Abstract
Of the many mysteries that surround the brain, few surpass the awe-inspiring complexity of its development. The intricate wiring of the brain at both the system and molecular level is both spatially and temporally regulated in perfect synchrony. How such a delicate, yet elegant, system arises from an embryo’s most basic cells remains at the forefront of neuroscientific research. At the cellular level, the competitive dance between synapses struggling to gain dominance seems to be refereed by both neurons themselves and microglia, the innate immune cells of the nervous system. Additionally, the unexpected complement cascade, a major effecter arm of the innate immune system, is almost certainly involved in synaptic remodeling by tagging destined neurons and synapses for destruction. As suddenly as they appear, the mechanisms of neurogenesis recede entering into adulthood. However, with age and insult, these mechanisms boisterously return, resulting in neurodegeneration. This review describes some of the mechanisms involved in synaptogenesis and wiring of the brain from the point of view of the innate immune system and then covers how similar molecular processes return with age and disease, specifically in the context of Alzheimer’s disease.
Keywords: Fractalkine, CX3CR1, CR1, CR3, CD11b, C1q, C3, phagocytosis
1. Introduction
Classically, the brain has been deemed an absolute immune privileged organ, due in part to the tight junction laden epithelial cells that line the cerebrovasculature, constituting the aptly named blood brain barrier (BBB). This concept, however, has begun to wane with the observations of infiltrating macrophages and T cells during BBB breakage. However, even with an intact BBB, neurons, astrocytes and resident microglia express a full range of immune markers including major histocompatability complexes (MHC), toll-like receptors (TLR), cytokines and their receptors and components of the complement cascade. This evidence suggests that in a normal state, the CNS modulates its own ‘neuroimmune’ system and thus its immune privilege state is only relative to other organs (Galea et al., 2007). This provides a second line of defense against infiltrating microbes and a clean up mechanism for maintaining the brain parenchyma.
Additionally, recent evidence has suggested a role for microglia, the brain’s resident immune cells, in synaptic development and maintenance through neuron-microglia interactions and complement-mediated mechanisms. Through unexpected means, microglia have the ability to influence wiring at the synaptic level and thus aid in the control of learning and memory.
On the other hand, this newly recognized function of the immune system can have detrimental effects on the delicate architecture of the brain as seen in many neurodegenerative diseases. Observations in the field of neuroinflammation suggest an intricate homeostatic balance between neurons and immune cells that if tipped either way, leads to neuronal dysfunction and cognitive deterioration. This has become a highly researched area within the realm of the most common form of dementia, Alzheimer’s disease (AD), where complement activation and microglial dysfunction seem to contribute significantly to disease onset and progression. If left unchecked, the mechanisms of microglia-mediated synaptic development described above can be pushed in a direction of pathological alteration of synapses and the surrounding brain milieu.
The wide array of functions and their effect on nervous system development and disease has sparked a massive interest into the cellular and molecular dynamics of the immune system residing within the walls of the BBB. This review will encompass these mechanisms in terms of microglial function in the developing brain and how dysfunction of this resident immune network leads to neurodegenerative diseases such as Alzheimer’s disease. We will also discuss the dichotomy of complement cascade function in development and neurodegenerative disease and how microglia influence this transition to pathology. The novel evidence at hand has ushered in the need for a more complete understanding of the relationship between microglia and complement in the development and disease progression of the nervous system.
2. Microglia influence synapse structure and function
The traditional view of microglial function has been one of defense against foreign invaders and maintenance of the healing process after nervous tissue injury. Microglia have the ability to react to any number of stimuli (Kreutzberg, 1996) in a matter of minutes (Morioka et al., 1991), mounting an immune response. Termed the “garbage men” of the brain, microglia are professional and highly efficient phagocytes, utilizing several methods for engulfing debris (Napoli and Neumann, 2009; Neumann et al., 2009).
The first implications for an alternate role of microglial phagocytosis in neuronal function came with studies by Franz Nissl (1889), who showed the close proximity of microglial rod cells to neuronal apical dendrites. Microglia seem to play an important role in the metabolically prudent mechanism of clearing a lesioned area of non-functional synaptic elements to allow regeneration of tissue. It has been shown that microglia migrate to the site of injury and engulf motor neuron synapses in a process called synaptic stripping (Schiefer et al., 1999); a process which can also occur during synaptic regeneration (Blinzinger and Kreutzberg, 1968). More recently, sound evidence has demonstrated that microglia may eliminate synapses, but not entire neurons, in response to an inflammatory stimulus (bacterial infection) in the cerebral cortex as a neuron-supportive mechanism (Trapp et al., 2007). In the non-pathological brain, microglia have been shown to transiently monitor synapses with brief, but consistent pauses, while maintaining connections for longer periods of time during ischemic insults (Wake et al., 2009).
Several molecular signaling pathways exist between neurons and microglia that determine the extent of their interaction and whether the response is pro- or anti-inflammatory. Microglia are in a prime position for manipulating neuronal function with their broad, gridded spatial distribution and plethora of receptors and signaling molecules. Numbering approximately one per neuron in the mammalian brain, microglia arrange themselves in a non-overlapping grid, and monitor their own parcel of brain parenchyma (Nimmerjahn et al., 2005), but do have the ability upon foreign insult to migrate towards a lesion. During mouse brain development, microglia increase in number and distribute themselves uniformly throughout the cortical grey matter and within white matter tracts (Milligan et al., 1991), with increased densities in the hippocampus, basal ganglia, substantia nigra and olfactory bulb (Lawson et al., 1990; Yang et al., 2012). No studies have conclusively determined the distribution of microglia in the adult human brain yet, but it seems they populate white matter more than grey matter (Mittelbronn et al., 2001). Additionally, microglia express a variety of classical neurotransmitter receptors (Pocock and Kettenmann, 2007), allowing them to constantly monitor levels of neuronal activity. This could explain the mechanism of pruning of inhibited dendrites in activity-dependent removal of synapses, discussed in the next section.
3. The brain’s “garbage men” in synapse and circuit development
The job of monitoring the brain parenchyma for damaged cells and toxins also includes the removal of newborn cells that have undergone developmentally programmed apoptosis. As the nervous system develops, it overestimates the number of neuronal connections it will need, and thus begins with excess. This is controlled for by programmed cell death. In the subgranular zone of the dentate gyrus, microglia engulf apoptotic neuroblasts that never integrate into the local hippocampal circuitry. This removal by ramified microglia is highly efficient as demonstrated by increased phagocytic index in later stages of apoptosis (91% of apoptotic neuroblasts) compared to earlier stages (5%–8%) (Sierra et al., 2010). Interestingly, neuronal precursors isolated from the embryonic brain migrate toward a gradient of microglial-conditioned media in vitro and differentiate into neurons rather than astrocytes (Aarum et al., 2003; Walton et al., 2006). This is reminiscent of a conductor in an orchestra and suggests microglia play an influential role in neural patterning.
As neuroblasts find their respective positions, neighboring axons are thought to compete with each other for post-synaptic targets, and following Hebb’s rule, the more synchronous connections survive and strengthen and the weaker ones are lost, as shown by Balice-Gordan and Lichtman at the neuromuscular junction (Balice-Gordon and Lichtman, 1994). The precedence that the final firing pattern of a circuit is due to competitive inhibition occurs in the cortex was set by Hubel and Wiesel in the 1960s when they showed that ocular dominance columns segregated relative to incoming, competing inputs (Wiesel and Hubel, 1965). Inactivity in one eye altered firing in that eye, whereas inactivity in both eyes had no effect on overall activity within the pathway; similar to the findings of Balice-Gordan and Lichtman at the synaptic level.
Just as an excess of neurons is a metabolic waste, it is also inefficient to hang on to non-functioning, weak synapses, thus they necessarily must be removed. As novice, amoeboid microglia enter the embryonic brain, they begin to ramify taking on a more specialized morphology (Giulian and Baker, 1986), while retaining their phagocytic capabilities. An important study in this regard showed that microglia actively phagocytose synapses in the developing mammalian brain. Conversely, mice with microglia lacking the ability to sense excess synaptic elements though select chemokine signaling consisted of excess immature synapses (Paolicelli et al., 2011). Ramified microglial processes have also been found directly apposed to synaptic clefts and time lapse in vivo microscopy showed changes in microglial morphology and phagocytosis of dendritic spines in response to changes in visual experience (Tremblay et al., 2010).
It has been well established since the studies of Hubel and Wiesel discussed above, that decreased synaptic activity results in the pruning of the inactive synapses and modification of the neural circuit so that the strongest synapses survive (Kerschensteiner et al., 2009; Maffei et al., 2006). Synaptic targets no longer receiving input from upstream axons because of visual deprivation would lack neurotransmitter spillover that would be detected by microglial neurotransmitter receptors. How microglia recognize the exact dendrite to engulf is not yet known, but one candidate stands out among the rest, which will be discussed below.
4. How do microglia recognize deficient synapses?
Over a decade ago, the first exclusively neuron-to-microglia signaling pathway was discovered. This cross-talk is mediated by a newly discovered, membrane-bound chemokine termed fractalkine (FKN), or CX3CL1 (Bazan et al., 1997). Its canonical G protein coupled receptor, CX3CR1, is found only on microglia in the CNS (Cardona et al., 2006). Since its discovery, the fractalkine pathway has been intensely studied in many neurodegenerative and neuroprotective settings. Interestingly, it seems that its role in mediating microglial function depends on the stimulus and/or the period of development (Gemma et al., 2010). In a model of facial motor nerve axotomy, CX3CR1(+) microglia showed a robust increase in intracellular calcium when adhering to damaged neurons in the presence of membrane-bound FKN, which was not seen with soluble FKN (Harrison et al., 1998). Interestingly, after the initial injury the smaller 36 kDa FKN fragment (presumably the soluble form containing the chemokine domain) was found most abundant up to day four post-injury. On the other hand, the larger 65 kDa fragment (membrane-bound form) was evident throughout the injury. The presence of the soluble fragment early on may reflect the need to attract microglia to the site of injury, whereas the consistent presence of the membrane-bound form would allow more efficient recognition through calcium-dependent adhesion to the damaged neuron. This interesting finding provides a mechanism by which microglia recognize distressed and damaged neurons via FKN/CX3CR1 signaling.
How might microglia use the above-mentioned mechanism of chemotaxis to and recognition of developing neurons? FKN is expressed in the brain at least by the fourth week of age in rodents (Labrada et al., 2002), but probably earlier. In the absence of CX3CR1 at postnatal (P)13-P16, mice showed significantly altered electrophysiology and an increased number of immature synapses in CA1 hippocampal subfield reminiscent of inadequately formed neural circuitry (Paolicelli et al., 2011). Presumably, microglia lacking the CX3CR1 receptor fail to recognize synapses displaying FKN, in either form. This study also showed that PSD95 puncta were surrounded by GFP-labeled microglia in three-dimensional reconstruction confocal microscopy and electron microscopy. However, it was unclear as to what signal initiated engulfment of synapses as after P28, there was no difference in the number of synapses or electrophysiological recordings between groups, suggesting a separate temporally dependent mechanism for synaptic regulation throughout development. Additionally, this study did not determine what forms of FKN are present in the developing synaptic milieu, which as seen during neuronal injury, could play an important role for a microglial response.
4.1. Complement in microglia-mediated synaptic remodeling
Promising molecular candidates for alternative mechanisms of synaptic pruning include the well-known adaptive immune entity MHC class I and components of the innate immune system’s complement cascade (MHC class III family). MHC I has been thoroughly studied by Shatz et al. and the reader is ushered to view that literature (reviewed in (Boulanger, 2009; Boulanger and Shatz, 2004). C1q, the initiator of the classical complement cascade has been suggested, along with its downstream partner, C3, to be necessary for synaptic refinement in the visual system during development. Astrocyte-mediated activation of C1q results in downstream activation of C3b, which then deposits on neurites, thus “tagging” them for elimination (Stevens et al., 2007). Mice lacking either C1q or C3 show increased synaptic connectivity and enhanced epileptiform activity due to failed synaptic pruning (Stevens et al., 2007; Chu et al., 2010). Although direct opsonization and elimination of synapses by C1q has been posited (Perry and O’Connor, 2008) and is likely mediated through Complement Receptor 1 (CR1) or the C1q Receptor (C1qRp) (Webster et al., 2000), it seems more likely that C3b is the effecter. C1q is only transiently expressed in developing neocortex between P4 and P10, while C3b manifests up to P30 in synaptic regions (Stevens et al., 2007). Because of the linearity of the complement cascade in this instance, C1q knock out (KO) mice would show the same deficits in wiring as C3 KO mice, suggesting a small role for the alternative complement pathway in development. Traditionally, C1q plays the role of initiating complement activation, while C3 acts to amplify the pathway, which could explain the extended deposition of C3b at synaptic sites. However, another indirect method of C1q-mediated synapse elimination involves the “disorganizing” of the synaptic cleft. Basically, C1q can bind neuronal pentraxins, a family of acute phase proteins involved in clustering AMPA receptors at the postsynaptic cleft, removing their stabilizing ability of dendritic spine receptors. This would ultimately decrease the amount of communication between the pre- and postsynapses and result in synaptic weakening (Yuzaki, 2010). Interestingly, this activity-dependent increase in C1q could be mediated by increased calcium influx and calcineurin-mediated transcription as seen with Cbln1, a part of the C1q-like family of proteins (Yuzaki, 2010).
Microglia, as the brain’s innate immune cells, possess an arsenal of phagocytic receptors. In the context of complement-mediated synaptic pruning, Complement Receptor 3 (CR3 or CD11b) plays a significant role. In the developing hippocampus, just as in the developing cerebellum (Marin-Teva et al., 2004), apoptosis is triggered by microglial CR3-mediated superoxide ion release, which is dependent on the DAP12 immunoreceptor (Wakselman et al., 2008). Although it was not shown, it could be speculated that this respiratory burst by microglia results from contact with C3b selectively deposited on neurons committed to die or synapses destined for pruning. Recent work supports this hypothesis. Genetic ablation of CR3 and C3 or pharmacological perturbations of these proteins results in microglial dysfunction and deficits in synaptic remodeling (Schafer et al., 2012). Microglia engulf synapses at peak neuronal activity and at the height of synaptic pruning in the developing nervous system. This remodeling is CR3/C3b-dependent and requires synaptic activity as demonstrated with pharmacological manipulation of signaling between competing synapses. These data suggest that complement-mediated synaptic remodeling represents an even more important mechanism underlying brain development as CR3 and C3 KO have sustained deficits in synaptic plasticity into adulthood, whereas it seems the impact of FKN signaling wanes after P28 as discussed above.
4.2. Putting the story of microglia-mediated brain development together
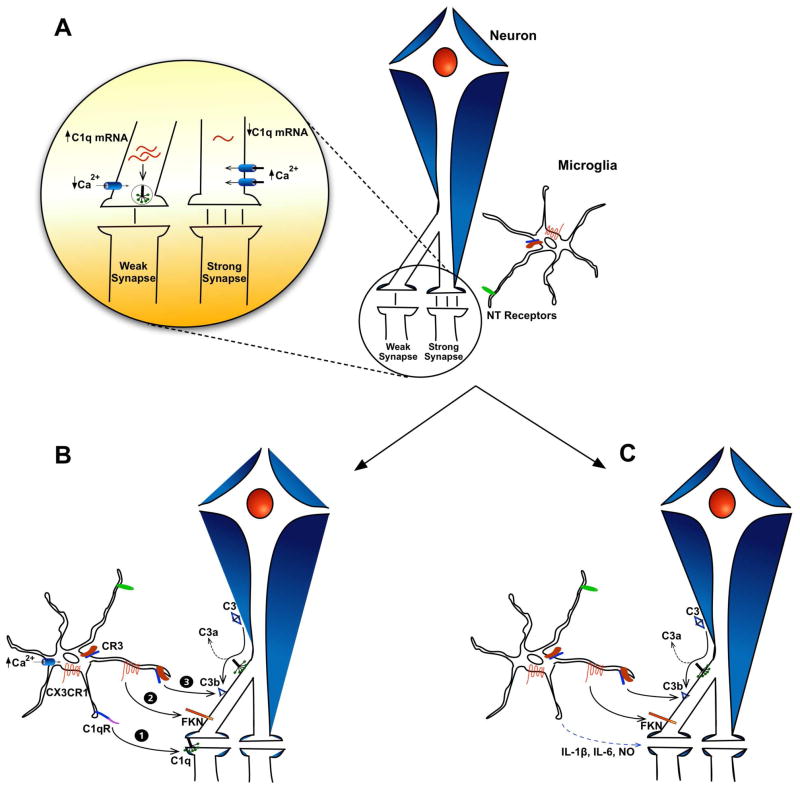
An intriguing mechanism for neural patterning and circuit development suggests that microglia direct the migration and differentiation of neural precursors. Once these precursors reach their destination, effectively into the vicinity of microglial processes, they apoptose through some unknown, programmed cellular signal or are tagged for phagocytosis; both scenarios seem to be mediated by microglia (Fig. 1). Apoptosing neurons are recognized by microglia through adenosine triphosphate (ATP) and uridine triphosphate (UDP) via P2X4 and P2Y6 receptor signaling, respectively, which result in chemotaxis and phagocytosis (Ohsawa et al., 2007, Koizumi et al., 2007). Those neurons that survive the initial selection process transition to a second trial of synaptic input and output testing. This could be mediated by two separate mechanisms or by their sequential occurrence: 1) As axons compete for dendrites, the stronger signaling axon prevails, which could cause transient downstream cellular transcription of the CX3CL1, which would then locate to the membrane of the weaker synapse. This has been shown to result in synaptic pruning through microglial activation (Paolicelli et al., 2011). 2) A more prolonged effect of synaptic pruning by microglial occurs through the same transcription mechanism, but for C1q, which can either translocate to the postsynaptic dendrite or activate downstream C3b that deposits on the presynaptic terminal. In any case, microglia recognize these “tags” via their respective receptors and effectively engulf their targets. More work will be required to tease apart the mechanisms of microglial recognition of “tagged” synapses and whether or not new neurons are directly involved in their death through transcription of complement factors, specifically C1q.
Fig. 1. Schematic of microglia- and complement-mediated synaptic remodeling during development.
A. Microglia can recognize active synapses through neurotransmitter-mediated signaling via receptors on their membrane, resulting in their avoidance. Concomitantly, active synapses experience higher influxes of calcium, which could repress calcineurin-mediated transcription of complement factors such as C1q, while decreased calcium would do the opposite (inset). B. Upon expression of C1q or fractalkine by neurites destined for destruction, microglia recognize these components through respective receptors (1) C1qR or (2) CX3CR1, which would mediate phagocytosis. Additionally, neuronal expression of C1q could also cleave C3 into its opsonin unit C3b, which deposits on targeted synapses for engulfment (3). C. Another mechanism through which microglia sensitize targeted synapses is through expression of pro-inflammatory molecules (i.e. IL-1β, IL-6 or NO), which stimulate neurons to express complement factors or fractalkine. This is not an exhaustive list, but details these selected mechanism of synaptic remodeling. NT = neurotransmitter; FKN = fractalkine; CX3CR1 = fractalkine receptor; C1qR = C1q Receptor; CR3 = Complement Receptor 3; IL-1β = interleukin 1β; IL-6 = interleukin 6; NO = nitric oxide.
5. The microglia-complement interaction: from development to disease
With all of the surfacing data presenting itself in the neurodevelopment literature, it might seem incomplete to concentrate on only two pathways that end at the fully developed brain. However, an interesting dichotomy presents itself when we look into the classical neuroinflammatory pathways mediating neurodegeneration—they are quite similar to those involved in neurogenesis and synaptic turnover and may reflect a molecular reversion back to an earlier developmental period. The reactivation of microglia and expression of related complement factors and receptors with age seems to push the brain’s homeostasis back to a removal of now essential neural components. Why microglia begin re-expressing complement receptors and other inflammatory mediators at the start of neurodegeneration is still under investigation, but could simply be due to senescence and aging of the brain milieu (Streit et al., 2009, Norden and Godbout, 2012) or other systemic factors such as global dysregulation in cholesterol homeostasis (Castello and Soriano, 2012), which may alter expression of such mediators and their regulators. It is well established, however, that microglia play a significant role in the aged and diseased brain. For example, microglia have been shown to bind and clear amyloid in Alzheimer’s disease (AD) models (Koenigsknecht et al., 2004; Koenigsknecht-Talboo et al., 2005; Mandrekar et al., 2009; Bamberger et al., 2003) with a myriad of immune receptors. Additionally, nearly all complement components have been studied in the context of AD pathogenesis. These complement-mediated disease mechanisms will be discussed below in relation to microglia activation.
5.1. Role of C1q in AD pathogenesis
It was first reported in the early 1980s that many of the complement proteins, including C1q and C3b, were localized in neuritic plaques (Eikelenboom and Stam, 1982), even before the identification of amyloid β (Aβ) as the main component of these deposits. This naturally suggested their importance for disease pathophysiology. Tenner and colleagues then went on to show the localization of C1q to astrocytes and microglia in the AD brain and interestingly to intact neurons, suggesting neuronal synthesis of this component (Afagh et al., 1996). This was confirmed and extended by Terai and colleagues (Terai et al., 1997)who showed that pyramidal neurons increase expression of C1q in AD versus normal controls. In a mouse model of AD crossed with a C1q knockout mouse (APPQ−/−), it was shown that amyloid plaque load could be reduced and neuronal integrity saved (Fonseca et al., 2004). However, due to the variability of this finding in other models, these data should be taken cautiously. Additionally, a separate study by the same group extended these findings and showed that lack of C1q resulted in increased C3 levels and coincident reduction in neuropathology compared to APPQ+/+ mice (Zhou et al., 2008).
In cell culture models, microglia were found to have more robust activation, increase in intracellular calcium suggestive of chemotaxis and release of proinflammatory mediators when stimulated with C1q (Farber et al., 2009), but this does not seem to increase uptake of Aβ by microglia stimulated with immobilized C1q and even decreases uptake via soluble C1q (Webster et al., 2000). These results may be explained by the aggregation state of Aβ, as Aβ40 in the fibrillar configuration is highly potent activator of complement (Webster et al., 1997). More work in this area is required, but a simple scenario presents in the form of aged and diseased neurons in AD increasing their expression of C1q, possibly in the presence of Aβ. Although it has not been observed in vivo, these C1q deposits on neurons (see also (Fonseca et al., 2004)) may induce microglial migration and phagocytosis of neurons or their dendrites (Fraser et al., 2010) leading to the cognitive dysfunction seen in AD. These data indicate a contrariety between the unfavorable classical pathway (C1q-mediated) and the beneficial alternative pathway (C3-mediated) on AD pathogenesis (Table 1).
Table 1.
Summary of microglial recognition components in development and disease
| Receptor/Ligand | Microglial response upon ligation | Role in development | Ref | Role in neurodegeneration | Ref |
|---|---|---|---|---|---|
| CX3CR1/CX3CL1 | Ca2+ influx and chemotaxis; regulates microglial activity by dampening inflammatory response. | Early contribution (between P13-16, but up to P28) to removal of supernumerary synapses. | Paolicelli et al. 2011 | Depending on the disease model: | |
| Deficiency is detrimental in tau mutant models and contributes to tau pathology. | Bhaskar et al. 2010 | ||||
| Deficiency is beneficial in Aβ mutant models and results in activated microglial Aβ clearance. | Liu et al. 2010 | ||||
|
| |||||
| CR1 or C1qRp/C1q | Phagocytosis; clearance; release of TNFα and IL-6. | Contributes to synapse remodeling between P4 and P10. | Stevens et al. 2007 | Classical activation may be detrimental due to the multitude of pro-inflammatory effecter functions carried out by the proximal arm of complement. | Zhou et al. 2008 |
|
| |||||
| CR3/C3b | Cell adhesion; phagocytosis; release of pro-inflammatory cytokines. | Contributes to prolonged synapse remodeling into adulthood. | Stevens et al. 2007 | Alternative activation may be beneficial up to a certain threshold; Detrimental depending on CR1 genotype, which could result in increased C3b levels. | Zhou et al. 2008, Ramaglia et al. 2012, Biffi et al. 2012 |
|
| |||||
| P2X4/ATP | Activation induces microglial chemotaxis | Programmed neuronal apoptosis releases ATP to be recognized by microglia | Ohsawa et al. 2007 | Neuronal damage during degeneration releases ATP and attracts microglia. | Ohsawa et al. 2007 |
|
| |||||
| P2Y6/ATP or UDP | Promotes microglial phagocytosis | Programmed neuronal apoptosis releases UDP to be recognized by microglia | Koizumi et al. 2007 | UDP release after neuronal injury elicits microglial phagocytosis. | Koizumi, et al. 2007 |
CR1 = Complement Receptor 1; CR3 = Complement Receptor 3; C1qRp = C1q Receptor (phagocytosis); TNFα = Tumor Necrosis Factor alpha; IL-6 = Interleukin 6; ATP = Adenosine triphosphate; UDP = Uridine triphosphate
5.2. C3b as a neuroprotective Aβ opsonin
Complement C3 is the central component of the complement cascade and the joining point of all three complement pathways. Upon activation either by classical C3-convertase mediated cleavage (C4b2a) or the alternative pathway C3bBb, C3 is cleaved into its larger fragment C3b, which acts as an opsonin and can bind many surfaces, and the smaller anaphylatoxin fragment C3a, which has many effecter functions in the innate immune system. Further cleavage of C3b results in smaller fragments designated iC3b, C3c and C3d, which may serve other unknown functions (Nishida et al., 2006). As with C1q, C3b and its cleavage product iC3b have been found deposited on AD-affected neurons (Loeffler et al., 2008), which would facilitate efficient removal by microglia.
Although the activation of C1q in AD described above seems to have detrimental effects on CNS homeostasis, the story of C3 activation seems more complicated and may only function properly within specific ranges. For example in a mouse model of AD, human amyloid precursor protein (hAPP) transgenic mice, C3 activation was suppressed with introduction of the soluble complement receptor-related protein y (sCrry). This led to a 2- to 3-fold increase in Aβ deposition and prominent neuronal degeneration in hAPP/sCrry mice compared to age-matched hAPP littermates (Wyss-Coray et al., 2002). A similar study with C3-deficient APP mice (APP;C3−/−) found increased levels of Aβ in the brain and plasma, along with a significant loss of hippocampal neurons and an altered microglial phenotype, including increases in the anti-inflammatory cytokines IL-4 and IL-10 (Maier et al., 2008). The results from these studies suggest a necessary role for C3- especially the opsonizing effects of C3b- in removal of Aβ, which may also be dependent on pro-inflammatory cytokines, but additionally that complement offers neuronal protection in the AD brain. Unfortunately, neither study measured levels of microglial CR3 expression, a receptor for C3b/iC3b. Other surface markers suggested a switch of microglia/macrophages to the M2 phenotype associated with scavenging of apoptotic cells and tissue repair.
On the other hand, abundant levels of C3b may be detrimental to neuronal integrity in a manner dependent on microglial activation. In a model of multiple sclerosis, Crry KO resulted in priming of microglia via the CR3/C3b interaction (Ramaglia et al., 2012). Microglia failed to show a classical activation phenotype, but instead one that showed microglia were ready for activation, or primed, and upon a second stimulus responded much more vigorously than wild type mice. They possessed thick ramifications and robust CR3 immunoreactivity, but lacked expression of pro-inflammatory mediators (Ramaglia et al., 2012). Depletion of Crry allowed the accumulation of C3b, which left unchecked, could bind to microglial CR3 and upon a second stimulus result in myelin phagocytosis and release of pro-inflammatory cytokines (van der Laan et al., 1996) or propagate to downstream membrane attack complex (MAC) consisting of complement components C5b-9. The MAC notoriously causes cellular injury through osmotic lysis.
Microglia-complement interaction may have downstream detrimental effects on the cerebrovasculature as well. In a human genetic study, allelic variation in the gene that encodes CR1, the human homolog of Crry, was strongly associated with the AD-related pathology, cerebral amyloid angiopathy (CAA) and CAA-associated intracerebral hemorrhage (ICH) (Biffi et al., 2012). In AD patients with CAA, in which 75% of CAA patients were homozygous for the CR1 risk allele (A/A), CR3 bound more C3b and Aβ compared to normal cognitive controls and AD patients without CAA (Zabel et al., 2012). In accordance with Ramaglia et al. (2012), microglia that bound C3b demonstrated a ramified phenotype (suggestive of priming), while the microglia in AD without CAA (which did not bind C3b), showed a much more activated, amoeboid phenotype. Interestingly, CR3 bound the same levels of Aβ in the absence of C3b in controls and AD only patients. Whether this binding was dependent on another complement component, such as iC3b is unknown. Not only did this increase in CR3/C3b/Aβ binding occur in AD with CAA patients, but these patients also manifested with increased levels of downstream MAC deposition on cortical and leptomeningeal arteries, which colocalized with smooth muscle cells, suggesting a specific mechanism of disease pathogenesis (Zabel et al., 2012). Additionally, it was determined that the MAC regulator, CD59, was not increased on the same blood vessels as would be expected with increased MAC activation (Zabel et al., 2012). It seems that a variation in the CR1 gene could lead to a CR1 protein product that is much less efficient at controlling C3b generation and thus an increase in C3b production. In theory, increased C3b seen in AD patients with CAA (Zabel et al., 2012) could be a beneficial compensatory mechanism that allows for more binding of Aβ to CR3 for clearance to the blood vessel. However, the use of C3b as the opsonin in this case results in downstream activation of the terminal lytic MAC in the absence of a CD59 response, which could explain the cerebral microbleeds and ICH seen so frequently in cases of CAA. Because Aβ clearance is necessary for brain physiology and C3 may augment this mechanism, a downstream inhibitor of MAC may be a good therapeutic target to leave more proximal aspects of complement functioning properly. More specific analysis of CAA specimens should directly compare CR1 risk genotype, C3b levels, MAC deposition and microbleeds.
6. Concluding Remarks
The mounting evidence for direct microglial function on brain integrity loosens the strict definition of these cells as simply immune effectors. Their elegant and selective ability to remodel the developing nervous system is crucial for appropriate CNS function as an adult. Aided by an unlikely cascade of proteins from the complement system, microglia hold tremendous responsibility in sculpting an immensely complicated organ. However, left unchecked throughout adulthood or compromised by unfortunate genetic variations, the complement system can storm out of control, coaxing microglia back into their engulfing phenotype, but now with much more vigor.
Like all biological systems, the perfectly calibrated titration of homeostatic mechanisms and temporal regulation are necessary for efficient operation at the molecular and cellular level. How the competitive signals generated by developing neurons result in exact time-dependent pruning by microglia is still under investigation. How microglia determine exactly which synapses to engulf is only now becoming clear. The next major undertaking will have to take into consideration that the mechanisms driving development can resurface in adulthood and result in disease. Why do mechanisms important for proper function in development return to cause havoc in the adult and aged brain? With the newly forming hypothesis that many neurodegenerative diseases manifest over decades of a person’s life, answers to this question will bring preventative therapies for the most dreaded neurological diseases. Understanding the development of the nervous system will be required for understanding the dysfunction of the nervous system.
Highlights.
Contrary to earlier hypotheses, the developing brain is a complex milieu of innate and adaptive immune molecules and cells.
The complement cascade is heavily involved in synaptic remodeling, especially the classical pathway via C1q and C3b.
Microglia are the effecter cells that recognize complement components on predestined synapses to be eliminated.
These same complement molecules and microglia can be reactivated during adulthood, resulting in neurodegeneration.
Both microglia and complement are involved in the pathogenesis of Alzheimer’s disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarum J, Sandberg K, Haeberlein SL, Persson MA. Migration and differentiation of neural precursor cells can be directed by microglia. Proc Natl Acad Sci U S A. 2003;100:15983–15988. doi: 10.1073/pnas.2237050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afagh A, Cummings BJ, Cribbs DH, Cotman CW, Tenner AJ. Localization and cell association of C1q in Alzheimer’s disease brain. Exp Neurol. 1996;138:22–32. doi: 10.1006/exnr.1996.0043. [DOI] [PubMed] [Google Scholar]
- Bachstetter AD, Morganti JM, Jernberg J, Schlunk A, Mitchell SH, Brewster KW, Hudson CE, Cole MJ, Harrison JK, Bickford PC, Gemma C. Fractalkine and CX 3 CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiol Aging. 2011;32:2030–2044. doi: 10.1016/j.neurobiolaging.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balice-Gordon RJ, Lichtman JW. Long-term synapse loss induced by focal blockade of postsynaptic receptors. Nature. 1994;372:519–524. doi: 10.1038/372519a0. [DOI] [PubMed] [Google Scholar]
- Bamberger ME, Harris ME, McDonald DR, Husemann J, Landreth GE. A cell surface receptor complex for fibrillar beta-amyloid mediates microglial activation. J Neurosci. 2003;23:2665–2674. doi: 10.1523/JNEUROSCI.23-07-02665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, Greaves DR, Zlotnik A, Schall TJ. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- Bhaskar K, Konerth M, Kokiko-Cochran ON, Cardona A, Ransohoff RM, Lamb BT. Regulation of tau pathology by the microglial fractalkine receptor. Neuron. 2010;68:19–31. doi: 10.1016/j.neuron.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffi A, Shulman JM, Jagiella JM, Cortellini L, Ayres AM, Schwab K, Brown DL, Silliman SL, Selim M, Worrall BB, Meschia JF, Slowik A, De Jager PL, Greenberg SM, Schneider JA, Bennett DA, Rosand J. Genetic variation at CR1 increases risk of cerebral amyloid angiopathy. Neurology. 2012;78:334–341. doi: 10.1212/WNL.0b013e3182452b40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinzinger K, Kreutzberg G. Displacement of synaptic terminals from regenerating motoneurons by microglial cells. Z Zellforsch Mikrosk Anat. 1968;85:145–157. doi: 10.1007/BF00325030. [DOI] [PubMed] [Google Scholar]
- Boulanger LM. Immune proteins in brain development and synaptic plasticity. Neuron. 2009;64:93–109. doi: 10.1016/j.neuron.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Boulanger LM, Shatz CJ. Immune signalling in neural development, synaptic plasticity and disease. Nat Rev Neurosci. 2004;5:521–531. doi: 10.1038/nrn1428. [DOI] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- Castello M, Soriano S. Rational heterodoxy: Cholesterol reformation of the amyloid doctrine. Ageing Res Rev. 2012;23:282–288. doi: 10.1016/j.arr.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Cho SH, Sun B, Zhou Y, Kauppinen TM, Halabisky B, Wes P, Ransohoff RM, Gan L. CX3CR1 modulates microglial activation and protects against plaque-independent cognitive deficits in a mouse model of Alzheimer’s disease. J Biol Chem. 2011 doi: 10.1074/jbc.M111.254268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Jin X, Parada I, Pesic A, Stevens B, Barres B, Prince DA. Enhanced synaptic connectivity and epilepsy in C1q knockout mice. Proc Natl Acad Sci U S A. 2010;107:7975–7980. doi: 10.1073/pnas.0913449107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikelenboom P, Stam FC. Immunoglobulins and complement factors in senile plaques. An immunoperoxidase study. Acta Neuropathol. 1982;57:239–242. doi: 10.1007/BF00685397. [DOI] [PubMed] [Google Scholar]
- Farber K, Cheung G, Mitchell D, Wallis R, Weihe E, Schwaeble W, Kettenmann H. C1q, the recognition subcomponent of the classical pathway of complement, drives microglial activation. J Neurosci Res. 2009;87:644–652. doi: 10.1002/jnr.21875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca MI, Kawas CH, Troncoso JC, Tenner AJ. Neuronal localization of C1q in preclinical Alzheimer’s disease. Neurobiol Dis. 2004a;15:40–46. doi: 10.1016/j.nbd.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Fonseca MI, Zhou J, Botto M, Tenner AJ. Absence of C1q leads to less neuropathology in transgenic mouse models of Alzheimer’s disease. J Neurosci. 2004b;24:6457–6465. doi: 10.1523/JNEUROSCI.0901-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser DA, Pisalyaput K, Tenner AJ. C1q enhances microglial clearance of apoptotic neurons and neuronal blebs, and modulates subsequent inflammatory cytokine production. J Neurochem. 2010;112:733–743. doi: 10.1111/j.1471-4159.2009.06494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea I, Bechmann I, Perry VH. What is immune privilege (not)? Trends Immunol. 2007;28:12–18. doi: 10.1016/j.it.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Gemma C, Bachstetter AD, Bickford PC. Neuron-Microglia Dialogue and Hippocampal Neurogenesis in the Aged Brain. Aging Dis. 2010;1:232–244. [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Baker TJ. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986;6:2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S, Thompson DA, Botti P, Bacon KB, Feng L. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci U S A. 1998;95:10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschensteiner D, Morgan JL, Parker ED, Lewis RM, Wong RO. Neurotransmission selectively regulates synapse formation in parallel circuits in vivo. Nature. 2009;460:1016–1020. doi: 10.1038/nature08236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsknecht J, Landreth G. Microglial phagocytosis of fibrillar beta-amyloid through a beta1 integrin-dependent mechanism. J Neurosci. 2004;24:9838–9846. doi: 10.1523/JNEUROSCI.2557-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsknecht-Talboo J, Landreth GE. Microglial phagocytosis induced by fibrillar beta-amyloid and IgGs are differentially regulated by proinflammatory cytokines. J Neurosci. 2005;25:8240–8249. doi: 10.1523/JNEUROSCI.1808-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K, Shinozaki Y, Ohsawa K, Tsuda M, Joshi BV, Jacobson KA, Kohsaka S, Inoue K. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature. 2007;446:1091–1095. doi: 10.1038/nature05704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrada L, Liang XH, Zheng W, Johnston C, Levine B. Age-dependent resistance to lethal alphavirus encephalitis in mice: analysis of gene expression in the central nervous system and identification of a novel interferon-inducible protective gene, mouse ISG12. Journal of virology. 2002;76:11688–11703. doi: 10.1128/JVI.76.22.11688-11703.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- Loeffler DA, Camp DM, Bennett DA. Plaque complement activation and cognitive loss in Alzheimer’s disease. J Neuroinflammation. 2008;5:9. doi: 10.1186/1742-2094-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Condello C, Schain A, Harb R, Grutzendler J. CX3CR1 in microglia regulates brain amyloid deposition through selective protofibrillar amyloid-beta phagocytosis. J Neurosci. 2010;30:17091–17101. doi: 10.1523/JNEUROSCI.4403-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Nataraj K, Nelson SB, Turrigiano GG. Potentiation of cortical inhibition by visual deprivation. Nature. 2006;443:81–84. doi: 10.1038/nature05079. [DOI] [PubMed] [Google Scholar]
- Maier M, Peng Y, Jiang L, Seabrook TJ, Carroll MC, Lemere CA. Complement C3 deficiency leads to accelerated amyloid beta plaque deposition and neurodegeneration and modulation of the microglia/macrophage phenotype in amyloid precursor protein transgenic mice. J Neurosci. 2008;28:6333–6341. doi: 10.1523/JNEUROSCI.0829-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar S, Jiang Q, Lee CY, Koenigsknecht-Talboo J, Holtzman DM, Landreth GE. Microglia mediate the clearance of soluble Abeta through fluid phase macropinocytosis. J Neurosci. 2009;29:4252–4262. doi: 10.1523/JNEUROSCI.5572-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Teva JL, Dusart I, Colin C, Gervais A, van Rooijen N, Mallat M. Microglia promote the death of developing Purkinje cells. Neuron. 2004;41:535–547. doi: 10.1016/s0896-6273(04)00069-8. [DOI] [PubMed] [Google Scholar]
- Milligan CE, Cunningham TJ, Levitt P. Differential immunochemical markers reveal the normal distribution of brain macrophages and microglia in the developing rat brain. The Journal of comparative neurology. 1991;314:125–135. doi: 10.1002/cne.903140112. [DOI] [PubMed] [Google Scholar]
- Mittelbronn M, Dietz K, Schluesener HJ, Meyermann R. Local distribution of microglia in the normal adult human central nervous system differs by up to one order of magnitude. Acta Neuropathologica. 2001;101:249–255. doi: 10.1007/s004010000284. [DOI] [PubMed] [Google Scholar]
- Morioka T, Kalehua AN, Streit WJ. The microglial reaction in the rat dorsal hippocampus following transient forebrain ischemia. J Cereb Blood Flow Metab. 1991;11:966–973. doi: 10.1038/jcbfm.1991.162. [DOI] [PubMed] [Google Scholar]
- Napoli I, Neumann H. Microglial clearance function in health and disease. Neuroscience. 2009;158:1030–1038. doi: 10.1016/j.neuroscience.2008.06.046. [DOI] [PubMed] [Google Scholar]
- Neumann H, Kotter MR, Franklin RJ. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain. 2009;132:288–295. doi: 10.1093/brain/awn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Nishida N, Walz T, Springer TA. Structural transitions of complement component C3 and its activation products. Proc Natl Acad Sci U S A. 2006;103:19737–19742. doi: 10.1073/pnas.0609791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, Godbout JP. Microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol Appl Neurobiol. 2012 doi: 10.1111/j.1365-2990.2012.01306.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa K, Irino Y, Nakamura Y, Akazawa C, Inoue K, Kohsaka S. Involvement of P2X4 and P2Y12 receptors in ATP-induced microglial chemotaxis. Glia. 2007;55:604–616. doi: 10.1002/glia.20489. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Perry VH, O’Connor V. C1q: the perfect complement for a synaptic feast? Nat Rev Neurosci. 2008;9:807–811. doi: 10.1038/nrn2394. [DOI] [PubMed] [Google Scholar]
- Pocock JM, Kettenmann H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007;30:527–535. doi: 10.1016/j.tins.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ramaglia V, Hughes TR, Donev RM, Ruseva MM, Wu X, Huitinga I, Baas F, Neal JW, Morgan BP. C3-dependent mechanism of microglial priming relevant to multiple sclerosis. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1111924109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JT, Morganti JM, Bachstetter AD, Hudson CE, Peters MM, Grimmig BA, Weeber EJ, Bickford PC, Gemma C. CX3CR1 deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity. J Neurosci. 2011;31:16241–16250. doi: 10.1523/JNEUROSCI.3667-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefer J, Kampe K, Dodt HU, Zieglgansberger W, Kreutzberg GW. Microglial motility in the rat facial nucleus following peripheral axotomy. Journal of neurocytology. 1999;28:439–453. doi: 10.1023/a:1007048903862. [DOI] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7:483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW, Barres BA. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Braak H, Xu QS, Bechmann I. Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer’s disease. Acta Neuropathol. 2009;118:475–486. doi: 10.1007/s00401-009-0556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp BD, Wujek JR, Criste GA, Jalabi W, Yin X, Kidd GJ, Stohlman S, Ransohoff R. Evidence for synaptic stripping by cortical microglia. Glia. 2007;55:360–368. doi: 10.1002/glia.20462. [DOI] [PubMed] [Google Scholar]
- Terai K, Walker DG, McGeer EG, McGeer PL. Neurons express proteins of the classical complement pathway in Alzheimer disease. Brain Res. 1997;769:385–390. doi: 10.1016/s0006-8993(97)00849-4. [DOI] [PubMed] [Google Scholar]
- Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Laan LJ, Ruuls SR, Weber KS, Lodder IJ, Dopp EA, Dijkstra CD. Macrophage phagocytosis of myelin in vitro determined by flow cytometry: phagocytosis is mediated by CR3 and induces production of tumor necrosis factor-alpha and nitric oxide. J Neuroimmunol. 1996;70:145–152. doi: 10.1016/s0165-5728(96)00110-5. [DOI] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakselman S, Bechade C, Roumier A, Bernard D, Triller A, Bessis A. Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor. J Neurosci. 2008;28:8138–8143. doi: 10.1523/JNEUROSCI.1006-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton NM, Sutter BM, Laywell ED, Levkoff LH, Kearns SM, Marshall GP, 2nd, Scheffler B, Steindler DA. Microglia instruct subventricular zone neurogenesis. Glia. 2006;54:815–825. doi: 10.1002/glia.20419. [DOI] [PubMed] [Google Scholar]
- Webster S, Bradt B, Rogers J, Cooper N. Aggregation state-dependent activation of the classical complement pathway by the amyloid beta peptide. J Neurochem. 1997;69:388–398. doi: 10.1046/j.1471-4159.1997.69010388.x. [DOI] [PubMed] [Google Scholar]
- Webster SD, Park M, Fonseca MI, Tenner AJ. Structural and functional evidence for microglial expression of C1qR(P), the C1q receptor that enhances phagocytosis. J Leukoc Biol. 2000a;67:109–116. doi: 10.1002/jlb.67.1.109. [DOI] [PubMed] [Google Scholar]
- Webster SD, Yang AJ, Margol L, Garzon-Rodriguez W, Glabe CG, Tenner AJ. Complement component C1q modulates the phagocytosis of Abeta by microglia. Exp Neurol. 2000b;161:127–138. doi: 10.1006/exnr.1999.7260. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J Neurophysiol. 1965;28:1029–1040. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Yan F, Lin AH, Lambris JD, Alexander JJ, Quigg RJ, Masliah E. Prominent neurodegeneration and increased plaque formation in complement-inhibited Alzheimer’s mice. Proc Natl Acad Sci U S A. 2002;99:10837–10842. doi: 10.1073/pnas.162350199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TT, Lin C, Hsu CT, Wang TF, Ke FY, Kuo YM. Differential distribution and activation of microglia in the brain of male C57BL/6J mice. Brain Structure & Function. 2012 doi: 10.1007/s00429-012-0446-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Yuzaki M. Synapse formation and maintenance by C1q family proteins: a new class of secreted synapse organizers. Eur J Neurosci. 2010;32:191–197. doi: 10.1111/j.1460-9568.2010.07346.x. [DOI] [PubMed] [Google Scholar]
- Zabel M, Schrag M, Crofton A, Tung S, Beaufond P, Van Ornam J, DiNinni A, Vinters HV, Coppola G, Kirsch WM. A shift in microglial β-amyloid binding in Alzheimer’s disease is associated with cerebral amyloid angiopathy. Brain Path. 2012 doi: 10.1111/bpa.12005. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Fonseca MI, Pisalyaput K, Tenner AJ. Complement C3 and C4 expression in C1q sufficient and deficient mouse models of Alzheimer’s disease. J Neurochem. 2008;106:2080–2092. doi: 10.1111/j.1471-4159.2008.05558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]