN-RetroQ vector (Clontech), and ID8/GFP-shRae1 cells were made by retroviral transduction of ID8/GFP cells. The generation of retrovirus using the packing cell line PT67 and the retroviral transduction were performed as previously described 34.
Preparation of genetically modified chNKG2D and wtNKG2D T cells
Spleen cells from a C57BL/6 mouse were stimulated with 1 μg/ml of Con A for 18 hrs and transduced as previously described 25. The chNKG2D construct was created by fusing the murine CD3ζ chain cytoplasmic region coding sequence to the full-length gene of murine NKG2D. Two days after transduction T cells were selected for 3 days in medium containing 0.5 mg/ml of G418 and 25 U/ml of recombinant human IL-2. Viable cells were isolated using Histopaque-1083 (Sigma-Aldrich), then the cells were expanded for 2 days without G418. The transfused NKG2D CAR T cells contain approximately 90% CD8+ and 10% CD4+ T cells.
Tumor cell injection and treatment of mice
To determine the effects of chNKG2D T cell treatment on the growth of ID8 ovarian tumors, mice received an intraperitoneal (i.p.) injection of 1 or 2 × 106 ID8/GFP, ID8/RFP, or ID8/GFP-shRae1 ovarian tumor cells and were treated i.p. with chNKG2D or wtNKG2D T cells (5 × 106), as indicated in each figure legend. For the tumor re-challenge experiments, tumor-surviving mice were re-challenged with 2 × 106 ID8/GFP-shRae1 cells i.p. 250 days after the initial ID8/GFP injection. After 8 weeks, the number of solid tumors and tumor cells in the peritoneal cavity were assessed.
For the determination of the direct effects of chNKG2D T cells on the growth of RMA or RMA/Rae1-GFP (RMA-RG), chNKG2D or wtNKG2D T cells (5 × 106) were mixed with RMA-RG (5 × 104) cells, RMA (1 × 104) tumor cells, or a 1:1 or 4:1 mixture of RMA-RG and RMA tumor cells, and they were immediately injected subcutaneously into the shaved right flank of recipient mice. Additional control mice were subcutaneously injected with RMA tumor cells (1 × 104 to 5 × 104) but did not receive T cell treatment. Tumor dimensions were measured using a caliper, and the tumor area was calculated for each recipient.
Flow cytometry
Cells isolated from the peritoneal wash were analyzed by flow cytometry for expression of RFP and/or GFP. To detect surface NKG2D ligand expression, cells were stained with APC-conjugated anti-Rae1 (R&D Systems). Cell fluorescence was monitored using a FACSCalibur cytometer (BD Biosciences) or C6 Accuri cytometer (BD Biosciences). Flow cytometry analysis was performed using FlowJo software (Tree Star, Ashland, OR) or C6 Accuri analysis software.
Cytokine production in secondary stimulation cultures
WtNKG2D or chNKG2D T cells (105 cells) were cultured with irradiated (120 Gy) ID8/GFP or ID8/GFP-shRae1 (2.5 × 104) tumor cells in a 96 well flat-bottom plate for 24 hours or with RMA or RMA-RG lymphoma cells (105) in a 96 well round-bottom plate. Cell-free supernatants were collected after 24 hours and assayed for IFN-γ by ELISA using DuoSet ELISA kits (R&D Systems).
Cytotoxicity by chNKG2D T cells
Lysis of ID8/GFP or ID8/GFP-shRae1 tumor cells was determined by 51Cr-release assays, as previously described 34.
Statistical analysis
Differences between groups were analyzed by a Student’s t-test, ANOVA and Newman-Keuls post-test, long-rank test, or a Dunn’s multiple comparison test, as detailed in each figure (GraphPad Software). Significant differences between individual groups are shown in each figure. Values of p < 0.05 were considered significant.
Acknowledgments
Financial Support: This work was supported by grants from the National Institutes of Health (CA130911, T32 AI007363) and the Norris Cotton Cancer Center. The views in this paper reflect the authors’ opinions and do not necessarily reflect the opinions of the National Institutes of Health.
We thank the NCI Biological Resource Branch for providing recombinant human IL-2, and the staff of the Center for Comparative Medicine and Research for assistance with animal care. The authors thank Dr. Ed Usherwood for thoughtful suggestions on the manuscript.
Footnotes
Conflict of Interest: The chimeric NKG2D technology used in this study is licensed to Celdara Medical LLC. CLS and Celdara are developing the technology for clinical use, for which he receives compensation. This arrangement is under compliance with guidelines from Dartmouth College.
References
- 1.Bosslet K, Schirrmacher V. Escape of metastasizing clonal tumor cell variants from tumor-specific cytolytic T lymphocytes. J Exp Med. 1981;154:557–62. doi: 10.1084/jem.154.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hines DL. Failure of specific adoptive immunotherapy owing to survival and outgrowth of variant cells. Cancer Immunol Immunother. 1989;28:241–7. doi: 10.1007/BF00205232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norell H, Carlsten M, Ohlum T, Malmberg KJ, Masucci G, Schedvins K, et al. Frequent loss of HLA-A2 expression in metastasizing ovarian carcinomas associated with genomic haplotype loss and HLA-A2-restricted HER-2/neu-specific immunity. Cancer Res. 2006;66:6387–94. doi: 10.1158/0008-5472.CAN-06-0029. [DOI] [PubMed] [Google Scholar]
- 4.Mackensen A, Meidenbauer N, Vogl S, Laumer M, Berger J, Andreesen R. Phase I study of adoptive T-cell therapy using antigen-specific CD8+ T cells for the treatment of patients with metastatic melanoma. J Clin Oncol. 2006;24:5060–9. doi: 10.1200/JCO.2006.07.1100. [DOI] [PubMed] [Google Scholar]
- 5.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002;99:16168–73. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21:233–40. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3:666–75. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100:8372–7. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scarlett UK, Cubillos-Ruiz JR, Nesbeth YC, Martinez DG, Engle X, Gewirtz AT, et al. In situ stimulation of CD40 and Toll-like receptor 3 transforms ovarian cancer-infiltrating dendritic cells from immunosuppressive to immunostimulatory cells. Cancer Res. 2009;69:7329–37. doi: 10.1158/0008-5472.CAN-09-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vonderheide RH, Flaherty KT, Khalil M, Stumacher MS, Bajor DL, Hutnick NA, et al. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clin Oncol. 2007;25:876–83. doi: 10.1200/JCO.2006.08.3311. [DOI] [PubMed] [Google Scholar]
- 15.Boon T, Van Pel A. Teratocarcinoma cell variants rejected by syngeneic mice: protection of mice immunized with these variants against other variants and against the original malignant cell line. Proc Natl Acad Sci U S A. 1978;75:1519–23. doi: 10.1073/pnas.75.3.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.el-Shami K, Tirosh B, Bar-Haim E, Carmon L, Vadai E, Fridkin M, et al. MHC class I-restricted epitope spreading in the context of tumor rejection following vaccination with a single immunodominant CTL epitope. Eur J Immunol. 1999;29:3295–301. doi: 10.1002/(SICI)1521-4141(199910)29:10<3295::AID-IMMU3295>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 17.Butterfield LH, Ribas A, Dissette VB, Amarnani SN, Vu HT, Oseguera D, et al. Determinant spreading associated with clinical response in dendritic cell-based immunotherapy for malignant melanoma. Clin Cancer Res. 2003;9:998–1008. [PubMed] [Google Scholar]
- 18.Disis ML, Grabstein KH, Sleath PR, Cheever MA. Generation of immunity to the HER-2/neu oncogenic protein in patients with breast and ovarian cancer using a peptide-based vaccine. Clin Cancer Res. 1999;5:1289–97. [PubMed] [Google Scholar]
- 19.Lally KM, Mocellin S, Ohnmacht GA, Nielsen MB, Bettinotti M, Panelli MC, et al. Unmasking cryptic epitopes after loss of immunodominant tumor antigen expression through epitope spreading. Int J Cancer. 2001;93:841–7. doi: 10.1002/ijc.1420. [DOI] [PubMed] [Google Scholar]
- 20.Disis ML, Gooley TA, Rinn K, Davis D, Piepkorn M, Cheever MA, et al. Generation of T-cell immunity to the HER-2/neu protein after active immunization with HER-2/neu peptide-based vaccines. J Clin Oncol. 2002;20:2624–32. doi: 10.1200/JCO.2002.06.171. [DOI] [PubMed] [Google Scholar]
- 21.Sadelain M. T-cell engineering for cancer immunotherapy. Cancer J. 2009;15:451–5. doi: 10.1097/PPO.0b013e3181c51f37. [DOI] [PubMed] [Google Scholar]
- 22.Sadelain M, Brentjens R, Riviere I. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol. 2009;21:215–23. doi: 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barber A, Meehan KR, Sentman CL. Treatment of multiple myeloma with adoptively transferred chimeric NKG2D receptor-expressing T cells. Gene Ther. 2011;18:509–16. doi: 10.1038/gt.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barber A, Sentman CL. Chimeric NKG2D T cells require both T cell- and host-derived cytokine secretion and perforin expression to increase tumor antigen presentation and systemic immunity. J Immunol. 2009;183:2365–72. doi: 10.4049/jimmunol.0900721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang T, Lemoi BA, Sentman CL. Chimeric NK-receptor-bearing T cells mediate antitumor immunotherapy. Blood. 2005;106:1544–51. doi: 10.1182/blood-2004-11-4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barber A, Rynda A, Sentman CL. Chimeric NKG2D expressing T cells eliminate immunosuppression and activate immunity within the ovarian tumor microenvironment. J Immunol. 2009;183:6939–47. doi: 10.4049/jimmunol.0902000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spear P, Barber A, Sentman CL. Collaboration of chimeric antigen receptor (CAR)-expressing T cells and host T cells for optimal elimination of established ovarian tumors. OncoImmunology. 2013;2:e23564. doi: 10.4161/onci.23564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khong HT, Restifo NP. Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nat Immunol. 2002;3:999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heppner GH. Tumor heterogeneity. Cancer Res. 1984;44:2259–65. [PubMed] [Google Scholar]
- 30.Barber A, Zhang T, DeMars LR, Conejo-Garcia J, Roby KF, Sentman CL. Chimeric NKG2D receptor-bearing T cells as immunotherapy for ovarian cancer. Cancer Res. 2007;67:5003–8. doi: 10.1158/0008-5472.CAN-06-4047. [DOI] [PubMed] [Google Scholar]
- 31.Spear P, Barber A, Rynda-Apple A, Sentman CL. Chimeric Antigen Receptor T Cells Shape Myeloid Cell Function within the Tumor Microenvironment through IFN-gamma and GM-CSF. J Immunol. 2012;188:6389–98. doi: 10.4049/jimmunol.1103019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang T, Barber A, Sentman CL. Chimeric NKG2D modified T cells inhibit systemic T-cell lymphoma growth in a manner involving multiple cytokines and cytotoxic pathways. Cancer Res. 2007;67:11029–36. doi: 10.1158/0008-5472.CAN-07-2251. [DOI] [PubMed] [Google Scholar]
- 33.Barber A, Zhang T, Sentman CL. Immunotherapy with chimeric NKG2D receptors leads to long-term tumor-free survival and development of host antitumor immunity in murine ovarian cancer. J Immunol. 2008;180:72–8. doi: 10.4049/jimmunol.180.1.72. [DOI] [PubMed] [Google Scholar]
- 34.Zhang T, Barber A, Sentman CL. Generation of antitumor responses by genetic modification of primary human T cells with a chimeric NKG2D receptor. Cancer Res. 2006;66:5927–33. doi: 10.1158/0008-5472.CAN-06-0130. [DOI] [PubMed] [Google Scholar]