Abstract
This study evaluates the role of scavenger receptor class A member 3 (SCARA3) in multiple myeloma (MM). SCARA3 expression was induced upon treatment with oxidative stressors (ionizing radiation and chemotherapeutic drugs). An epigenetic inactivation of SCARA3 was noted in MM.1S myeloma cells. Myeloma cell killing by dexamethasone and bortezomib was inhibited by up-regulation of SCARA3 while SCARA3 knockdown sensitized myeloma cells to the drugs. Clinical samples showed an inverse correlation between SCARA3 gene expression, myeloma progression, and favorable clinical prognosis. In MM, SCARA3 protects against oxidative stress-induced cell killing and can serve as predictor of MM progression and therapeutic response.
Keywords: Multiple myeloma, SCARA3, dexamethasone, bortezomib, ionizing radiation, oxidative stress
1. Introduction
Multiple myeloma (MM) is an aggressive human B-cell neoplasm with a relative five-year survival rate of only 40% [1]. MM evolves through a multistage process in which normal plasma cells (NPC) transform into monoclonal gammopathy of undetermined significance (MGUS) which proceeds to clinically overt MM [2]. Overall survival in patients with MM has improved significantly in the last decade due to a better understanding of the biology of this malignancy, its interactions with the microenvironment, and emergence of new classes of drugs [3-5]. Gene-expression profiling (GEP) studies have been used to determine the underlying genetic and epigenetic alterations that lead to initiation, progression, and drug resistance in MM [6-9]. A better understanding on the gene expression and signaling pathway signature can lead to improved clinical monitoring, inhibit myeloma progression, and a more effective treatment outcome for myeloma patients.
Studies have shown that persistent high-levels of oxidative stress can lead to adverse events such as oncogene activation and genomic instability culminating in cancer progression and therapy resistance [10,11]. MM patients show higher circulating levels of malondialdehyde, a by-product resulting from of lipid peroxidation and lower levels of antioxidant enzymes including superoxide dismutases (SODs), glutathione peroxidases (GPxs), and catalase CAT) relative to healthy controls [12-15]. In a clinical study, myeloma patients treated with vincristine-adriamycin-dexamethasone combination therapy showed an inhibition of function in antioxidant enzymes including SODs, GPxs, and CAT [16]. These clinical observations suggest that increased steady-state levels of pro-oxidants may be causally associated with MM progression and disease relapse. The bone marrow (BM) microenvironment, to which plasma cells and their malignant counterparts home, affects MM tumor cell development, disease progression, and treatment response [3,17]. We have recently demonstrated that interleukin 6 (IL-6), a myeloma survival factor produced in the BM, plays an important role in re-establishing intracellular redox homoeostasis in the context of myeloma therapy [18]. We have also found that combination chemotherapy with dexamethasone (Dex) and bortezomib (BTZ) can attenuate basal and therapy-induced nuclear factor (NF)-κB activity leading to inhibition of emergence of chemo- and radio-resistance in MM via reduced expression of IL-6 and manganese superoxide dismutase (MnSOD) [19].
Scavenger receptor class A, member 3 (SCARA3) is a member of the scavenger receptor family [20,21]. Oxidative stress has been shown to induce the expression of SCARA3 [20]. SCARA3 is alternatively referred to as a cellular stress response (CSR) gene since it protects cells by scavenging reactive oxygen species (ROS) and other harmful products of oxidation [20]. Various lipoprotein modifications have been described in cancer, and these lipoproteins are abundant in tumor-bearing hosts [22,23] and also detected in myeloma patients [4,14,15]. SCARA3 binds to polyanionic ligands [20] and represents a major route used by dendritic cells and macrophages to acquire fatty acids [24].
In this study we report that a re-activation of SCARA3 expression occurs in myeloma cells after treatment with oxidative stressors such as IR, H2O2, and chemotherapy drugs. In MM.1S cells, epigenetic inactivation of SCARA3 was noted. Inhibition of SCARA3 in combination with oxidative chemotherapy led to increased killing of myeloma cells. GEP analysis of clinical MM samples showed that myeloma progression is associated with decreased SCARA3 mRNA expression. GEP analysis also demonstrated that MM samples from “high-risk” patients (those exhibiting progressive myeloma disease with shorter durations of complete remission, event-free survival, and overall survival) showed decreased SCARA3 mRNA expression as compared to samples from “low-risk” myeloma patients. To our knowledge, this is the first study that identifies an antioxidant role for SCARA3 in myeloma cells and suggests that inhibition of SCARA3 in combination with oxidative chemotherapy could lead to improved remission rates in MM.
2. Materials and methods
2.1. Cell lines and tissue culture
The myeloma cell lines RPMI-8226 (8226, CCL-155), H929 (CRL-9068), IM9 (CCL-159), and MM.1S (CRL-2974) as well as the bone marrow stromal cell line (HS-5, CRL-11882) were obtained from the American Type Culture Collection (ATCC, Manassas, VA). Stable SCARA3 KD (knockdown) of 8226 and H929 cells was generated using the pre-validated human pLKO.1 lentiviral SCARA3 shRNA target set (RHS4533, Open Biosystems, Huntsville, USA) using standard methods [18]. All cell lines were grown in RPMI complete medium in a humidity-controlled incubator (37°C, 5% CO2) [19,25].
2.2 Quantitative real-time PCR (qPCR) and RT-PCR
Gene expression patterns between control and irradiated 8226 cells were compared using a TaqMan array containing 92 antioxidant mechanism-associated genes and four control genes (4418764, Applied Biosystems, Foster City, CA). Briefly, total RNA was isolated using a Qiagen RNeasy kit and cDNA was synthesized and subjected to qPCR analysis as described before [18]. Samples were run on a 7900HT fast real-time PCR system (Applied Biosystems). The Ct values for the target gene in all of the samples were normalized based on abundance of the 18S transcript and the fold difference (relative abundance) was calculated using the formula 2–ΔΔCT and plotted. The up-regulation of SCARA3 was analyzed in triplicate using the SCARA3 TaqMan gene qPCR expression assay (4351372, Applied Biosystems).
For epigenetic studies, MM.1S cells were treated with 5-aza-2′-deoxycytidine (aza-dC, Sigma-Aldrich, St Louis, MO, 2.5 μM for 48 h), or trichostatin A (TSA, Sigma-Aldrich, 100 nM for 24 h); control cells received no drug treatment followed by SCARA3 qPCR and results were analyzed as described previously [18].
For semi-quantitative RT-PCR, total RNA was isolated and cDNA synthesis was performed using the iSCRIPT™ cDNA synthesis kit using 2 μg RNA, 0.2 ng of random hexamer primer, and 50 U of SuperScript II reverse transcriptase (Invitrogen, Grand Island, NY). PCR reactions were performed using 2 μL of cDNA, 200 nM of each primer, and 1U of Taq DNA polymerase (Invitrogen). Various members of the SR-A sub-family were PCR amplified with primers described by DeWitte-Orr et al [26]. As control, 18S RNA was amplified using the forward 5′-GAAGACGATCAGATACCGTCGTAG-3′ and reverse 5′-CACTTGTCCCTCTAAGAACTTGGG-3′ primers. In specific experiments, 8226 cells were treated with 200 μM hydrogen peroxide (H2O2) for 6 h and N-acetylcysteine (NAC, 10 mM, Sigma-Aldrich) was added 1 h before H2O2 treatment. For drug studies, 8226 cells were treated with Dex (5 μM, Sigma-Aldrich), BTZ (20 nM, LC labs, Woburn, MA), or arsenic trioxide (ATO, 2 μM, Sigma-Aldrich) for 12 h. RT-PCR analysis of SCARA3 variant 2 (SCARA3 v2) was performed using total RNA for cells harvested at 12 h post treatment.
2.3. MTS cell viability assay
Myeloma cell lines (wild type, WT), variants with SCARA3 KD, or SCARA3 over-expression (O/E, obtained by treatment with 50 μM H2O2 for 24 h) were exposed to Dex (1 μM) or BTZ (10 nM); MTS assays were performed at 48 h using a commercially available kit from Promega (Madison, WI). All treatments were performed in triplicate and the mean ± SD was determined.
2.4. Western blot analysis
Protein immunoblotting was performed according to standard protocols as described previously [18,19,25]. Briefly, equal amounts of protein were electrophoresed in a 10% reducing SDS-PAGE gel. Proteins were transferred to PVDF membranes, non-specific binding was blocked with 5% skim milk in TBST buffer (4 mM Tris base, 10 mM NaCl, pH 7.5, 0.1% Tween-20), and incubated overnight at 4°C with primary antibodies against SCARA3 (WH0051435M1, Sigma-Aldrich) or tubulin (Developmental Studies Hybridoma Bank, University of Iowa) and then incubated with secondary antibody for 1 h at RT. Blots were developed using an enhanced chemiluminescence assay (Thermo Scientific). Bands were visualized by autoradiography. For illustrations, Adobe Photoshop CS4 was used to convert figures to grayscale, crop to an appropriate size, and then were contrast corrected using Photoshop’s “Auto Contrast” tool.
2.5. Microarray analysis of SCARA3 gene expression in clinical myeloma samples
The GEP data of primary human myeloma samples was analyzed for SCARA3 expression. In these studies, purified plasma cells were obtained from normal healthy subjects, patients with MGUS, or from patients with overt myeloma requiring therapy. Samples were run on the Affymetrix U133Plus2.0 microarray (Santa Clara, CA) [27,28]. This data is deposited in the NIH Gene Expression Omnibus under accession number GSE2658.
In addition, the GEP data from primary human myeloma samples was analyzed for SCARA3 gene expression in a “high-risk” group of myeloma patients – patients with shorter durations of complete remission, event-free survival, and overall survival – and compared with a corresponding “low-risk” group of myeloma patients. In this study myeloma patients were treated under an NIH-sponsored clinical trial (UARK 03-033) utilizing induction regimen followed by melphalan-based tandem auto-transplantations, consolidation chemotherapy, and maintenance treatment [27].
2.6. Statistical analysis
GraphPad Prizm 4.0 software (GraphPad Software) was used for data handling, analysis, and presentation. Statistical significance was determined using two-tailed unpaired t-test with a confidence interval of 95%.
3. Results
3.1. SCARA3 is up-regulated in myeloma cell lines after treatment with IR
The pattern of gene expression between control and irradiated 8226 cells was compared using a TaqMan antioxidant mechanism array. Genes induced greater than 4-fold by irradiation were collated (Fig. 1A). Irradiation increased SCARA3 gene expression by 5.3-fold in myeloma cells. Two other genes that were expressed greater that 4-fold were NME-5, a nucleoside-diphosphate kinase, and phosphoinositide-binding protein PIP3-E (Fig. 1A, shown by solid circles). IR-mediated up-regulation of SCARA3 in 8226 cells was confirmed in triplicate where IR resulted in approximately 4.4 fold increase in SCARA3 mRNA expression (Fig. 1B). In other myeloma cells lines (H929 and IM9), IR resulted in approximately 3.5-fold increase in SCARA3 expression (Fig. 1B). A well-established myeloma cell line (MM.1S) did not show induction of SCARA3 upon irradiation (Fig. 1B); however, treatment with the DNA methyltransferase inhibitor, 5-aza-2′-deoxycytidine (aza-dC), or the histone deacetylase inhibitor, trichostatin A (TSA), increased SCARA3 mRNA expression (Fig. 1C). Overall, Figure 1 shows that irradiation induces SCARA3 expression in myeloma cells and SCARA3 gene expression is epigenetically regulated in MM.1S cells.
Fig 1.
SCARA3 gene expression in myeloma cell lines. (A) Composite representation of the expression level of various genes between control (group 1, sham irradiated) and irradiated (group 2, 6 Gy, 12 h) 8226 cells on a TaqMan human antioxidant mechanisms array plate. The lines indicate a four-fold increase or decrease in gene expression. Arrows indicate fold change in SCARA3 and control 18S RNA. (B) qPCR analysis of SCARA3 mRNA normalized to control (untreated) cells in myeloma cell lines (8226, H929, IM9, and MM.1S) exposed to 6 Gy of IR and analyzed at 12 h post IR. (C) MM.1S cells treated with aza-dC or TSA. For panels B and C, the data are shown as the mean +/− SEM and are representative of three independent experiments. *P<0.001 or ! P<0.05 versus control.
3.2. Myeloma cell lines express various SR-A sub-family members
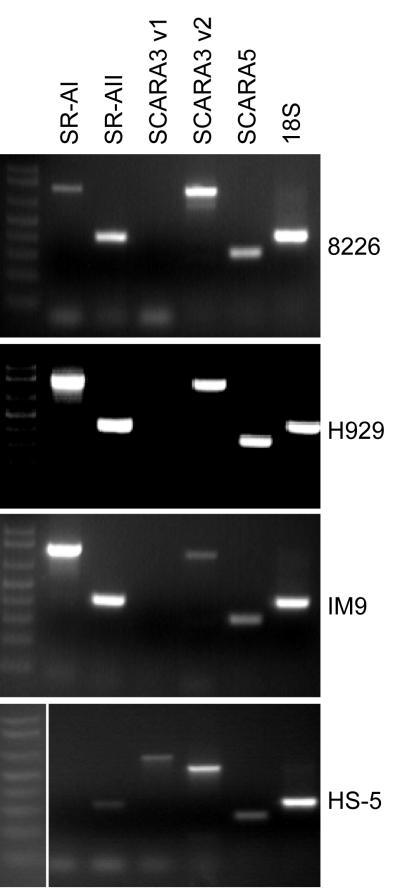
Five different species of SR-As have been identified (SR-AI/II/III, MARCO, SCARA3, SCARA4 and SCARA5) in mammalian cells [26]. There are two separate variants of SCARA3: variant 1 (v1, long form) and variant 2 (v2, short form). SCARA3 v2 shares the membrane-spanning region and an α-helical coiled-coil domain with v1 but lacks the collagen-like domain [20]. We determined whether myeloma cells express few of these SR-A isoforms using RT-PCR analysis (Fig. 2). In three myeloma cell lines (8226, H929, and IM9), mRNA of SR-AI, SR-AII, SCARA3 v2, and SCARA5 was detected. Interestingly, all these myeloma cell lines lacked expression of SCARA3 v1; however v1 was expressed in the HS-5 bone marrow stromal cell line (Fig. 2).
Fig. 2.
Myeloma cells express various SR-A members. RT-PCR analysis of SR-AI (787 bp), SR-AII (446 bp), SCARA3 v1 (877 bp), SCARA3 v2 (510 bp), SCARA5 (315 bp) and control 18S RNA (411 bp) in myeloma cell lines (8226, H929, IM9) and a bone marrow stromal cell line (HS-5). For each gel, the fragment sizes were determined using a1 kb DNA ladder.
3.3. Oxidative stressors increase SCARA3 expression in myeloma cells that may attenuate overall therapy response to anti-myeloma agents
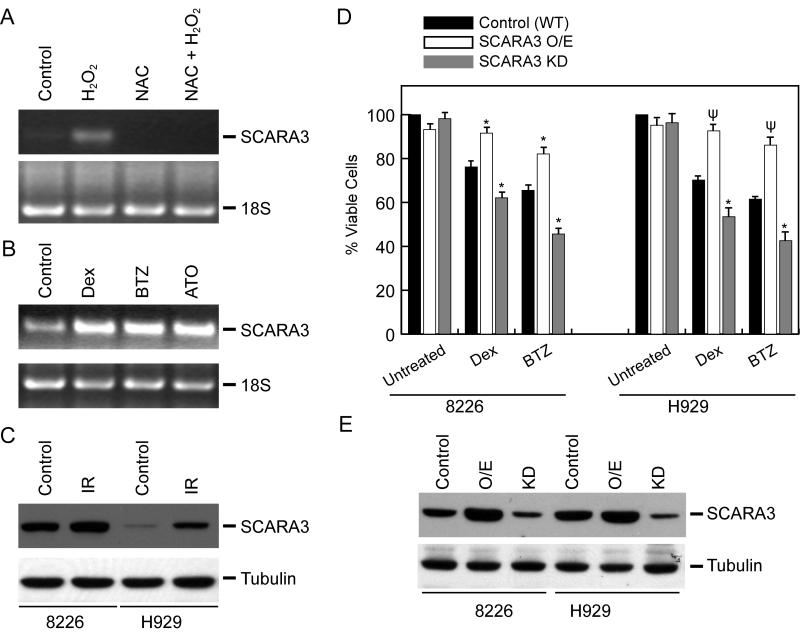
Similar to IR, H2O2 treatment produces different types of ROS (i.e. superoxide anion radicals, H2O2 and hydroxyl radicals), which are capable of damaging DNA, protein and lipid membranes in cells [29]. In 8226 cells, treatment with H2O2 resulted in increased SCARA3 v2 mRNA expression; this increase was abrogated by pretreatment with the glutathione precursor N-acetylcysteine (NAC, Fig. 3A). Similar induction of SCARA3 mRNA expression by H2O2 treatment was detected in the 5TGM1 murine myeloma cell line by qPCR analysis (data not shown).
Fig. 3.
Oxidative stressors up-regulate SCARA3 expression in myeloma cells, altering response to chemotherapy. RT-PCR at 12 h after treatment of 8226 cells with (A) H2O2 (200 ! M for 6 h) or NAC (10 mM) + H2O2, and (B) Dex (5 ! M), BTZ (10 nM), or ATO (2 ! M). Equal amounts of sample were loaded as determined by the amount of 18S RNA. (C) 8226 or H929 cells were either sham-irradiated or exposed to 6 Gy of IR.; SCARA3 protein expression was detected by immunoblotting at 24 h post-IR. (D) Cytotoxic effects of Dex (1 ! M) and BTZ (10 nM) assessed at 48 h by MTS assay in 8226 and H929 variants (WT, SCARA3 O/E, or SCARA3 KD). Values are presented as the mean of triplicate readings ! SD or are representative of three independent experiments. ! P<0.05 or ! P<0.005 versus Dex or BTZ treatment on WT cells. (E) SCARA3 protein expression was detected by immunoblotting in variant myeloma cells. For SCARA3 O/E, cells were treated with H2O2 (50 ! M for 6 h) and cell lysate was made at 24 h.
Therapeutic modalities that generate ROS (i.e. radiation and specific chemotherapeutic drugs) have been shown to be selectively cytotoxic to malignant B-cells [4]. We have previously reported Dex-mediated generation of ROS in myeloma cells and studies by other groups have suggested a role of ROS in cytotoxicity of B-cell malignancies by BTZ and ATO [4]. Since an up-regulation in SCARA3 expression was observed in normal human fibroblasts exposed to UV irradiation or H2O2 [20] and in myeloma cells by IR (Fig. 1A and B) and H2O2 (Fig. 2A), we next determined if SCARA3 mRNA would be induced by various ROS inducing anti-myeloma chemotherapeutic drugs. 8226 cells were treated with Dex, BTZ, or ATO for 12 h and SCARA3 expression was detected by RT-PCR. As shown in Figure 3B, treatments with Dex, BTZ, and ATO up-regulated SCARA3 v2 mRNA expression suggesting that SCARA3 may be a stress response gene in myeloma cells. Immunoblotting indicated that irradiation increased SCARA3 protein levels in both 8226 and H929 cells at 24 h (Fig. 3C). Taken together, these results show that in myeloma cells, increase in oxidative stress results in an up-regulation of mRNA and protein expression of SCARA3.
We next determined the specific role of SCARA3 in altering therapy responses in MM. In both 8226 and H929 cells, SCARA3 expression was either induced by H2O2 treatment (O/E) or knocked-down using a SCARA3-specific lentiviral shRNAs. Chemotherapy-induced cell death was assessed in WT or SCARA3 O/E, or SCARA3 KD myeloma cells by MTS assay at 48 h. Lentiviral inhibition of SCARA3 expression rendered myeloma cells susceptible to Dex or BTZ-mediated cell killing while non-cytotoxic doses of H2O2 resulted in chemo-resistance in myeloma cells (Fig. 3D). Immunoblotting confirmed the alterations in SCARA3 expression by pharmacological and genetic interventions when compared to the wild-type (WT) cells (Fig. 3E). Taken together, the results presented in Figure 3 demonstrate SCARA3 to be an oxidative stress response gene in myeloma cells and indicate that SCARA3 expression impacts on the emergence of therapy resistance in MM.
3.4. Expression level of SCARA3 in primary human myeloma samples
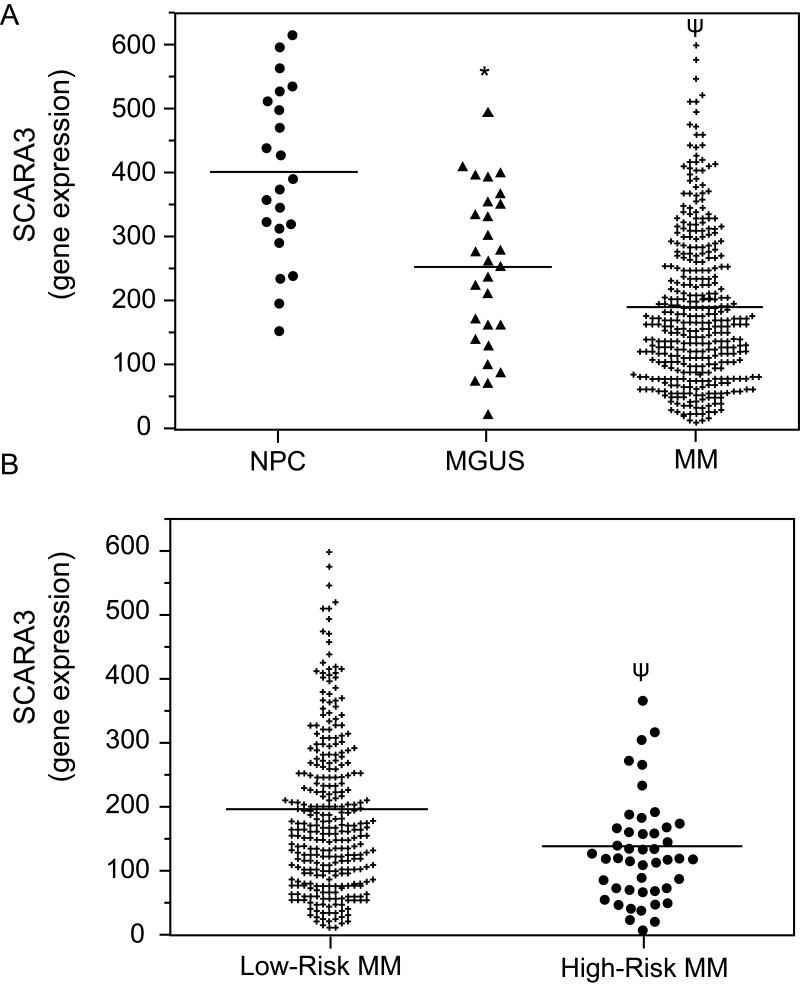
The GEP data from primary samples were analyzed for SCARA3 expression. Expression of SCARA3 mRNA was found to inversely correlate with myeloma progression as NPCs exhibited SCARA3 expression > MGUS > MM (Fig. 4A). This result suggests that decreased expression of the SCARA3 gene may play a role in myeloma disease progression. Using retrospective analysis, we next asked whether down-regulation of SCARA3 is an independent predictor of response to myeloma chemotherapy. The GEP data of human myeloma samples were analyzed for SCARA3 expression in a “high-risk” group versus “low-risk” group of patients identified by the 70-gene model [27]. High-risk myeloma patients exhibited lower levels of SCARA3 gene expression relative to low-risk patients (Fig. 4B). This result suggests that decreased mRNA expression of SCARA3 is associated with poor survival of MM patients. We speculate that SCARA3 mRNA and/or protein expression may represent a useful biomarker to predict the clinical outcome of myeloma patients.
Fig. 4.
In primary human myeloma samples, lower levels of SCARA3 expression correlate with disease progression and high-risk MM. GEP analysis of the relative expression levels of SCARA3 mRNA in (A) NPC (n=22), MGUS (n=44), or myeloma patients (n=351), or (B) myeloma patients with high-risk (n=46) versus low-risk (n=305) disease. !P<0.001 versus NPC or ! P<0.01 versus MGUS or low-risk MM.
4. Discussion
To date, MM remains an incurable disease claiming more than 10,000 lives each year in the USA [1]. The focus of this study was to obtain fundamental insights into MM disease progression, decipher why frontline therapies are failing to treat MM, and provide a strong biochemical understanding of MM to be used to design more effective therapies. In this study we propose a role of scavenger receptor SCARA3 in myeloma disease progression and emergence of therapy resistance. SR-A levels have been shown to be induced by oxidative stress, modified forms of low density lipoprotein (LDL) including acetylated LDL and oxidized LDL, macrophage colony-stimulating factor, and treatment with phorbol esters [30-33]; a pro-atherogenic role of SR-A has been proposed in macrophages [34]. We demonstrate that SCARA3 expression is up-regulated in myeloma cells after irradiation and other oxidative stress-inducing chemotherapies that may be associated with cellular defense mechanisms to detoxify IR-induced ROS and prevent cellular oxidative damage.
Studies have suggested that MM patients may be under systemic oxidative stress at diagnosis and relapse [12-16]. MM patients also show higher levels of lipid peroxidation with reduced activities of cellular antioxidant systems, suggesting a role for free radicals in the progression of MM [14]. Myeloma patients also exhibit higher serum levels of interleukin-1β (IL-1β), soluble interleukin-2 receptor, IL-6, IL-8, and tumor necrosis factor-α as compared to healthy controls; furthermore, a correlation between excessive production of pro-inflammatory cytokines, increased ROS production, and tumorigenesis has been documented [35]. Thus, altered cellular redox homeostasis and oxidative stress may contribute to the progression of MGUS to symptomatic MM and also with myeloma relapse after therapies.
Under physiological conditions, cellular redox homeostasis is robustly maintained by cellular antioxidant systems that include detoxifying enzymes such as SODs, GPxs, CAT, and small molecule antioxidants such as glutathione, peroxiredoxins, and thioredoxin [36]. In this study we compared gene expression patterns between control and irradiated myeloma cells using a TaqMan array containing 92 antioxidant mechanism associated genes. Irradiation of 8226 cells resulted in a 1.5 to 2.5-fold up-regulation of many the well-characterized antioxidant genes such SODs and H2O2 metabolizing enzymes (i.e. GPxs, CAT, and peroxyredoxins) (data not shown). SCARA3 showed a significant induction (> 5-fold) after irradiation, indicating that SCARA3 up-regulation may play a significant role in myeloma cell defense to IR-induced ROS perturbations. We have shown that radiotherapy- and chemotherapy-induced increases in intracellular pro-oxidant levels is associated with increased NF-κB-dependent MnSOD up-regulation and therapy resistance in MM [18]. SCARA3 promoter/enhancer region has binding sites for redox-sensitive factors like NF-κB and AP-1 [37] and it remains to be determined if these transcription factors are causally linked to increased SCARA3 mRNA expression seen with radiotherapy and chemotherapy in MM.
We have previously shown that myeloma cells can be rendered susceptible to ROS-mediated cytotoxicity with combination treatment (153-Sm-EDTMP skeletally-targeted radiotherapy and chemotherapy with Dex or BTZ), resulting in killing of myeloma cells while protecting normal bone marrow hematopoiesis [4,25,38]. In this study, we demonstrate that a re-activation of SCARA3 gene occurs in myeloma cells after treatment with oxidative stressors such as IR, H2O2, and chemotherapy drugs (Dex, BTZ and ATO) resulting in decreased myeloma cell killing. Interestingly SCARA3 gene expression was found to be is epigenetically regulated in the MM.1S myeloma cells. Silencing of the SCARA3 gene by promoter methylation has been reported in prostate cancer [39]. We speculate that re-activation of SCARA3 and/or other antioxidant genes by oxidative stress-inducing agents functions to maintain cellular redox homeostasis and promotes therapy resistance in MM. A better understanding of the gene expression signature of myeloma clones that are resistant to current therapies may assist in the development of early treatment strategies designed to delay and prevent the development of MM.
In a recent study, Bock et al [40] reported an increase in SCARA3 levels with disease progression in ovarian cancer. Upon retrospective analysis of human clinical myeloma samples, we observed that myeloma progression is associated with decreased SCARA3 mRNA expression; furthermore, a “high-risk” group of myeloma patients had lower levels of SCARA3 mRNA expression as compared with “low-risk” myeloma patients. Given that myeloma patients have increased markers of systemic oxidative stress, we speculate that SCARA3 is a tumor-repressor gene in MM that may represent a useful biomarker to predict progression from precursor disease to MM as well as clinical outcome. In established MM disease, inhibition of SCARA3 and/or other antioxidant genes in combination with oxidative chemotherapy could potentially lead to improved remission rates in MM.
In summary, using gene-expression studies, we have identified a novel candidate gene, SCARA3, that appears to be causally linked to myeloma progression and the emergence of therapy resistance in MM. Our studies demonstrate that inhibition of SCARA3 expression can enhance oxidative stress-induced cell killing in myeloma cells. Hence, designing complementary therapeutic approaches based on fundamental differences in oxidative metabolism between myeloma versus normal cells will aid in the development of effective treatment strategies that may also inhibit the emergence of in vivo resistance in myeloma cells in their supportive microenvironment leading to improved clinical responses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Fonseca R, Barlogie B, Bataille R, Bastard C, Bergsagel PL, Chesi M, Davies FE, Drach J, Greipp PR, Kirsch IR. Genetics and cytogenetics of multiple myeloma: a workshop report. Cancer Res. 2004;64:1546–58. doi: 10.1158/0008-5472.can-03-2876. others. [DOI] [PubMed] [Google Scholar]
- 3.Abe M. Targeting the interplay between myeloma cells and the bone marrow microenvironment in myeloma. Int J Hematol. 2011;94:334–43. doi: 10.1007/s12185-011-0949-x. [DOI] [PubMed] [Google Scholar]
- 4.Goel A, Spitz DR, Weiner GJ. Manipulation of cellular redox parameters for improving therapeutic responses in B-cell lymphoma and multiple myeloma. J Cell Biochem. 2012;113:419–25. doi: 10.1002/jcb.23387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahindra A, Laubach J, Raje N, Munshi N, Richardson PG, Anderson K. Latest advances and current challenges in the treatment of multiple myeloma. Nat Rev Clin Oncol. 2012;9:135–43. doi: 10.1038/nrclinonc.2012.15. [DOI] [PubMed] [Google Scholar]
- 6.Chauhan D, Auclair D, Robinson EK, Hideshima T, Li G, Podar K, Gupta D, Richardson P, Schlossman RL, Krett N. Identification of genes regulated by dexamethasone in multiple myeloma cells using oligonucleotide arrays. Oncogene. 2002;21:1346–58. doi: 10.1038/sj.onc.1205205. others. [DOI] [PubMed] [Google Scholar]
- 7.De Vos J, Couderc G, Tarte K, Jourdan M, Requirand G, Delteil MC, Rossi JF, Mechti N, Klein B. Identifying intercellular signaling genes expressed in malignant plasma cells by using complementary DNA arrays. Blood. 2001;98:771–80. doi: 10.1182/blood.v98.3.771. [DOI] [PubMed] [Google Scholar]
- 8.Mulligan G, Mitsiades C, Bryant B, Zhan F, Chng WJ, Roels S, Koenig E, Fergus A, Huang Y, Richardson P. Gene expression profiling and correlation with outcome in clinical trials of the proteasome inhibitor bortezomib. Blood. 2007;109:3177–88. doi: 10.1182/blood-2006-09-044974. others. [DOI] [PubMed] [Google Scholar]
- 9.Zhan F, Hardin J, Kordsmeier B, Bumm K, Zheng M, Tian E, Sanderson R, Yang Y, Wilson C, Zangari M. Global gene expression profiling of multiple myeloma, monoclonal gammopathy of undetermined significance, and normal bone marrow plasma cells. Blood. 2002;99:1745–57. doi: 10.1182/blood.v99.5.1745. others. [DOI] [PubMed] [Google Scholar]
- 10.Gius D, Spitz DR. Redox signaling in cancer biology. Antioxid Redox Signal. 2006;8:1249–52. doi: 10.1089/ars.2006.8.1249. [DOI] [PubMed] [Google Scholar]
- 11.Spitz DR, Azzam EI, Li JJ, Gius D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: a unifying concept in stress response biology. Cancer Metastasis Rev. 2004;23:311–22. doi: 10.1023/B:CANC.0000031769.14728.bc. [DOI] [PubMed] [Google Scholar]
- 12.Kuku I, Bayraktar MR, Kaya E, Erkurt MA, Bayraktar N, Cikim K, Aydogdu I. Serum proinflammatory mediators at different periods of therapy in patients with multiple myeloma. Mediators Inflamm. 2005;2005:171–4. doi: 10.1155/MI.2005.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma A, Tripathi M, Satyam A, Kumar L. Study of antioxidant levels in patients with multiple myeloma. Leuk Lymphoma. 2009;50:809–15. doi: 10.1080/10428190902802323. [DOI] [PubMed] [Google Scholar]
- 14.Zima T, Spicka I, Stipek S, Crkovska J, Platenik J, Merta M, Tesar V. Antioxidant enzymes and lipid peroxidation in patients with multiple myeloma. Neoplasma. 1996;43:69–73. [PubMed] [Google Scholar]
- 15.Gangemi S, Allegra A, Alonci A, Cristani M, Russo S, Speciale A, Penna G, Spatari G, Cannavo A, Bellomo G. Increase of novel biomarkers for oxidative stress in patients with plasma cell disorders and in multiple myeloma patients with bone lesions. Inflamm Res. 2012;61:1063–7. doi: 10.1007/s00011-012-0498-7. others. [DOI] [PubMed] [Google Scholar]
- 16.Kuku I, Aydogdu I, Bayraktar N, Kaya E, Akyol O, Erkurt MA. Oxidant/antioxidant parameters and their relationship with medical treatment in multiple myeloma. Cell Biochem Funct. 2005;23:47–50. doi: 10.1002/cbf.1127. [DOI] [PubMed] [Google Scholar]
- 17.Kuehl WM, Bergsagel PL. Multiple myeloma: evolving genetic events and host interactions. Nat Rev Cancer. 2002;2:175–87. doi: 10.1038/nrc746. [DOI] [PubMed] [Google Scholar]
- 18.Brown CO, Salem K, Wagner BA, Bera S, Singh N, Tiwari A, Choudhury A, Buettner GR, Goel A. Interleukin-6 counteracts therapy-induced cellular oxidative stress in multiple myeloma by up-regulating manganese superoxide dismutase. Biochem J. 2012;444:515–27. doi: 10.1042/BJ20112019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salem K, Brown CO, Schibler J, Goel A. Combination chemotherapy increases cytotoxicity of multiple myeloma cells by modification of nuclear factor (NF)-kappaB activity. Exp Hematol. 2012 doi: 10.1016/j.exphem.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han HJ, Tokino T, Nakamura Y. CSR, a scavenger receptor-like protein with a protective role against cellular damage causedby UV irradiation and oxidative stress. Hum Mol Genet. 1998;7:1039–46. doi: 10.1093/hmg/7.6.1039. [DOI] [PubMed] [Google Scholar]
- 21.DeWitte-Orr SJ, Collins SE, Bauer CM, Bowdish DM, Mossman KL. An accessory to the ‘Trinity’: SR-As are essential pathogen sensors of extracellular dsRNA, mediating entry and leading to subsequent type I IFN responses. PLoS Pathog. 6:e1000829. doi: 10.1371/journal.ppat.1000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delimaris I, Faviou E, Antonakos G, Stathopoulou E, Zachari A, Dionyssiou-Asteriou A. Oxidized LDL, serum oxidizability and serum lipid levels in patients with breast or ovarian cancer. Clin Biochem. 2007;40:1129–34. doi: 10.1016/j.clinbiochem.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Motta M, Pistone G, Franzone AM, Romeo MA, Di Mauro S, Giugno I, Ruello P, Malaguarnera M. Antibodies against ox-LDL serum levels in patients with hepatocellular carcinoma. Panminerva Med. 2003;45:69–73. [PubMed] [Google Scholar]
- 24.Murphy JE, Tedbury PR, Homer-Vanniasinkam S, Walker JH, Ponnambalam S. Biochemistry and cell biology of mammalian scavenger receptors. Atherosclerosis. 2005;182:1–15. doi: 10.1016/j.atherosclerosis.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 25.Bera S, Greiner S, Choudhury A, Dispenzieri A, Spitz DR, Russell SJ, Goel A. Dexamethasone-induced oxidative stress enhances myeloma cell radiosensitization while sparing normal bone marrow hematopoiesis. Neoplasia. 2010;12:980–92. doi: 10.1593/neo.101146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeWitte-Orr SJ, Collins SE, Bauer CM, Bowdish DM, Mossman KL. An accessory to the ‘Trinity’: SR-As are essential pathogen sensors of extracellular dsRNA, mediating entry and leading to subsequent type I IFN responses. PLoS Pathog. 2010;6:e1000829. doi: 10.1371/journal.ppat.1000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaughnessy JD, Jr., Zhan F, Burington BE, Huang Y, Colla S, Hanamura I, Stewart JP, Kordsmeier B, Randolph C, Williams DR. A validated gene expression model of high-risk multiple myeloma is defined by deregulated expression of genes mapping to chromosome 1. Blood. 2007;109:2276–84. doi: 10.1182/blood-2006-07-038430. others. [DOI] [PubMed] [Google Scholar]
- 28.Zhan F, Barlogie B, Mulligan G, Shaughnessy JD, Jr., Bryant B. High-risk myeloma: a gene expression based risk-stratification model for newly diagnosed multiple myeloma treated with high-dose therapy is predictive of outcome in relapsed disease treated with single-agent bortezomib or high-dose dexamethasone. Blood. 2008;111:968–9. doi: 10.1182/blood-2007-10-119321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cadenas E. Biochemistry of oxygen toxicity. Annu Rev Biochem. 1989;58:79–110. doi: 10.1146/annurev.bi.58.070189.000455. [DOI] [PubMed] [Google Scholar]
- 30.de Villiers WJ, Fraser IP, Hughes DA, Doyle AG, Gordon S. Macrophage-colony-stimulating factor selectively enhances macrophage scavenger receptor expression and function. J Exp Med. 1994;180:705–9. doi: 10.1084/jem.180.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mietus-Snyder M, Gowri MS, Pitas RE. Class A scavenger receptor up-regulation in smooth muscle cells by oxidized low density lipoprotein. Enhancement by calcium flux and concurrent cyclooxygenase-2 up-regulation. J Biol Chem. 2000;275:17661–70. doi: 10.1074/jbc.275.23.17661. [DOI] [PubMed] [Google Scholar]
- 32.Terpstra V, van Amersfoort ES, van Velzen AG, Kuiper J, van Berkel TJ. Hepatic and extrahepatic scavenger receptors: function in relation to disease. Arterioscler Thromb Vasc Biol. 2000;20:1860–72. doi: 10.1161/01.atv.20.8.1860. [DOI] [PubMed] [Google Scholar]
- 33.Via DP, Pons L, Dennison DK, Fanslow AE, Bernini F. Induction of acetyl-LDL receptor activity by phorbol ester in human monocyte cell line THP-1. J Lipid Res. 1989;30:1515–24. [PubMed] [Google Scholar]
- 34.Moore KJ, Freeman MW. Scavenger receptors in atherosclerosis: beyond lipid uptake. Arterioscler Thromb Vasc Biol. 2006;26:1702–11. doi: 10.1161/01.ATV.0000229218.97976.43. [DOI] [PubMed] [Google Scholar]
- 35.Kundu JK, Surh YJ. Inflammation: gearing the journey to cancer. Mutat Res. 2008;659:15–30. doi: 10.1016/j.mrrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P. Redox regulation of cell survival. Antioxid Redox Signal. 2008;10:1343–74. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mietus-Snyder M, Friera A, Glass CK, Pitas RE. Regulation of scavenger receptor expression in smooth muscle cells by protein kinase C: a role for oxidative stress. Arterioscler Thromb Vasc Biol. 1997;17:969–78. doi: 10.1161/01.atv.17.5.969. [DOI] [PubMed] [Google Scholar]
- 38.Goel A, Dispenzieri A, Geyer SM, Greiner S, Peng KW, Russell SJ. Synergistic activity of the proteasome inhibitor PS-341 with non-myeloablative 153-Sm-EDTMP skeletally targeted radiotherapy in an orthotopic model of multiple myeloma. Blood. 2006;107:4063–70. doi: 10.1182/blood-2005-09-3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu G, Tseng GC, Yu YP, Gavel T, Nelson J, Wells A, Michalopoulos G, Kokkinakis D, Luo JH. CSR1 suppresses tumor growth and metastasis of prostate cancer. Am J Pathol. 2006;168:597–607. doi: 10.2353/ajpath.2006.050620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bock AJ, Nymoen DA, Brenne K, Kaern J, Davidson B. SCARA3 mRNA is overexpressed in ovarian carcinoma compared with breast carcinoma effusions. Hum Pathol. 2012;43:669–74. doi: 10.1016/j.humpath.2011.06.003. [DOI] [PubMed] [Google Scholar]