Abstract
Arterial aging, characterized by stiffening of large elastic arteries and the development of arterial endothelial dysfunction, increases cardiovascular disease (CVD) risk. We tested the hypothesis that spermidine, a nutrient associated with the anti-aging process autophagy, would improve arterial aging. Aortic pulse wave velocity (aPWV), a measure of arterial stiffness, was ~20% greater in old (O, 28 months) compared with young C57BL6 mice (Y, 4 months, P < 0.05). Arterial endothelium-dependent dilation (EDD), a measure of endothelial function, was ~25% lower in O (P < 0.05 vs. Y) due to reduced nitric oxide (NO) bioavailability. These impairments were associated with greater arterial oxidative stress (nitrotyrosine), superoxide production, and protein cross-linking (advanced glycation end-products, AGEs) in O (all P < 0.05). Spermidine supplementation normalized aPWV, restored NO-mediated EDD and reduced nitrotyrosine, superoxide, AGEs and collagen in O. These effects of spermidine were associated with enhanced arterial expression of autophagy markers, and in vitro experiments demonstrated that vascular protection by spermidine was autophagy-dependent. Our results indicate that spermidine exerts a potent anti-aging influence on arteries by increasing NO bioavailability, reducing oxidative stress, modifying structural factors and enhancing autophagy. Spermidine may be a promising nutraceutical treatment for arterial aging and prevention of age-associated CVD.
Keywords: arterial stiffness, endothelial dysfunction, nitric oxide, oxidative stress
1. Introduction
Aging causes two key changes in arteries that significantly increase the risk of cardiovascular diseases (CVD): stiffening of the large elastic arteries (aorta and carotid arteries) and the development of vascular endothelial dysfunction (Lakatta and Levy, 2003; North and Sinclair, 2012). Arterial stiffening results from age-related changes in the arterial wall including increases in collagen deposition, reductions in elastin and cross-linking of these and other structural proteins via formation of advanced glycation end-products (AGEs) (O'Rourke and Hashimoto, 2007). Vascular endothelial dysfunction develops with age primarily due to reduced nitric oxide (NO) bioavailability, as reflected by impaired NO-mediated endothelium-dependent dilation (EDD) (Brandes et al., 2005; Lakatta, 2003a).
Although the mechanisms underlying arterial aging are incompletely understood, the characteristics of age-associated vascular dysfunction are consistent with dysregulated cellular protein homeostasis, i.e., oxidative stress and increased molecular damage that ultimately impair cell and tissue function (Koga et al., 2010; Lakatta, 2003a; Seals et al., 2011b). Autophagy, the cellular process of recycling damaged biomolecules, is a major mechanism for protein homeostasis and defense against oxidative stress (Koga et al., 2010; Mizushima and Komatsu, 2011) and may, therefore, play an important role in arterial aging. Indeed, numerous longevity pathways exert their effects through autophagy (Rubinsztein et al., 2011), and recent work from our laboratory suggests that impaired autophagy contributes to arterial aging (LaRocca et al., 2012). Thus, therapeutic strategies aimed at improving protein quality control by enhancing autophagy may have the potential to prevent/reverse age-associated arterial dysfunction and CVD.
Because many known autophagy inducers have off-target effects (e.g., rapamycin) or uncertain translational promise (e.g., caloric restriction), there is considerable interest in natural food components or “nutraceuticals” that promote autophagy (Galluzzi and Kroemer, 2012; Sudarsanam and Johnson, 2010). Recently, the polyamine spermidine has been identified as a potent and specific inducer of autophagy (Eisenberg et al., 2009; Madeo et al., 2010). Spermidine is a natural dietary compound found in high concentrations in Mediterranean and Asian diets (Binh, 2010; Soda et al., 2010). Supplementation with spermidine extends lifespan in yeast and flies by an autophagy-dependent mechanism and reduces oxidative stress (Eisenberg et al., 2009; Guo et al., 2011; Minois et al., 2012). The mechanism of action for spermidine involves enhanced transcription of autophagy-relevant proteins and is contingent on de-acetylation of histone H3 (Eisenberg et al., 2009; Morselli et al., 2011). However, the potential for spermidine to promote autophagy and exert anti-aging effects in arteries is entirely unknown.
Here, we tested the hypothesis that supplementation with spermidine would reduce arterial stiffness and improve vascular endothelial function in old mice. The results of these experiments provide the first evidence that spermidine may hold efficacy for treating age-associated arterial dysfunction by enhancing autophagy, reducing oxidative stress and increasing NO bioavailability.
2. Materials and Methods
2.1 Animals
Young (4–6 months) and old (27–29 months; ~50% survival rate) male C57BL6 mice, an established model of aging and vascular dysfunction (Sindler et al., 2011b; Sprott and Ramirez, 1997), were obtained from the National Institute on Aging rodent colony. Control animals received regular drinking water, whereas treated animals received water supplemented with 3 mM spermidine (Sigma-Aldrich, St Louis, MO, USA), a previously reported dose (Eisenberg et al., 2009), for a period of 4 weeks. Spermidine supplemented water was replaced every 3 days, prepared from a 1 M aqueous stock solution (spermidine/HCl pH 7.4) stored at −20° C. Mice were housed in an animal care facility at the University of Colorado at Boulder on a 12:12 h light-dark cycle. All procedures conformed to the Guide for the Care and Use of Laboratory Animals (NIH publication no. 85-23, revised 2011) and were approved by the University of Colorado at Boulder Animal Care and Use Committee.
2.2 Aortic pulse wave velocity (arterial stiffness)
Aortic pulse wave velocity was measured as previously described (Fleenor et al., 2012; Reddy et al., 2003). In brief: mice were anesthetized with 2% isoflurane and positioned supine on a heating board (37° C) with limbs secured to ECG electrodes. Doppler probes were used to detect flow velocity signals at the transverse aortic arch and the abdominal aorta while simultaneously recording ECG (MouseDoppler acquisition system, Indus Instruments, Wester, TX, USA). Time elapsed between the ECG R-wave and the foot of the Doppler signal was determined for each site (Fig. 1), and pulse wave velocity was calculated as the distance between the two probes divided by the difference in time elapsed at each site.
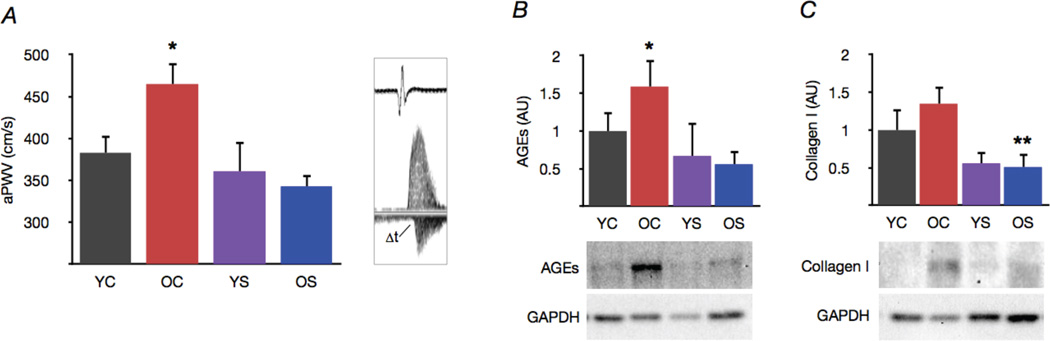
Figure 1. Spermidine supplementation normalizes large elastic artery stiffness and structural proteins.
(A) Aortic pulse wave velocity in young and old control (YC and OC) and young and old spermidine supplemented (YS and OS) mice. Representative ECG and Doppler flow signals at right. (B) Advanced glycation end products (AGEs), and (C) protein expression of collagen I in whole aortas. Representative Western blot images below. Protein expression data expressed relative to GAPDH and normalized to YC mean value. Values are means ± SEM (n = 7–8 per group). *P < 0.05 vs. YC. **P < 0.05 vs. OC.
2.3 Vascular endothelial function
EDD and endothelium-independent dilation were determined ex vivo in isolated carotid arteries as previously described (LaRocca et al., 2012; Rippe et al., 2010b). Mice were anesthetized with isoflurane and sacrificed by exsanguination via cardiac puncture. Carotid arteries were dissected free of surrounding tissue, cleaned and cannulated onto glass micropipettes in myograph chambers (DMT, Aarhus, Denmark). Arteries were pressurized to 50 mmHg at 37°C in physiological saline solution and allowed to equilibrate for 1 h. After preconstriction with phenylephrine (2 µM), NO-mediated EDD was determined by measuring increases in luminal diameter in response to acetylcholine (ACh, 1 ×10−9 − 1×10−4 M, Sigma-Aldrich) in the presence or absence of N G-nitro-L-arginine methyl ester (L-NAME; 0.1 mM, 30 min incubation to block NO production, Sigma-Aldrich) or the superoxide dismutase mimetic TEMPOL (1 mM, 60 min incubation to scavenge superoxide, Sigma-Aldrich). Endothelium-independent dilation was determined as dilation in response to the exogenous NO donor sodium nitroprusside (SNP, 1×10−10 − 1×10−4 M, Sigma-Aldrich), and is used as a measure of vascular smooth muscle sensitivity to NO. Dose-response data are presented on a percentage basis to account for differences in carotid artery diameter between young and old animals. NO-dependent dilation was determined from maximal EDD with or without L-NAME as: NO-dependent dilation (%) = max dilationACh - max dilationACh+L-NAME.
2.4 Arterial superoxide production
Superoxide production was assessed by electron paramagnetic resonance (EPR) spectroscopy as previously described (Fleenor et al., 2012; LaRocca et al., 2012). Freshly dissected and cleaned 2 mm aortic segments were incubated for 60 min at 37°C in Krebs-HEPES buffer with the superoxide-specific spin probe 1-hydroxy-3-methoxycar- bonly-2,2,5,5-tetramethylpyrrolidine (0.5 mM; Enzo Life Sciences, Farmingdale, NY, USA). EPR signal amplitude was analyzed immediately on an MS300 X-band EPR spectrometer (Magnettech, Berlin, Germany) with the following settings: centerfield, 3350 G; sweep, 80 G; microwave modulation, 3000 mG; microwave attenuation, 7 dB. Data are expressed relative to the mean of the young control group.
2.5 Arterial protein expression
Measurements of protein expression were performed on cleaned mouse aortas (a representative large elastic artery) to provide sufficient tissue for analysis. Thoracic aortas were excised, cleaned of surrounding tissue and analyzed by standard Western blotting techniques as previously described (LaRocca et al., 2012; Rippe et al., 2010b). Briefly: whole aortas were homogenized in radio-immunoprecipitation assay lysis buffer with protease and phosphatase inhibitors. 10 µg protein was loaded onto 4–12% polyacrylamide gels, separated by electrophoresis and transferred to nitrocellulose membranes (Criterion System; Bio-Rad, Hercules, CA, USA). Membranes were incubated overnight at 4° C with primary antibodies: collagen-I (1:1000 dilution; Abcam, Cambridge, MA, USA), AGEs (1:2000; Abcam), nitrotyrosine (1:500; Abcam), lipid-modified microtubule-associated protein light chain 3 (LC3-II, 1:2000; Cell Signaling, Danvers, MA, USA), p62 adaptor protein (1:2000; MBL International, Woburn, MA, USA), acetylated (Lys9) histone H3 (1:500; Cell Signaling), autophagy protein Atg3 (1:1000; Cell Signaling). Proteins were visualized on a digital acquisition system (ChemiDoc-It; UVP, Upland, CA, USA) using horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA, USA) and ECL chemiluminescent substrate (Pierce, Rockford, IL, USA). Protein expression is presented normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 1:1000; Cell Signaling), and data expressed as a ratio of the mean of the young control group.
2.6 In vitro tissue culture experiments
Aortas were excised from young mice and perivascular fat removed from the arteries. Equal length segments of thoracic aorta were incubated in DMEM (with antibiotics, Sigma-Aldrich) in a humidified incubator at 37°C and 5% CO2 with or without: pyocyanin (10 µM, Sigma-Aldrich) to induce oxidative stress, as previously described in vascular cells and tissue (Gryglewski et al., 1992; Muller, 2002); chloroquine (50 µM), to inhibit autophagy; or spermidine (3 mM). Half of each aortic segment was removed after 1 h and analyzed for superoxide production by EPR as described above. The remaining half of each segment was incubated for 48 h before analysis.
2.7 Statistical analyses
Statistical analysis was performed with SPSS 19.0 software. For dose responses, group differences were determined by repeated measures ANOVA. For aortic pulse wave velocity, maximal dilation, superoxide production and protein expression, comparisons between groups were made using appropriate ANOVA. Significance was determined using P < 0.05.
3. Results
3.1 Arterial stiffness and wall structural factors
Aortic pulse wave velocity was ~20% greater in old compared with young control mice (Fig. 1A) and was associated with increased formation of AGEs in the aorta (Fig. 1B). Spermidine supplementation normalized both aortic pulse wave velocity and AGEs in old mice without affecting young animals. Aortic collagen I tended to increase with age, and spermidine treatment markedly reduced expression in aortas of old mice (Fig. 1C). These results suggest that spermidine supplementation reverses age-associated stiffening of large elastic arteries, and that these improvements are associated with reductions in structural factors in the arterial wall that promote stiffness.
3.2 Arterial endothelial function
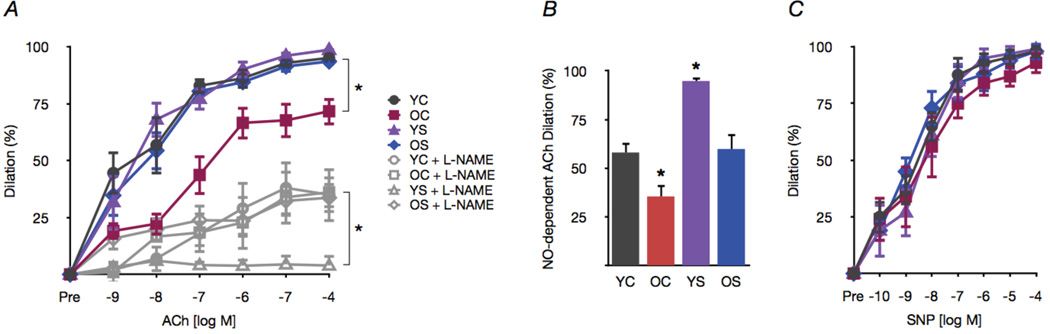
Carotid artery EDD in response to ACh was ~25% lower in old mice (P < 0.05 vs. young controls, Fig. 2A). Impaired EDD in old animals was a result of reduced NO, indicated by a smaller reduction in EDD upon co-incubation with the NO inhibitor L-NAME (Fig. 2A,B). Spermidine supplementation restored NO-mediated EDD in old mice to levels observed in young control mice. Spermidine did not influence EDD in young animals, but increased the NO component of dilation (Fig. 2A,B). Endothelium-independent dilation to the NO donor sodium nitroprusside was similar among the groups, indicating no differences in vascular smooth muscle sensitivity to NO (Fig. 2C). These observations indicate that spermidine supplementation restores EDD in old mice by restoring NO bioavailability.
Figure 2. Spermidine supplementation restores NO-mediated EDD in old mice.
(A) Dose responses (EDD) to acetylcholine (ACh) with/without the NO inhibitor L-NAME in carotid arteries of young and old control (YC and OC) and young and old spermidine supplemented (YS and OS) mice. (B) NO-dependent dilation (max dilationACh - max dilationACh+L-NAME). (C) Endothelium-independent dilation to the NO donor sodium nitroprusside (SNP). All data presented on a percent basis to account for differences in arterial diameter among groups. Values are means ± SEM (n = 7–8 per group). *P < 0.05 vs. YC.
3.3 Oxidative stress
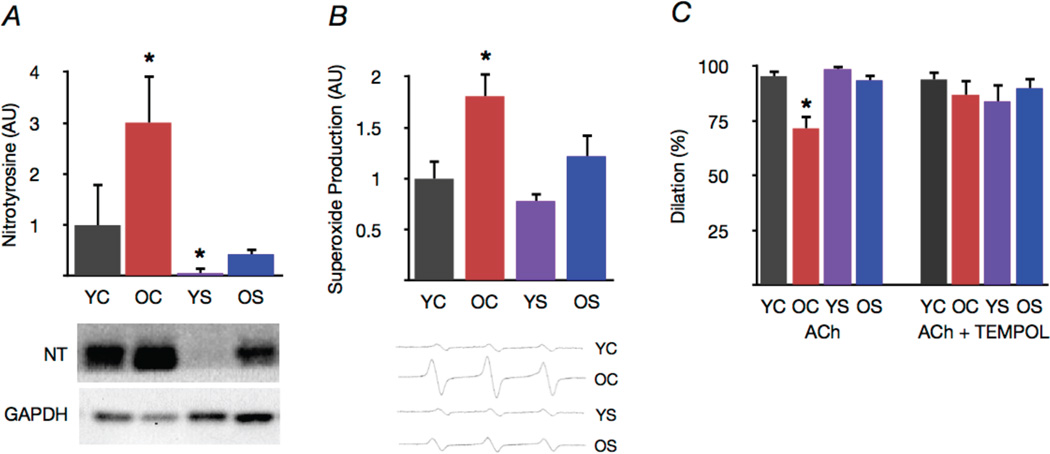
Aortas from old control animals had markedly greater levels of nitrotyrosine, a protein marker of superoxide-associated oxidative stress (Fig. 3A), and demonstrated increased superoxide production compared with young controls (Fig. 3B). Spermidine supplementation ameliorated the age-associated increases in both aortic nitrotyrosine levels and superoxide production, while also reducing nitrotyrosine in young animals (Fig. 3A,B). Consistent with these observations, co-incubation with the superoxide scavenger TEMPOL restored maximum carotid artery EDD to acetylcholine in old control mice, suggesting excessive superoxide-mediated suppression of EDD with aging. In contrast, TEMPOL had no effect in spermidine supplemented old or young animals, indicating an absence of superoxide-related impairment of EDD in those groups (Fig. 3C). Collectively, these data demonstrate that spermidine supplementation exerts a powerful antioxidant influence on arteries that appears to mediate improvements in arterial endothelial function.
Figure 3. Spermidine supplementation reduces arterial oxidative stress in old mice.
(A) Nitrotyrosine, a marker of oxidative protein damage, in aorta of young and old control (YC and OC) and young and old spermidine supplemented (YS and OS) mice. Representative Western blot images below. (B) Mean EPR signal for superoxide in aortic rings. Representative EPR signals below. (C) Maximal carotid artery dilation (EDD) to acetylcholine (ACh) in the presence/absence of the superoxide scavenger TEMPOL. Protein expression data expressed relative to GAPDH. Protein expression and EPR data normalized to YC mean value. Values are means ± SEM (n = 7–8 per group). *P < 0.05 vs. YC.
3.4 Autophagy
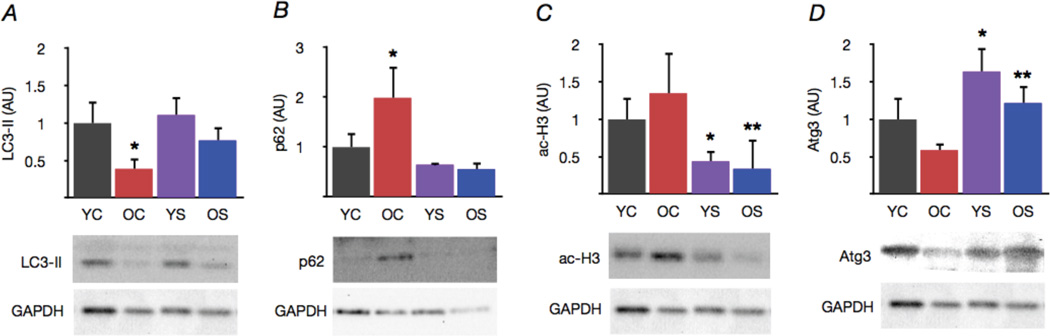
Expression of the autophagy marker LC3-II was reduced in aorta of old mice, whereas p62, a marker of undegraded autophagy substrates, was increased relative to young controls (Fig. 4A,B). Spermidine supplementation restored aortic expression of LC3-II and reduced p62 in old mice, while having no effect in young animals. These effects of spermidine were associated with reduced acetylation of histone H3 and increased expression of the core autophagy machinery protein Atg3 in both young and old mice (Fig. 4C,D). Taken together, these findings suggest that spermidine supplementation is associated with increased activation of autophagy regulatory systems and corresponding enhancement of protein markers of autophagic processes in arteries.
Figure 4. Spermidine supplementation increases markers of autophagy in old mice.
(A) Protein expression of the autophagy marker LC3-II in aorta of young and old control (YC and OC) and young and old spermidine supplemented (YS and OS) mice. (B) The p62 adapter protein, a marker of undegraded autophagy substrates. (C) Acetylation of the autophagy-relevant histone H3. (D) Expression of the core autophagy machinery protein Atg3. Data expressed relative to GAPDH and normalized to YC mean value. Representative Western blot images below. Values are means ± SEM (n = 7–8 per group). *P < 0.05 vs. YC. **P < 0.05 vs. OC.
3.5 Short- vs. long-term effects of spermidine
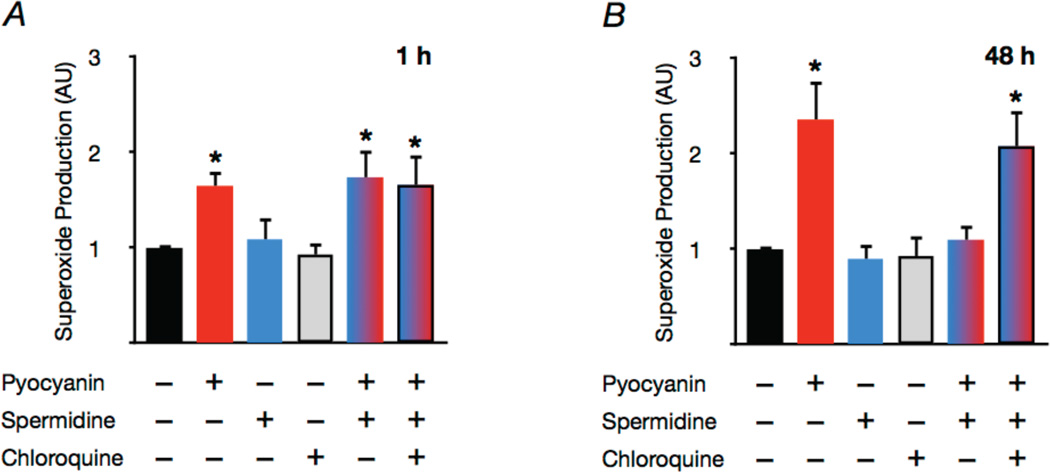
Pyocyanin induced “aging-like” oxidative stress in arterial segments isolated from young mice, as indicated by an increase in superoxide production similar in magnitude to that which we observed in aortas of old animals (Fig. 5A,B). Co-incubation with spermidine had no short-term effect on pyocyanin-induced superoxide production (Fig. 5A). In contrast, over a period of 48 h, spermidine treatment normalized superoxide production in pyocyanin-treated arteries (Fig. 5B). This protective effect of spermidine was abolished upon co-incubation with the autophagy inhibitor chloroquine (Fig. 5B). These findings suggest that spermidine does not directly scavenge superoxide, but may reduce oxidative stress in the long-term via autophagy-dependent antioxidant actions.
Figure 5. The protective effects of spermidine are time- and autophagy-dependent.
Mean EPR signal for superoxide in cultured aortic rings incubated for 1 or 48 h with or without the oxidative stress inducer pyocyanin (10 µM), spermidine (3 mM) or the autophagy inhibitor chloroquine (50 µM). Data expressed relative to control condition for each trial. Values are means ± SEM (n = 6 per group). *P < 0.05 vs. control.
4. Discussion
Age is the most important determinant of CVD risk (Lloyd-Jones et al., 2010) due in large part to stiffening of large elastic arteries and the development of vascular endothelial dysfunction (Lakatta and Levy, 2003). Suboptimal lifestyle and diet exacerbate these processes, contributing significantly to the global CVD burden (Mozaffarian et al., 2011). Thus, identifying dietary patterns and selected nutrients that may prevent or reverse arterial aging is an important research objective.
Spermidine and other polyamines are naturally occurring biomolecules involved in numerous cellular functions including growth, development, protein/nucleic acid synthesis and cell signaling (Minois and Carmona-Gutierrez, 2011). Higher intake of polyamines in Mediterranean and Asian diets is related to increased longevity and reduced CVD risk (Binh, 2010; Soda, 2010; Tognon et al., 2010), and recent reports indicate that spermidine in particular is a powerful inducer of autophagy, an important longevity-enhancing pathway (Eisenberg et al., 2009). Polyamine levels decline with aging in some tissues (Minois and Carmona-Gutierrez, 2011) and polyamine supplementation has been reported to reduce mortality in aged mice (Soda et al., 2009). Moreover, plasma spermidine levels may be related to longevity in human subjects (Pucciarelli et al., 2012).
The results of the present study extend previous observations by providing the first evidence for the therapeutic potential of polyamine supplementation in the treatment of arterial aging. Our findings here also are the first to show that spermidine per se exerts anti- effects that include normalization of arterial function. Finally, our results provide initial insight into the mechanisms by which spermidine may ameliorate arterial aging, namely by reversing superoxide-associated oxidative stress and restoring NO-bioavailability, perhaps, in part, via enhancement of autophagy.
4.1 Large elastic artery stiffness
Stiffening of large elastic arteries is a major cause of CVD in otherwise healthy people (O'Rourke and Hashimoto, 2007). Indeed, aortic pulse wave velocity, the benchmark clinical measure of large elastic artery stiffness, is a strong independent predictor of incident CVD risk in older adults (Mitchell et al., 2010). The present findings are consistent with previous reports from our laboratory (Fleenor et al., 2012; Sindler et al., 2011a) showing that aortic pulse wave velocity increases with aging in mice and is associated with greater expression of collagen I and AGEs, i.e., changes that contribute to increased stiffness of the aortic wall (O'Rourke and Hashimoto, 2007). Here, we show for the first time that spermidine supplementation reverses age-associated increases in aortic pulse wave velocity and normalizes levels of aortic collagen I and AGEs. These findings are consistent with previous reports linking Mediterranean diet to lower aortic pulse wave velocity (Lydakis et al., 2012), as well as limited in vitro observations of the effects of polyamine administration on collagen and AGEs (Gugliucci and Menini, 2003; Santhanam et al., 2008). Thus, the present findings establish pre-clinical support for the beneficial effects of spermidine on age-associated arterial stiffness, collagen and AGEs, and provide an experimental basis for assessing the potential de-stiffening effects of this nutraceutical in humans.
4.2 Vascular endothelial dysfunction
Vascular endothelial function (EDD) declines progressively with age and is predictive of future CVD risk (Seals et al., 2011a; Widlansky et al., 2003). In agreement with previous reports from our laboratory, we observed impaired carotid artery EDD in old mice as a result of reduced NO-mediated dilation (Fleenor et al., 2012; LaRocca et al., 2012; Rippe et al., 2010a). In the present study, we extend these findings by showing that spermidine supplementation restores NO-dependent EDD in old mice, as indicated by improved acetylcholine-mediated dilation in the presence of NO, but not during inhibition of NOS-associated NO production by L-NAME. Spermidine had no effect on endothelium-independent dilation, suggesting a direct effect on endotheliumassociated NO bioavailability. The NO-enhancing effects of spermidine also were observed in young animals, although this was not associated with greater dilation relative to their already normal levels.
One possible explanation for these observations is that by reducing protein nitration (nitrotyrosine), spermidine may suppress the influence of redox-sensitive cyclooxygenases on vasomotor function (Frein et al., 2005; Schildknecht and Ullrich, 2009), thus leading to greater NO-dependent EDD. Our observations also could reflect the overlap of NO and polyamine metabolic pathways. L-arginine is the substrate for both NO production by NOS and polyamine synthesis (Minois and Carmona-Gutierrez, 2011). It is, therefore, possible that spermidine supplementation reduces the activity of polyamine-synthesizing enzymes, thereby increasing L-arginine availability for NO production (Soda, 2010). These possibilities require further investigation, but, in any case, the present findings demonstrate that spermidine supplementation may be an effective therapeutic strategy for reversing age-associated vascular endothelial dysfunction.
4.3 Oxidative stress
Oxidative stress is a key mechanism underlying the development of both arterial stiffening and vascular endothelial dysfunction with age (Lakatta, 2003b; North and Sinclair, 2012; Seals et al., 2011a). Age-associated vascular oxidative stress reduces NO bioavailability, induces biomolecular damage as a consequence of increased superoxide bioactivity and accelerates the formation of AGEs (Lakatta, 2003b; Seals et al., 2011a). We recently reported that supplementation with the superoxide dismutase mimetic TEMPOL improves aortic pulse wave velocity and EDD in old mice (Fleenor et al., 2012), suggesting a central role for superoxide in arterial oxidative stress-associated vascular dysfunction with aging. The present findings are consistent with these observations, showing both increased levels of oxidative protein damage (nitrotyrosine) and elevated superoxide production, as well as selective rescue of EDD by ex vivo TEMPOL treatment in arteries of old mice. Here, we identify a potential novel treatment for arterial oxidative stress. Spermidine supplementation reversed the age-associated increase in arterial superoxide production and reduced nitrotyrosine in arteries of old mice, while also reducing nitrotyrosine in young mice. Given the key role of superoxide in arterial aging, these data suggest that suppression of oxidative stress may be an important mechanism underlying the beneficial effects of spermidine on both age-associated arterial stiffening and vascular endothelial dysfunction. Previous reports suggest that spermidine supplementation may reduce oxidative stress in mice and flies (Eisenberg et al., 2009; Guo et al., 2011). However, the present observations are the first to show the antioxidant effects of spermidine in vascular tissue and to directly link this action with an improvement of physiological function.
The potential mechanisms by which novel therapeutic agents such as spermidine reduce oxidative stress and improve vascular function often involve both direct and indirect effects (Dinkova-Kostova and Talalay, 2008; Forstermann, 2008). Previous reports utilizing cell-free systems indicate that at concentrations used in the present study, spermidine does not directly scavenge superoxide (Das and Misra, 2004; Kafy et al., 1986). In agreement with these observations, we found that short-term spermidine treatment in vitro did not prevent the aging-like increase in arterial superoxide production caused by pyocyanin. Together, these observations suggest an important role for autophagy-dependent indirect actions in mediating the long-term protective and antioxidant effects of spermidine.
4.4 Autophagy
As the major cellular process for degradation and recycling of damaged biomolecules (Mizushima and Komatsu, 2011), autophagy is a common effector mechanism for numerous cellular stress resistance and homeostasis pathways (Yen and Klionsky, 2008). Although altered autophagy is implicated in many aging and disease processes, its exact role in a particular context requires careful interpretation (Scherz-Shouval and Elazar, 2011). Elevated autophagy occurs in advanced atherosclerotic plaques (Meyer and Martinet, 2009), and autophagy and polyamines are increased in cancer cells, an observation that may reflect the increased survival capacity of oncogenic cells (Minois and Carmona-Gutierrez, 2011). However, accumulating evidence indicates that aging per se involves a decline in autophagy that contributes to the progression of age-related cellular dysfunction (Koga et al., 2010; Rubinsztein et al., 2011; Yen and Klionsky, 2008).
We recently described a role for impaired autophagy in arterial aging (LaRocca et al., 2012). Here, we extend these findings by demonstrating that spermidine supplementation restores the expression of autophagy markers in arteries of old mice. Given the critical role of autophagy in the disposal of damaged biomolecules and defense against oxidative stress, it is possible that enhanced autophagy underlies the reductions in AGEs, nitrotyrosine and superoxide that we observed in spermidine supplemented mice. Our present findings are consistent with this possibility. Although we detected no short-term effects of spermidine on superoxide production in vascular tissue in vitro, we did observe a potent, autophagy-dependent antioxidant influence over a longer term. Consistent with the ideas that these events occur in vivo, we found that spermidine reduced acetylation of histone H3, the key mechanism involved in spermidine-mediated autophagy (Eisenberg et al., 2009). Other mechanisms by which spermidine could induce long-term changes in vascular autophagy include modulation of gene transcription and/or protein modification by NO or peroxynitrite (Frein et al., 2005; Pacher et al., 2007; Sarkar et al., 2011). These potential actions remain to be confirmed experimentally. However, the present data provide initial support for the possibility that autophagy plays a protective role in aging arteries, and that autophagy-enhancing therapies hold promise for the prevention of age-associated arterial dysfunction and CVD.
5. Conclusions
In summary, supplementation with the polyamine and potential nutraceutical spermidine reverses large elastic artery stiffening, restores NO-mediated endothelial function and reduces oxidative stress, while enhancing autophagy in arteries of old mice. These novel findings provide the necessary preclinical evidence to support future translational studies on the efficacy of spermidine for treating age-associated arterial dysfunction and prevention CVD in older adults.
Highlights.
-
!!
We assessed the potential for spermidine supplementation to prevent/reverse arterial aging.
-
!!
Spermidine reduced arterial stiffness and improved arterial endothelial function in old mice.
-
!!
Spermidine also reduced oxidative stress and enhanced markers of autophagy.
-
!!
Supplementation with spermidine may be a novel strategy for treatment of arterial aging.
Acknowledgments
This work was supported by the National Institutes of Health: AG013038, AG039210. We especially thank Melanie Zigler for her technical assistance.
Abbreviations
- ACh
acetylcholine
- AGEs
advanced glycation end-products
- Atg3
autophagy-related protein 3
- CVD
cardiovascular disease
- EDD
endothelium-dependent dilation
- EPR
electron paramagnetic resonance
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- L-NAME
N-G-nitro-L-arginine methyl ester
- LC3-II
lipid-modified microtuble-associated protein light chain 3
- NO
nitric oxide
- SNP
sodium nitroprusside
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We have no conflicts of interest or disclosures.
References
- Binh PNT. Relationship between food polyamines and gross domestic product in association with longevity in Asian countries. Health. 2010;02:1390–1396. [Google Scholar]
- Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovascular Research. 2005;66:286–294. doi: 10.1016/j.cardiores.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Das KC, Misra HP. Hydroxyl radical scavenging and singlet oxygen quenching properties of polyamines. Molecular and cellular biochemistry. 2004;262:127–133. doi: 10.1023/b:mcbi.0000038227.91813.79. [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Talalay P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol Nutr Food Res. 2008;52(Suppl 1):S128–S138. doi: 10.1002/mnfr.200700195. [DOI] [PubMed] [Google Scholar]
- Eisenberg T, Knauer H, Schauer A, Büttner S, Ruckenstuhl C, Carmona-Gutierrez D, Ring J, Schroeder S, Magnes C, Antonacci L, Fussi H, Deszcz L, Hartl R, Schraml E, Criollo A, Megalou E, Weiskopf D, Laun P, Heeren G, Breitenbach M, Grubeck-Loebenstein B, Herker E, Fahrenkrog B, Fröhlich K-U, Sinner F, Tavernarakis N, Minois N, Kroemer G, Madeo F. Induction of autophagy by spermidine promotes longevity. Nature Cell Biology. 2009;11:1305–1314. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- Fleenor BS, Seals DR, Zigler ML, Sindler AL. Superoxide-lowering therapy with TEMPOL reverses arterial dysfunction with aging in mice. Aging Cell. 2012;11:269–276. doi: 10.1111/j.1474-9726.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstermann U. Oxidative stress in vascular disease: causes, defense mechanisms and potential therapies. Nature clinical practice. Cardiovascular medicine. 2008;5:338–349. doi: 10.1038/ncpcardio1211. [DOI] [PubMed] [Google Scholar]
- Frein D, Schildknecht S, Bachschmid M, Ullrich V. Redox regulation: a new challenge for pharmacology. Biochem Pharmacol. 2005;70:811–823. doi: 10.1016/j.bcp.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Kroemer G. Autophagy Mediates the Metabolic Benefits of Endurance Training. Circulation Research. 2012;110:1276–1278. doi: 10.1161/RES.0b013e318259e70b. [DOI] [PubMed] [Google Scholar]
- Gryglewski RJ, Zembowicz A, Salvemini D, Taylor GW, Vane JR. Modulation of the pharmacological actions of nitrovasodilators by methylene blue and pyocyanin. Br J Pharmacol. 1992;106:838–845. doi: 10.1111/j.1476-5381.1992.tb14422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gugliucci A, Menini T. The polyamines spermine and spermidine protect proteins from structural and functional damage by AGE precursors: a new role for old molecules? Life Sci. 2003;72:2603–2616. doi: 10.1016/s0024-3205(03)00166-8. [DOI] [PubMed] [Google Scholar]
- Guo X, Harada C, Namekata K, Kimura A, Mitamura Y, Yoshida H, Matsumoto Y, Harada T. Spermidine alleviates severity of murine experimental autoimmune encephalomyelitis. Investigative ophthalmology & visual science. 2011 doi: 10.1167/iovs.10-6015. [DOI] [PubMed] [Google Scholar]
- Kafy AM, Haigh CG, Lewis DA. In vitro interactions between endogenous polyamines and superoxide anion. Agents and actions. 1986;18:555–559. doi: 10.1007/BF01964964. [DOI] [PubMed] [Google Scholar]
- Koga H, Kaushik S, Cuervo AM. Protein homeostasis and aging: The importance of exquisite quality control. Ageing Research Reviews. 2010:1–11. doi: 10.1016/j.arr.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003a;107:490–497. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003b;107:490–497. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and Cardiac Aging: Major Shareholders in Cardiovascular Disease Enterprises Part I: Aging Arteries: A ℋ Set Up” for Vascular Disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- LaRocca TJ, Henson GD, Thorburn A, Sindler AL, Pierce GL, Seals DR. Translational evidence that impaired autophagy contributes to arterial ageing. The Journal of Physiology. 2012;590:3305–3316. doi: 10.1113/jphysiol.2012.229690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel- Smoller S, Wong ND, Wylie-Rosett J. Heart Disease and Stroke Statistics--2010 Update: A Report From the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- Lydakis C, Stefanaki E, Stefanaki S, Thalassinos E, Kavousanaki M, Lydaki D. Correlation of blood pressure, obesity, and adherence to the Mediterranean diet with indices of arterial stiffness in children. European journal of pediatrics. 2012;171:1373–1382. doi: 10.1007/s00431-012-1735-3. [DOI] [PubMed] [Google Scholar]
- Madeo F, Eisenberg T, Büttner S, Ruckenstuhl C, Kroemer G. Spermidine: a novel autophagy inducer and longevity elixir. Autophagy. 2010;6:160–162. doi: 10.4161/auto.6.1.10600. [DOI] [PubMed] [Google Scholar]
- Meyer GRYD, Martinet W. Autophagy in the cardiovascular system. BBA - Molecular Cell Research. 2009;1793:1485–1495. doi: 10.1016/j.bbamcr.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Minois N, Carmona-Gutierrez D. Polyamines in aging and disease. AGING. 2011 doi: 10.18632/aging.100361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minois N, Carmona-Gutierrez D, Bauer MA, Rockenfeller P, Eisenberg T, Brandhorst S, Sigrist SJ, Kroemer G, Madeo F. Spermidine promotes stress resistance in Drosophila melanogaster through autophagy-dependent and -independent pathways. Cell death & disease. 2012;3:e401. doi: 10.1038/cddis.2012.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GF, Hwang S-J, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Komatsu M. Autophagy: Renovation of Cells and Tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- Morselli E, Marino G, Bennetzen MV, Eisenberg T, Megalou E, Schroeder S, Cabrera S, Benit P, Rustin P, Criollo A, Kepp O, Galluzzi L, Shen S, Malik SA, Maiuri MC, Horio Y, Lopez-Otin C, Andersen JS, Tavernarakis N, Madeo F, Kroemer G. Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. The Journal of Cell Biology. 2011;192:615–629. doi: 10.1083/jcb.201008167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Appel LJ, Van Horn L. Components of a Cardioprotective Diet: New Insights. Circulation. 2011;123:2870–2891. doi: 10.1161/CIRCULATIONAHA.110.968735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M. Pyocyanin induces oxidative stress in human endothelial cells and modulates the glutathione redox cycle. Free Radic Biol Med. 2002;33:1527–1533. doi: 10.1016/s0891-5849(02)01087-0. [DOI] [PubMed] [Google Scholar]
- North BJ, Sinclair DA. The Intersection Between Aging and Cardiovascular Disease. Circulation Research. 2012;110:1097–1108. doi: 10.1161/CIRCRESAHA.111.246876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. Journal of the American College of Cardiology. 2007;50:1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucciarelli S, Moreschini B, Micozzi D, De Fronzo GS, Carpi FM, Polzonetti V, Vincenzetti S, Mignini F, Napolioni V. Spermidine and spermine are enriched in whole-blood of nona/centenarians. Rejuvenation Res. 2012 doi: 10.1089/rej.2012.1349. [DOI] [PubMed] [Google Scholar]
- Reddy AK, Li YH, Pham TT, Ochoa LN, Trevino MT, Hartley CJ, Michael LH, Entman ML, Taffet GE. Measurement of aortic input impedance in mice: effects of age on aortic stiffness. American journal of physiology. Heart and circulatory physiology. 2003;285:H1464–H1470. doi: 10.1152/ajpheart.00004.2003. [DOI] [PubMed] [Google Scholar]
- Rippe C, Lesniewski L, Connell M, LaRocca T, Donato A, Seals D. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell. 2010a;9:304–312. doi: 10.1111/j.1474-9726.2010.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippe C, Lesniewski L, Connell M, Larocca T, Donato A, Seals D. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell. 2010b:1–9. doi: 10.1111/j.1474-9726.2010.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein DC, Mariño G, Kroemer G. Autophagy and Aging. Cell. 2011;146:682–695. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- Santhanam L, Christianson DW, Nyhan D, Berkowitz DE. Arginase and vascular aging. J Appl Physiol. 2008;105:1632–1642. doi: 10.1152/japplphysiol.90627.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Korolchuk VI, Renna M, Imarisio S, Fleming A, Williams A, Garcia-Arencibia M, Rose C, Luo S, Underwood BR, Kroemer G, O'Kane CJ, Rubinsztein DC. Complex inhibitory effects of nitric oxide on autophagy. Mol Cell. 2011;43:19–32. doi: 10.1016/j.molcel.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherz-Shouval R, Elazar Z. Regulation of autophagy by ROS: physiology and pathology. Trends in Biochemical Sciences. 2011;36:30–38. doi: 10.1016/j.tibs.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Schildknecht S, Ullrich V. Peroxynitrite as regulator of vascular prostanoid synthesis. Arch Biochem Biophys. 2009;484:183–189. doi: 10.1016/j.abb.2008.10.023. [DOI] [PubMed] [Google Scholar]
- Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond) 2011a;120:357–375. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clinical Science. 2011b;120:357–375. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindler AL, Fleenor BS, Calvert JW, Marshall KD, Zigler ML, Lefer DJ, Seals DR. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell. 2011a;10:429–437. doi: 10.1111/j.1474-9726.2011.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindler AL, Fleenor BS, Calvert JW, Marshall KD, Zigler ML, Lefer DJ, Seals DR. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell. 2011b doi: 10.1111/j.1474-9726.2011.00679.x. no-no. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soda K. Polyamine intake, dietary pattern, and cardiovascular disease. Medical Hypotheses. 2010;75:299–301. doi: 10.1016/j.mehy.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Soda K, Dobashi Y, Kano Y, Tsujinaka S, Konishi F. Polyamine-rich food decreases age-associated pathology and mortality in aged mice. Experimental Gerontology. 2009;44:727–732. doi: 10.1016/j.exger.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Soda K, Phan Nguyen Thanh B, Masanobu K. Mediterranean diet and polyamine intake: possible contribution of increased polyamine intake to inhibition of age-associated disease. Nutrition and Dietary Supplements. 2010:1. [Google Scholar]
- Sprott RL, Ramirez I. Current Inbred and Hybrid Rat and Mouse Models for Gereontological Research. ILAR journal / National Research Council, Institute of Laboratory Animal Resources. 1997;38:104–109. doi: 10.1093/ilar.38.3.104. [DOI] [PubMed] [Google Scholar]
- Sudarsanam S, Johnson DE. Functional consequences of mTOR inhibition. Current opinion in drug discovery & development. 2010;13:31–40. [PubMed] [Google Scholar]
- Tognon G, Rothenberg E, Eiben G, Sundh V, Winkvist A, Lissner L. Does the Mediterranean diet predict longevity in the elderly? A Swedish perspective. AGE. 2010;33:439–450. doi: 10.1007/s11357-010-9193-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- Yen WL, Klionsky DJ. How to live long and prosper: autophagy, mitochondria, and aging. Physiology (Bethesda) 2008;23:248–262. doi: 10.1152/physiol.00013.2008. [DOI] [PubMed] [Google Scholar]