Abstract
Study Objectives
Patients with postural tachycardia syndrome (POTS) commonly complain of fatigue, unrefreshing sleep, daytime sleepiness and diminished quality of life. The study objective was to assess sleep quality in POTS patients using wrist actigraphy.
Design
Prospective study with control group.
Methods
Patients with POTS (n=36) and healthy subjects (n=36) completed a detailed sleep log and actigraphy for 7 days.
Results
Compared with healthy subjects, POTS patients have more self-reported problems including days with restless sleep (53±30% vs. 21±20%; P<0.001) and tiredness (75±23% vs. 39±27%; P<0.001). Using actigraphy, POTS patients have lower sleep efficiency (73±13% vs. 79±6%; P= 0.01).. Actigraphy determined sleep onset latency (SOL) did not vary significantly in the two groups, but subjective SOL was higher in POTS patient (56±66 min vs. 13±9 min; P=0.001). In POTS patients, there was a significant correlation between subjective complaints of tiredness and actigraphic sleep efficiency (Rs=−0.36; R2=0.15; P=0.01), significant correlations between actigraphic SOL and upright norepinephrine levels (P=0.040), and between wake after sleep onset and standing heart rate (P=0.02).
Conclusions
POTS patients have more sleep-related symptoms and poor sleep efficiency. The pattern of subjective vs. objective SOL mismatch is suggestive of sleep-state misperception. High norepinephrine correlated with actigraphic SOL, and this activation of the stress system may contribute significantly to a hyperarousal state with consequent insomnia, poor mental and physical health in POTS patients.
INTRODUCTION
Postural Tachycardia Syndrome (POTS) is the most common disorder among patients seen at centers specializing in diseases of the autonomic nervous system. POTS is defined by an excessive increase in heart rate (HR; ≥30 beats/min) with upright posture in the absence of orthostatic hypotension, and have symptoms of sympathetic activation. This disorder is associated with orthostatic symptoms including palpitation, chest pain syndrome, dyspnea on standing, mental clouding and difficulties with concentration. It can produce substantial disability among otherwise healthy people.1,2 The pathophysiology of POTS is not completely known. Many patients suffer from either a primary or secondary increase in sympathetic nervous system tone3, with elevated levels of plasma norepinephrine, particularly when upright. One subgroup of patients has a primary hyperadrenergic state.
Recent studies have shown that subjective reports of poor sleep are associated with reduced physical performance, greater functional limitation, and increased risk for cardiovascular diseases; poor sleep may also predict mortality.4–7 Patients with insomnia have increased nocturnal catecholamine levels as compared with controls.8
Our recent questionnaire based study demonstrated that patients with POTS have higher subjective daytime sleepiness, fatigue, worse sleep and health related quality of life (HRQL).9 Sleep problems contributed significantly to the diminished HRQL, accounting for about 50% of the variability in the HRQL. To date, there are no published objective data on the quality of sleep or sleep disturbances in patients with POTS.
An objective assessment of sleep disturbance is crucial as data obtained from questionnaires and sleep diaries can be confounded. An individual may sleep well objectively, but subjectively feel that he has poor sleep and vice versa. 10 Wrist actigraphy is a relatively inexpensive and non-invasive method of estimating sleep patterns based on recording motion data. One advantage of actigraphy is that it allows for the study of sleep patterns in the patient’s own home environment for multiple nights. Studies have shown that actigraphy-based estimates of sleep correlate well with data obtained from overnight polysomnograms.11–13
The aim of this study was to formally assess sleep using actigraphy in patients with POTS as compared with healthy control subjects. We hypothesized that the objective measures of sleep disturbance will be worse in patients with POTS.
METHODS
Study Subjects
The study sample consisted of 36 subjects with POTS and 36 control subjects. Patients with POTS who met the conventional criteria were included in the study. 14,15 POTS patients were recruited from the Vanderbilt University Autonomic Dysfunction Center. Patients developed symptoms of orthostatic intolerance accompanied by a HR rise ≥30 bpm which occurred within the first 10 minutes of upright posture, without any evidence of orthostatic hypotension (a fall in blood pressure [BP] of ≥20/10 mm Hg). Patients had at least a 6-month history of symptoms, in the absence of another chronic debilitating disorder or prolonged bed rest, and were at least 18 years of age. Common symptoms in POTS include rapid palpitation from tachycardia, exercise intolerance, lightheadedness, extreme fatigue, headache and mental clouding. Patients with POTS all underwent a Posture Study to assess their orthostatic tolerance (details below) and an assessment of cardiovagal function (sinus arrhythmia) on the Elliot V. Newman Clinical Research Center (CRC) at Vanderbilt University. Healthy control subjects (who did not meet criteria for POTS) were similar in age and gender to the POTS patients, but were not matched to individual patients. Control subjects were recruited from healthy volunteers known to the Vanderbilt Dysautonomia Center, through the Vanderbilt Research Volunteer Database, and through advertisements in the Vanderbilt community. All controls subjects described themselves as “healthy” and without known sleep problems.
The research protocol was approved by the Vanderbilt Institutional Review Board, and written informed consent was obtained from each subject before the study began. This study is registered with ClinicalTrials.gov (NCT00692471).
Supine and Upright Posture Study
HR, BP, and plasma norepinephrine and epinephrine were assessed after overnight rest in the supine position and again after standing up to 30 minutes (as tolerated) in the subjects with POTS. The standing test was performed in order to assess the hemodynamic and biochemical responses to gravity-induced central hypovolemia. For catecholamine measurements, blood was collected in plastic syringes and immediately transferred to chilled vacuum tubes with EGTA and reduced glutathione (Amersham International PLC, Amersham, UK) and immediately put on ice. The plasma was separated by refrigerated centrifugation at −4°C and stored at −70°C until the assay. Concentrations of norepinephrine and epinephrine were measured by batch alumina extraction followed by high performance liquid chromatography for separation with electrochemical detection and quantification.16
Autonomic Testing
Cardiovagal function was assessed in all patients with POTS by testing sinus arrhythmia.17 During continuous electrocardiographic monitoring, patients were asked to breathe deeply at 6/minute and the maximum and minimum HR was measured. Sinus arrhythmia was quantified as the SAdelta (maximum HR- minimum HR) and as SAratio (maximum HR/minimum HR).
Sleep Log
During the recorded period, subjects were asked to fill the sleep log daily within 30 minutes of awakening in the morning. The purpose of the sleep log was to record sleep and wake times and to rate the sleep quality and morning tiredness on each day. The additional recording of sleep-onset and offset with the sleep log allowed for more robust analysis of the actigraphy data. Additional teaching was provided as needed for the subject to be successful with the sleep log forms prior to beginning the study.
The following subjective measures were assessed:
Tiredness: Subjects reported ‘yes’ or ‘no’ to complaints of tiredness for each day. Percentage of days tested when the subjects reported ‘yes’ to subjective tiredness were calculated.
Restless sleep: Subjects reported ‘yes’ or ‘no’ to complaints of restless sleep. Percentage of nights tested when the subjects reported ‘yes’ to restless sleep were calculated.
Average minutes to sleep: Average minutes to fall asleep as reported on the sleep log.
Average awakenings: Average number of awakenings/night as reported on the sleep log.
Actigraphy
All subjects wore the AW-64 Actiwatch® device (Philips Respironics, Bend, OR) on the non-dominant wrist for a minimum of seven days. Subjects were instructed to push the event-marker button on the device to mark time-into and time-out of bed. Each device contains an accelerometer, which detects motion and translates it into an electrical signal, stored in memory within the device as actigraphy counts. The devices were configured using one-minute epochs with medium threshold and the validated software algorithm 18 was used to estimate sleep parameters, based on thresholds for wake and sleep, as described in prior work 13,19,20
The data from the actigraphy device was downloaded to a secure computer for off-line analysis and incorporated with information from the sleep log. The event-marker points and data from the sleep log were used to set the time spent in bed. The scoring was confirmed by an experienced scorer. If there was only one source of information for the night (for example, if event marker button was not pushed), the scorer referred to the only source provided. If neither source was available, then the night was not counted. All subjects were required to have at least 6 days of suitable actigraphy data to be included in the overall analysis. Light-off refers to the time the subject went to bed and switched off the lights. Sleep End refers to the time the subject last woke up in the morning.
Total sleep time (TST) is the sum in minutes, of all sleep epochs between sleep onset and sleep end. The following actigraphy measures were assessed:
Sleep Onset Latency: Time required (min) for the onset of sleep after first attempting to go to sleep (i.e. from lights out time).
Sleep Efficiency (SE expressed as a percentage): Ratio of total nighttime sleep duration to the total time in bed.
Wake Time After Sleep Onset (WASO): The amount of wake time in minutes from Sleep Onset to Sleep End. It is the sum of all wake epochs during the sleep period and reflects the number of minutes scored as awake.
Statistical Analysis
This study was an exploratory objective study to evaluate sleep in patients with POTS; hence we analyzed multiple objective measures of sleep. The sleep onset latency, sleep efficiency, wake after sleep onset (WASO), and subjective complaints of tiredness or restless sleep were compared in POTS patients and healthy control subjects. In order to better understand the relationship between these subjective complaints and actigraphic measures, Spearman correlations were performed. Data were analyzed with Student’s t-tests (if the data were normally distributed) or a Mann-Whitney U test (if they were not normally distributed).
Sample Size Calculation
There are no preliminary data available on sleep actigraphy in patients with POTS. Therefore, we used data from a recent study on sleep actigraphy in patients with chronic musculoskeletal pain syndrome in order to estimate the needed samples size.21 The patient group had a sleep latency of 21±15 min. We estimated that this will be seen in our patients with POTS, and that the sleep latency in healthy control subjects would be 10 min (about half that of the POTS patients). A two group Student’s t-test with a 0.05 two-sided significance level provided 80% power to detect this difference when the sample size was 30 in each group. To account for potential dropout, we aimed to recruit 36 patients to each group. Data are presented as mean ± SD unless otherwise specified.
RESULTS
Subjects with POTS (n=36; 33 female; 36 ± 9 years) and control subjects (n=36; 33 female; 31 ± 9 years) were of comparable age and body mass index and body mass index (23.7 vs. 22.0 kg/m2; P=0.1). The baseline autonomic characteristics of the subjects with POTS are listed in Table 1. The values are similar to that seen in our prior publications with cohorts of POTS patients. The standing hemodynamic parameters and catecholamines in patients with POTS are significantly higher than previously reported values in healthy control subjects.3
Table 1.
Supine and standing hemodynamic parameters and catecholamine levels in patients with Postural Tachycardia Syndrome.
| Supine | Stand | P | |
|---|---|---|---|
| Heart Rate (bpm) | 74 ± 2 | 123 ± 3 | <0.001 |
| Systolic Blood Pressure (mmHg) | 110 ± 2 | 113 ± 3 | 0.384 |
| Diastolic Blood Pressure (mmHg) | 68 ± 1 | 75 ± 2 | 0.004 |
| Norepinephrine (pg/ml) | 273 ± 27 | 883 ± 82 | <0.001 |
| Epinephrine (pg/ml) | 24 ± 4 | 71 ± 13 | <0.001 |
Continuous data are presented as the mean ± SEM, and were analyzed using Student’s t-test; POTS: Postural Tachycardia Syndrome.
Sleep Assessments by Sleep Logs (Table 2)
Table 2.
Subjective diary and objective actigraphy parameters of the subjects with Postural Tachycardia Syndrome and healthy controls.
| Parameters | POTS | Controls | P |
|---|---|---|---|
| A. Subjective Diary Characteristics | |||
| Restless (%) | 53 ± 30 | 21 ± 20 | <0.001 |
| Tired (%) | 75 ± 23 | 39 ± 27 | <0.001 |
| Average time to sleep (min) | 56 ± 66 | 13 ± 9 | <0.001 |
| Average awakenings | 3 ± 2 | 1 ± 1 | <0.001 |
| B. Objective Actigraphy Characteristics | |||
| Sleep onset latency (min) | 37 ± 41 | 31 ± 31 | 0.49 |
| Sleep Efficiency (%) | 73 ± 13 | 79 ± 6 | 0.01 |
| WASO (min) | 63 ± 33 | 50 ± 20 | 0.05 |
POTS – Postural Tachycardia Syndrome; WASO: Wake after sleep onset; Data are presented as the mean ± SD. Continuous variables between POTS patients and controls were compared using the Wilcoxon Rank Sum test.
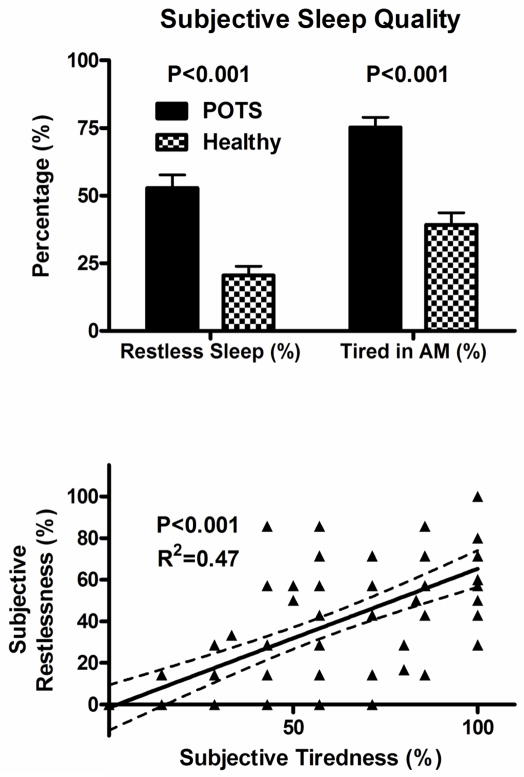
As seen in Figure 1, POTS patients had significantly higher greater number of days (reported as percentage of total days) with restless sleep than healthy control subjects (53% vs. 21%; P<0.001). Morning tiredness was reported in a greater percentage of days in patients with POTS (75 ± 23%) than control subjects (39 ± 27%; P<0.001). Average sleep latency was greater in POTS than control subjects (56 ± 66 min vs. 13 ± 9 min; P<0.001), as was the number of awakenings/night reported by POTS patients compared to controls (3 ± 2 vs. 1 ± 1; P<0.001).
Figure 1.
Sleep Log Parameters in Postural Tachycardia Syndrome (POTS) patients and healthy controls subjects, including subjective sleep complaints of restless sleep and tiredness in morning (top panel), and Spearman correlations between subjective complaints of restless sleep and tiredness (bottom panel).
Sleep Assessments by Actigraphy (Table 2)
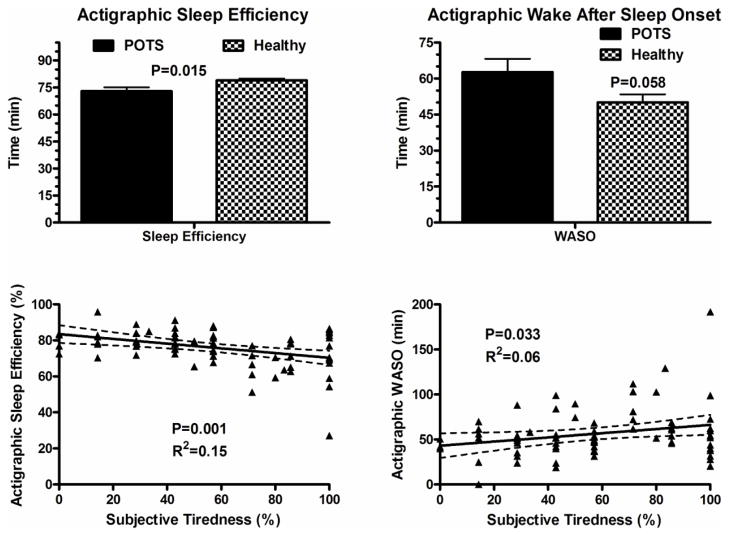
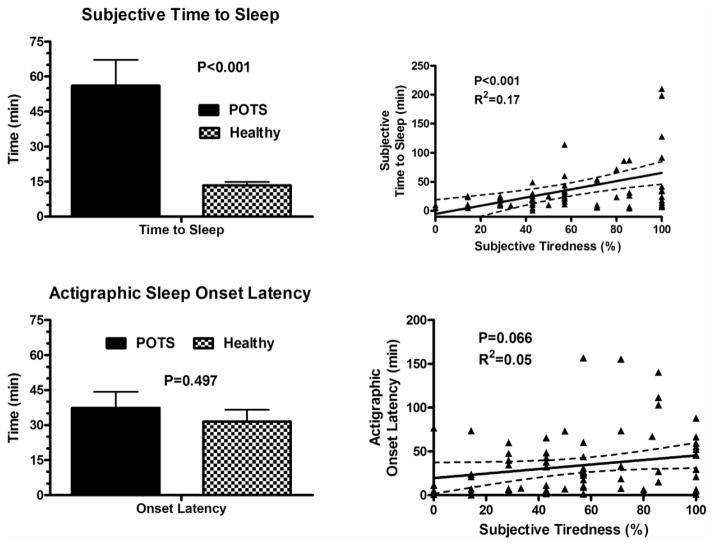
The average number of days the actigraphy device was worn in subjects with POTS was 7.1± 0.6 days, compared with 7.4± 0.6 days in controls. Sleep efficiency was significantly lower in subjects with POTS (73 ± 13%) as compared with controls (79 ± 6%; P=0.01; Figure 2). There was a trend towards higher WASO in POTS patients (63 ± 33 min) as compared with controls (50 ± 20 min; p=0.058; Figure 2). There was no difference in sleep onset latency between the POTS patients (37 ± 10 min) and control subjects (31 ± 9 min; P=0.49; Figure 3).
Figure 2.
Actigraphic Parameters in Postural Tachycardia Syndrome (POTS) patients and healthy controls subjects including sleep efficiency and wake after sleep onset (WASO) (top panel), and Spearman correlations between subjective complaints of tiredness and actigraphic sleep efficiency, and tiredness and WASO (bottom panel). Data are presented as mean±SEM, and were analyzed using the Student’s t-test.
Figure 3.
Sleep log based subjective time to sleep in Postural Tachycardia Syndrome (POTS) patients and healthy controls subjects, and Spearman correlations between subjective complaints of tiredness and subjective time to sleep (top panel). Actigraphic sleep onset latency in POTS patients and healthy controls subjects, and Spearman correlations between subjective tiredness and actigraphic sleep onset latency (bottom panel).
Correlation between Subjective Sleep Quality, Actigraphy and Autonomic data
Overall, there were positive correlations between the percentage of days with subjective complaints of tiredness and restless sleep in POTS patients and controls (overall group Rs= 0.7; P<0.001; Figure 1). In patients with POTS, there was significant negative correlations between percentage of patients with subjective tiredness and sleep efficiency (P=0.001; Figure 2) and significant positive correlations between percentage of patients with subjective tiredness and WASO (P=0.033; Figure 2). There was no significant correlation between subjective tiredness and actigraphic sleep onset latency (Figure 3). However, there were significant positive correlations between subjective tiredness and subjective sleep latencies (Rs=0.17; P<0.001; Figure 3).
In POTS patients, there were significant positive correlations between actigraphic sleep onset latency and upright norepinephrine levels (P=0.040; Fig 4 top), and WASO and maximum standing heart rate (P=0.02; Fig 4 bottom). These data are shown in Table 3.
Figure 4.
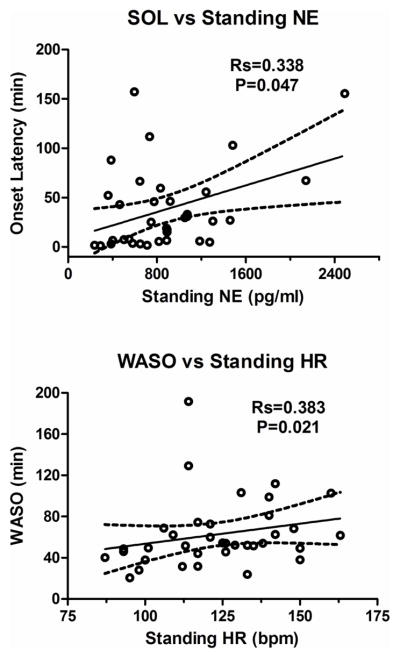
Spearman correlations (Rs) are presented for actigraphic sleep onset latency (SOL) versus standing norepinephrine (NE) levels (top panel) and actigraphic wake after sleep onset (WASO) and standing heart rate (HR; bottom panel).
Table 3.
Spearman correlation coefficients between actigraphic sleep measures in POTS patients and autonomic parameters. Spearman Correlation Coefficient
| SE | WASO | SOL | |
|---|---|---|---|
| Max standing HR | −0.272 (P=0.10) | 0.383 * (P=0.02) | 0.091 (P=0.59) |
| Upright NE | −0.032 (P=0.86) | 0.074 (P=0.67) | 0.33 * (P=0.04) |
POTS – Postural Tachycardia Syndrome; SE: actigraphic sleep efficiency; WASO: actigraphy determined wake after sleep onset; SOL: actigraphy determined sleep onset latency Correlation is significant at a 0.05 level (*).
DISCUSSION
In this study, we characterized the sleep pattern in subjects with POTS compared to a control group using actigraphy and sleep logs. Our results provide objective data to confirm that subjects with POTS have sleep pattern changes including poor sleep efficiency and a trend towards increased sleep fragmentation (as measured by WASO). We have previously shown that patients with POTS have diminished health-related quality of life, higher fatigue levels and daytime sleepiness, and that sleep problems contributed significantly to the diminished health-related quality of life.22
Poor Sleep Quality
Poor sleep is a common complaint in patients with POTS. To our knowledge, this is the first study comparing both subjective and objective measures of sleep in POTS patients and control subjects. Compared with healthy control subjects, POTS patients have significantly more sleep problems, including subjective complains of restless sleep and morning tiredness, perceptions of longer sleep onset latency and greater number of awakenings per night.
Actigraphy analysis confirmed poor sleep efficiency and increased WASO, but no differences in the actigraphic sleep onset latency in patients with POTS and controls were noted.
Possible Mechanisms of Poor Sleep Quality
One possible hypothesis for these sleep disturbances is that patients with POTS may exhibit higher arousal rates at night resulting in non-restorative sleep, similar to reports in patients with chronic fatigue syndrome.23 Sleep disruption or frequent arousals are associated with significant increases in plasma cortisol levels.24 In normal subjects, waking periods and stage-N1 sleep accompany cortisol increases, whereas slow-wave sleep is associated with declining plasma cortisol levels.24,25
Another plausible explanation for the sleep disturbances in POTS patients is their state of heightened sympathetic activation.3 Vgontzas et al. found that the 24-hour urinary levels of catecholamine metabolites, dihydroxyphenyglycol (DHPG) and 3, 4-dihydroxyphenylacetic acid (DOPAC), were positively correlated with percent of stage N1 sleep and WASO, and the 24-hour urinary free cortisol levels were positively correlated with the total wake time. These data suggest disturbances in the HPA axis and sympathetic nervous system activity are associated with objective sleep disturbance.26 Evidence from several other studies suggests that insomnia is a disorder of hyperarousal.27 The central tenet of the NeuroCognitive Model of Insomnia is that insomnia is a state of cortical, cognitive and somatic arousal. 28 The cortical arousal may in turn permit cognitive processing that does not occur with normal sleep. The increased sensory and information processing during non-REM sleep, or the attenuation of the mesograde amnesia to sleep, may in turn produce a sleep state misperception. Insomnia patients have a relative increase of beta power (high-frequency) EEG activity, with a decrease in the slow EEG activity. Insomnia patients have higher levels or beta and gamma power EEG activity during REM sleep as compared with subjects with normal sleep.27,29 Using functional neuroimaging, insomnia patients had elevation of global cerebral metabolism as compared with subjects with normal sleep suggestive of a state of hyper-arousal during sleep.30
In this study, POTS patients report poor sleep quality including daytime tiredness and restless sleep. The most interesting incidental finding is that although there were no significant differences noted in the objective (actigraphic) sleep latencies in POTS patients and controls, the subjective (sleep log) sleep latencies were significantly prolonged. This pattern of mismatch between subjective and objective findings is suggestive of sleep-state misperception which can be explained by the NeuroCognitive model of Insomnia. The actigraphic sleep onset latency was positively correlated with the upright norepinephrine levels, and WASO was positively correlated to the maximum standing heart rate. Activation of the stress system may contribute significantly to this hyperarousal state and consequent insomnia, poor mental and physical health in patients with POTS. Based on these data, it is possible that pharmacological measures that reduce the HPA hyperactivity or high cortisol levels in POTS patients may also improve symptoms of insomnia by decreasing the central nervous system hyperarousal state.
Limitations
The major limitation of this study is that formal overnight polysomnography was not performed to assess the nature of the underlying sleep disturbance. This will be the focus of future studies.
Conclusions
POTS patients report poor sleep quality with restless sleep, increased nocturnal awakenings, longer sleep-onset latencies and daytime fatigue. However, the most interesting finding of this study is that there is no difference noted in the objective sleep latencies in POTS patient and controls, raising the possibility of a state of sleep-state misperception. Using actigraphy, this study documents that patients with POTS have decreased sleep efficiency and a trend towards increased wake after sleep onset as compared with healthy controls, which could be contributing factors for their restless sleep and daytime fatigue. Changes in markers of autonomic activity in POTS patients, including upright norepinephrine levels and maximum standing heart rate, may be factors contributing to the hyper-arousal state. Further studies with overnight polysomnography are needed to further define the nature and etiology of the sleep disturbances in patients with POTS.
Supplementary Material
Acknowledgments
Supported in part by NIH grants R01 HL102387 (SRR), R01 HL071784 (DR), P01 HL56693 (DR), 1 UL1 TR000445 (Clinical and Translational Science Award), and the Paden Dysautonomia Center.
We would like to thank our patients and the staff of the Elliot V. Newman Clinical Research Center.
Footnotes
Conflicts of Interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Low PA, Opfer-Gehrking TL, Textor SC, et al. Postural tachycardia syndrome (POTS) Neurology. 1995;45:S19–25. [PubMed] [Google Scholar]
- 2.Raj SR. The Postural Tachycardia Syndrome (POTS): pathophysiology, diagnosis & management. Indian Pacing Electrophysiol J. 2006;6:84–99. [PMC free article] [PubMed] [Google Scholar]
- 3.Garland EM, Raj SR, Black BK, Harris PA, Robertson D. The hemodynamic and neurohumoral phenotype of postural tachycardia syndrome. Neurology. 2007;69:790–8. doi: 10.1212/01.wnl.0000267663.05398.40. [DOI] [PubMed] [Google Scholar]
- 4.Haseli-Mashhadi N, Dadd T, Pan A, Yu Z, Lin X, Franco OH. Sleep quality in middle-aged and elderly Chinese: distribution, associated factors and associations with cardio-metabolic risk factors. BMC Public Health. 2009;9:130. doi: 10.1186/1471-2458-9-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldman SE, Stone KL, Ancoli-Israel S, et al. Poor sleep is associated with poorer physical performance and greater functional limitations in older women. Sleep. 2007;30:1317–24. doi: 10.1093/sleep/30.10.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dew MA, Hoch CC, Buysse DJ, et al. Healthy older adults’ sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Med. 2003;65:63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- 7.Haseli-Mashhadi N, Pan A, Ye X, et al. Self-Rated Health in middle-aged and elderly Chinese: distribution, determinants and associations with cardio-metabolic risk factors. BMC Public Health. 2009;9:368. doi: 10.1186/1471-2458-9-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Irwin M, Clark C, Kennedy B, Christian Gillin J, Ziegler M. Nocturnal catecholamines and immune function in insomniacs, depressed patients, and control subjects. Brain Behav Immun. 2003;17:365–72. doi: 10.1016/s0889-1591(03)00031-x. [DOI] [PubMed] [Google Scholar]
- 9.Bagai K, Song Y, Ling JF, et al. Sleep disturbances and diminished quality of life in postural tachycardia syndrome. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2011;7:204–10. [PMC free article] [PubMed] [Google Scholar]
- 10.Trajanovic NN, Radivojevic V, Kaushansky Y, Shapiro CM. Positive sleep state misperception - a new concept of sleep misperception. Sleep Med. 2007;8:111–8. doi: 10.1016/j.sleep.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Blackwell T, Redline S, Ancoli-Israel S, et al. Comparison of sleep parameters from actigraphy and polysomnography in older women: the SOF study. Sleep. 2008;31:283–91. doi: 10.1093/sleep/31.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanchez-Ortuno MM, Edinger JD, Means MK, Almirall D. Home is where sleep is: an ecological approach to test the validity of actigraphy for the assessment of insomnia. J Clin Sleep Med. 6:21–9. [PMC free article] [PubMed] [Google Scholar]
- 13.Lichstein KL, Stone KC, Donaldson J, et al. Actigraphy validation with insomnia. Sleep. 2006;29:232–9. [PubMed] [Google Scholar]
- 14.Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology. 1993;43:132–7. doi: 10.1212/wnl.43.1_part_1.132. [DOI] [PubMed] [Google Scholar]
- 15.Raj SR, Biaggioni I, Yamhure PC, et al. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation. 2005;111:1574–82. doi: 10.1161/01.CIR.0000160356.97313.5D. [DOI] [PubMed] [Google Scholar]
- 16.Jacob G, Robertson D, Mosqueda-Garcia R, Ertl AC, Robertson RM, Biaggioni I. Hypovolemia in syncope and orthostatic intolerance role of the renin-angiotensin system. Am J Med. 1997;103:128–33. doi: 10.1016/s0002-9343(97)00133-2. [DOI] [PubMed] [Google Scholar]
- 17.Mosqueda-Garcia R. Evaluation of Autonomic Failure. In: Robertson D, Biaggioni I, editors. Disorders of the Autonomic Nervous System. Luxembourg: Harwood Academic Publishers GmbH; 1995. pp. 25–59. [Google Scholar]
- 18.PhillipsRespironics. Actiware / Actiware-CT: Actiwatch Communication and Sleep Analysis Software Instruction Manual. 2010. [Google Scholar]
- 19.Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2:389–96. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 20.Mezick E, Matthews K, Hall M, et al. Intra-Individual Variability in Sleep Duration and Fragmentation: Associations with Stress. Psychoneuroendocrinology. 2009;34:1346–54. doi: 10.1016/j.psyneuen.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai SY, Labyak SE, Richardson LP, et al. Actigraphic sleep and daytime naps in adolescent girls with chronic musculoskeletal pain. J Pediatr Psychol. 2008;33:307–11. doi: 10.1093/jpepsy/jsm117. [DOI] [PubMed] [Google Scholar]
- 22.Bagai K, Song Y, Ling JF, et al. Sleep disturbances and diminished quality of life in postural tachycardia syndrome. J Clin Sleep Med. 7:204–10. [PMC free article] [PubMed] [Google Scholar]
- 23.Fossey M, Libman E, Bailes S, et al. Sleep quality and psychological adjustment in chronic fatigue syndrome. J Behav Med. 2004;27:581–605. doi: 10.1007/s10865-004-0004-y. [DOI] [PubMed] [Google Scholar]
- 24.Follenius M, Brandenberger G, Bandesapt JJ, Libert JP, Ehrhart J. Nocturnal cortisol release in relation to sleep structure. Sleep. 1992;15:21–7. doi: 10.1093/sleep/15.1.21. [DOI] [PubMed] [Google Scholar]
- 25.Born J, Kern W, Bieber K, Fehm-Wolfsdorf G, Schiebe M, Fehm HL. Night-time plasma cortisol secretion is associated with specific sleep stages. Biol Psychiatry. 1986;21:1415–24. doi: 10.1016/0006-3223(86)90333-1. [DOI] [PubMed] [Google Scholar]
- 26.Vgontzas AN, Tsigos C, Bixler EO, et al. Chronic insomnia and activity of the stress system: a preliminary study. J Psychosom Res. 1998;45:21–31. doi: 10.1016/s0022-3999(97)00302-4. [DOI] [PubMed] [Google Scholar]
- 27.De Gennaro L, Ferrara M, Bertini M. The boundary between wakefulness and sleep: quantitative electroencephalographic changes during the sleep onset period. Neuroscience. 2001;107:1–11. doi: 10.1016/s0306-4522(01)00309-8. [DOI] [PubMed] [Google Scholar]
- 28.Perlis ML, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. J Sleep Res. 1997;6:179–88. doi: 10.1046/j.1365-2869.1997.00045.x. [DOI] [PubMed] [Google Scholar]
- 29.Lamarche CH, Ogilvie RD. Electrophysiological changes during the sleep onset period of psychophysiological insomniacs, psychiatric insomniacs, and normal sleepers. Sleep. 1997;20:724–33. [PubMed] [Google Scholar]
- 30.Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161:2126–8. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.