Abstract
Psychiatric and neurodegenerative disorders, including intellectual disability (ID), autism spectrum disorders (ASD), schizophrenia (SZ), and Alzheimer's disease (AD), pose an immense burden to society. Symptoms of these disorders become manifest at different stages of life: early childhood, adolescence, and late adulthood, respectively. Progress has been made in recent years toward understanding the genetic substrates, cellular mechanisms, brain circuits, and endophenotypes of these disorders. Multiple lines of evidence implicate excitatory and inhibitory synaptic circuits in the cortex and hippocampus as key cellular substrates of pathogenesis in these disorders. Excitatory/inhibitory balance – modulated largely by dopamine – critically regulates cortical network function, neural network activity (i.e. gamma oscillations) and behaviors associated with psychiatric disorders. Understanding the molecular underpinnings of synaptic pathology and neuronal network activity may thus provide essential insight into the pathogenesis of these disorders and can reveal novel drug targets to treat them. Here we discuss recent genetic, neuropathological, and molecular studies that implicate alterations in excitatory and inhibitory synaptic circuits in the pathogenesis of psychiatric disorders across the lifespan.
Keywords: dendritic spine, mental disorder, glutamatergic, postmortem, genetic, intellectual disability, autism, schizophrenia, Alzheimer's disease, neurodegenerative, ErbB4, neuregulin, GABAergic interneuron, gamma oscillations, dopamine
Introduction
Abnormalities in synapses of excitatory and inhibitory neurons have emerged as key cellular substrates in the pathogenesis of several psychiatric and neurodegenerative disorders (Penzes et al. 2011). Indeed, disease-specific disruptions in synaptic morphology and function accompany a large number of brain disorders, suggesting that such alterations may serve as substrates for many psychiatric and neurological disorders, particularly those that involve deficits in information processing. In support of this view, recent studies found altered dendritic spine density on cortical pyramidal neurons in individuals with ID, ASD, SZ and AD (Penzes et al. 2011). In addition, structural and functional changes in GABAergic inhibitory circuits have been described in ASD and SZ (Chattopadhyaya and Cristo 2012). Among these, dysfunction of inhibitory parvalbumin-positive (PV-positive) basket cells is thought to play a major role in both ASD and SZ (Lewis and Gonzalez-Burgos 2008; Gogolla et al. 2009). Alterations in GABAergic circuits in ASD are supported by findings of significantly reduced GAD65 and GAD67 in the parietal cortex and cerebellum (Fatemi et al. 2002; Yip et al. 2007) and alterations in GABAA and GABAB receptors in post-mortem brains of autistic subjects (Collins et al. 2006; Fatemi et al. 2009; Oblak et al. 2010), combined with reduced benzodiazepine binding to GABAA receptors (Guptill et al. 2007). A reduction in multipolar interneuronal dendritic length and reduced GAD67 levels occur in SZ and may underlie the dysfunction of inhibitory circuits in the disease (Kalus et al. 2002; Lewis et al. 2005; Akbarian and Huang 2006).
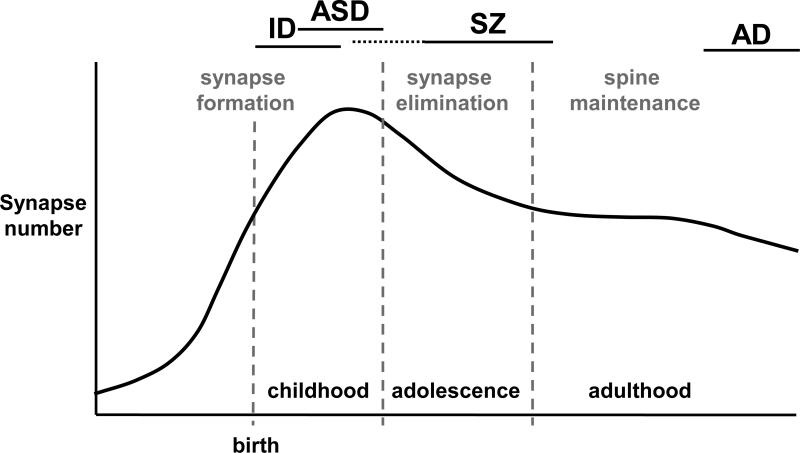
Here we discuss synaptic alterations in ID, ASD, SZ, and psychosis in AD (AD+P). These disorders become manifest at different stages across the life: ID and ASD in early childhood, positive SZ symptoms in adolescence and young adulthood, and AD in late adulthood (Figure 1). Although spine formation and elimination occur throughout a normal lifespan, perinatal and postnatal net synapse proliferation is followed by a period of protracted net synapse elimination that lasts throughout childhood and into adolescence in some brain regions. This is followed by synapse maintenance in the adult brain that preserves circuitry established earlier in life. Disruption of synapse morphogenesis and function during a particular time during development, or in a particular brain region, may dictate the subsequent neuropathological symptoms that arise. Thus, the timing of synapse pathology can lead to disease-specific synaptic and cellular dysfunction, neuronal circuit alterations, and ultimately cognitive and behavioral symptoms.
Figure 1. Trajectory of synapse number across the lifespan.
Synapse numbers increase before and after birth; synapse are selectively eliminated during childhood and adolescence to adult levels. Bars on the top indicate the period of emergence of symptoms of specific disorders.
Consistent with the idea that synaptic circuits are common substrates of mental disorders, a multitude of genetic studies over the past few years have implicated “neuroplasticity” genes in the etiology of these disorders. Indeed, it is now widely accepted that rare (copy number variants and point mutations) and common variants in genes that control the development and plasticity of synapses and dendrites increase risk for mental disorders (Gilman et al. 2011; Gai et al. 2012; Guilmatre et al. 2009; Kirov et al. 2012).
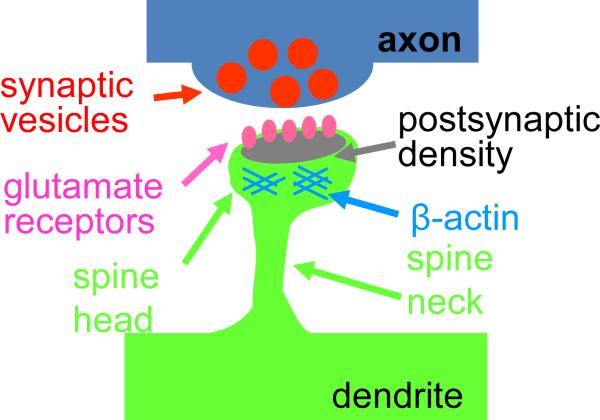
Many psychiatric and neurological disorders are associated with disruptions of dendritic spine numbers and morphology. In the mammalian forebrain, most glutamatergic excitatory synapses occur on small protrusions on dendrites called dendritic spines (Figure 2). Changes in spine morphology occur in synaptogenesis, maintenance, plasticity and elimination. During development, dynamic spines allow dendrites to actively participate in synaptogenesis, thereby contributing to the establishment of connectivity within neuronal circuits (Holtmaat and Svoboda 2009). Activity-dependent spine maintenance or elimination contributes to the remodeling of neuronal circuits during postnatal development and adolescence (Zuo et al. 2005; Alvarez and Sabatini 2007). Spines undergo experience-dependent morphological changes in live animals (Holtmaat and Svoboda 2009; Alvarez and Sabatini 2007) and spine morphology is intimately linked to human cognitive development and function (Lewis and Gonzalez 2008). Spine dynamics are regulated by molecular pathways that control cytoskeletal remodeling, trans-synaptic adhesion, receptor trafficking, protein translation, ubiquitination, and gene expression (Penzes et al. 2008; Tada and Sheng 2006; Penzes and Jones 2008).
Figure 2. Schematic structure of a spiny synapse.
Alterations in the mechanisms that control the formation, maintenance, and elimination of spiny synapses are likely to contribute to synaptic pathology in mental disorders.
In this mini-review we elaborate on recent findings presented by some of the authors at the 5th Special Neurochemistry Conference entitled “Synapses and dendritic spines in health and disease” held in Buenos Aires, Argentina. We review genetic studies that address etiology, postmortem neuropathological studies that reveal the pathophysiological outcome of the disease process, and functional studies in model systems that investigate the underlying neurobiological mechanisms. We propose that while these disorders have largely distinct symptom profiles, they collectively share the common substrate of abnormal function of synaptic circuits.
Synaptic dysfunction in ID and ASD
One of the most common neurodevelopmental disorders is ID. Patients affected by ID have an intelligence quotient of 70 or below and often exhibit deficits in behaviour related to adaptive functioning, which includes ASD. Over 25% of ID and ASD cases are caused by genetic factors (Rauch et al. 2006; Pinto et al. 2010) and up to 60% have unknown etiologies. Several single-genes causing syndromic or nonsyndromic ID have been identified over the past 15 years. Most of these genes are located on the X chromosome and are responsible for X-linked intellectual disabilities (XLID). Interestingly, more then 50% of the ID-related proteins that are not transcription or chromatin-remodelling factors are clearly present in the pre- or post-synaptic compartments and appear to be implicated in synaptic functions by regulating actin cytoskeleton rearrangement, synaptic plasticity or synapse formation (Ropers and Hamel 2005). In this review we will describe the molecular mechanisms by which dysfunctions in some of these synaptic proteins – including Shank, IL1RAPL1, oligophrenin-1 and TSPAN7 – contribute to ID and ASD.
SHANK3 haploinsufficiency is now considered to be the main cause of the neurobehavioral symptoms of the Phelan-McDermid syndrome (PMS, also called 22q13.3 deletion syndrome), although other genes may also be lost by the chromosomal deletion (Bonaglia et al. 2001; Wilson et al. 2003; Durand et al. 2007; Delahaye et al. 2009). Indeed, a number of de novo mutations in SHANK3 (Durand et al. 2007; Moessner et al. 2007; Gauthier et al. 2009), SHANK2 (Berkel et al. 2010), and in SHANK1 (Sato et al. 2012) have been identified in individuals with ASD and ID. A number of knock out mice have been created for the three SHANK genes. The first described mouse, lacking Shank1, has small dendritic spines, weakened synaptic transmission, enhanced learning (Hung et al. 2008), and defects in social communication (Wöhr et al. 2011). In addition, more recent studies show that mice with heterozygous or homozygous disruption of Shank3 have self-injurious repetitive grooming, deficits in social interaction, alterations in learning and memory formation, and defects in synaptic transmission (Bozdagi et al. 2010; Peça et al. 2011; Wang et al. 2011). The behavioral defects correlate with impaired basal synaptic transmission in CA3-CA1 connections, reduced GluR1 clusters and protein levels in the hippocampus, altered activity-dependent AMPAR synaptic plasticity and major changes in striatal and cortico-striatal synapses (Bozdagi et al. 2010; Peça et al. 2011; Wang et al. 2011). Shank3 knocked down in rodent neuronal cultures by RNA interference (shRNA) specifically reduced the expression of mGluR5 receptors and also impaired DHPG-induced phosphorylation of ERK1/2 and CREB (Verpelli et al. 2011). Finally, the Shank2 knockout mice, like their Shank3 knockout counterparts, show abnormalities in behavior tests, impairment in social activities, hyperactivity, and defects in synaptic transmission (Bockers et al. 2004; Schmeisser et al. 2012; Won et al. 2012). These studies demonstrate that mice with mutations in the SHANK genes cause alterations in synaptic morphology and signalling, as well as changes in behavior characteristics, indicating that they are good animal models for the study of ID and ASD.
Most of the XLID are attributable to the Fragile X and Rett syndromes; however, mutations of several other genes on the X chromosome have been found to strongly associate with ID, with an estimated 50% of the XLID genes coding for synaptic proteins (Laumonnier et al. 2007). For instance, cognitive impairments ranging from nonsyndromic ID to ASD have been found in patients with mutations in the interleukin-1 receptor accessory protein-like 1 gene (IL1RAPL1) (Carrie et al. 1999; Bhat et al. 2008; Piton et al. 2008; Franek et al. 2011). The IL1RAPL1 protein is structurally formed by three extracellular Ig-like domains, a transmembrane domain, and an intracellular Toll/IL-1R homology domain (TIR domain). IL1RAPL1 binds postsynaptic density protein 95 (PSD-95) and regulates its phosphorylation and synaptic association by activating the c-Jun terminal kinase (JNK) (Pavlowsky et al. 2010). Instead the extracellular domain of IL1RAPL1 induces presynaptic differentiation by binding the receptor tyrosine phosphatase δ (PTPδ), which is localized at the presynaptic terminal, while the TIR domain binds to RhoGAP2 and regulates dendritic spine formation (Valnegri et al. 2011b; Yoshida et al. 2011). All these findings suggest that the IL1RAPL1 complex mediates trans-synaptic signalling that regulates excitatory synapse formation and function.
Some forms of XLID are caused by mutations or deletions in the synaptic RhoGTPase-activating protein oligophrenin-1 (Nadif Kasri and Van Aelst 2008), indicating that signalling involving member A of the Ras homologue gene family (RhoA) is involved in ID. Oligophrenin-1 is a negative regulator of RhoA, Rac and Cdc42, and also interacts with the postsynaptic adaptor protein Homer (Govek et al. 2004). Knockdown of oligophrenin-1 in CA1 pyramidal neurons significantly reduces spine length and this effect is mimicked by a constitutively active form of RhoA and can be rescued by the presence of constitutively dominant negative RhoA, which leads to an inhibition of the RhoA effector Rho-kinase (ROCK1) (Govek et al. 2004). Considering the important role of ROCK1 in actin remodelling, these results strongly suggest that RhoA regulates the actin cytoskeleton of spines, possibly through effects on the LIM kinase, myosin light chain (MLC), or MLC phosphatase (Govek et al. 2004; Nadif Kasri and Van Aelst 2008). A new role of oligophrenin-1 in regulating the activity of the circadian clock protein Rev-erbα has also recently been shown, suggesting that the etiology of intellectual disability could be related to the interaction between synaptic activity and circadian oscillators (Valnegri et al. 2011a). Very recently, Powell et al. showed that oligophrenin-1-deficient mice have changes in the number of vesicles in the readily releasable pool and also have altered availability of secretory vesicles (Powell et al. 2012). Thus, alterations in oligophrenin-1 expression result in multiple deficits of synaptic activity and plasticity that depend on oligophrenin-1 being expressed in both the pre- and postsynaptic terminals.
TSPAN7 is directly associated with cognitive defects in humans because several alterations to its gene (TM4SF2) – TM4SF2 inactivation by an X:2 balanced translocation, a premature stop codon TGA (gly218-to-ter), (Zemni et al. 2000) and a 2-bp deletion (564delGT) resulting in a premature stop codon at position 192 (Abidi et al. 2002) – are directly associated with non-syndromic intellectual disability. The gly218-to-ter nonsense mutation and the 2-bp deletion may predict a truncated TSPAN7 lacking cytoplasmic C-terminal tail and the fourth transmembrane domain. TSPAN7 promotes filopodia and dendritic spine formation in cultured hippocampal neurons, and is required for spine stability and normal synaptic transmission. TSPAN7 directly interacts with the PDZ domain of protein interacting with C kinase 1 (PICK1), and associates with AMPAR subunit GluA2 and β1-integrin. Interestingly TSPAN7 regulates AMPA receptor trafficking by modulating the PICK1 and GluA2/3 association. These findings identify TSPAN7 as a key player in the morphological and functional maturation of glutamatergic synapses; possibly explaining why its deletion is strongly associated with ID in humans (Bassani et al. 2012).
In conclusion, several genetic and functional studies demonstrate that mutations associated with intellectual disabilities occur in molecules that play an essential role in regulating brain synapse formation and plasticity.
Abnormalities of spiny synapses in schizophrenia
Schizophrenia is a complex developmental psychiatric disorder generally characterized by positive (i.e., hallucinations, disorganized thoughts, delusions) and negative (i.e., diminished affect, social withdrawal) symptoms, and deficits in executive and cognitive functions (Harrison and Weinberger 2005; Owen and O'Donovan 2005). Schizophrenia affects approximately 0.5-1% of the population. Positive symptoms (i.e. hallucinations, delusions) typically emerge in late adolescence or early adulthood, while negative symptoms (i.e. impaired social interactions, low affect) and deficits in cognitive function can be observed earlier in development. Disturbances in glutamate, GABA and dopamine neurotransmission have been implicated in the observed functional ‘dysconnectivity’ in neural circuits observed in schizophrenia (Kantrowitz and Javitt 2010; Bergeron and Coyle 2012; Seeman 2009).
As synapses are the basic units of neural circuits and are intimately involved in neurotransmitter signal transduction, dendritic spine dysfunction may play an important etiological role in schizophrenia. Indeed, structural MRI studies have consistently shown gray matter reductions in schizophrenic patients. Among the ultrastructural changes thought to directly contribute to the lower gray matter are reductions in spine density (Selemon and Goldman-Rakic 1999). Several postmortem studies have examined spine density in brain regions with pronounced gray matter loss. Spine loss has been reported in the dorsolateral prefrontal cortex (DLPFC), particularly in layer 3 neurons (Glantz and Lewis 2000). Schizophrenia patients also show a profound reduction in spine density on pyramidal neurons in the superior temporal gyrus (STG), particularly in the primary auditory cortex (Sweet et al. 2009), which could potentially be associated with auditory hallucinations (Barta et al. 1990). Several studies have shown reductions in hippocampal volume in schizophrenia (Steen et al. 2006). Within the hippocampus, reduced spine density on subicular dendrites, reduced CA3 spine density (Law et al. 2004; Kolomeets et al. 2005), as well as reduced spine size (Kolomeets et al. 2005) have been reported. A lower density of synaptic contacts formed by individual mossy fiber tracts on CA3 pyramidal neurons (Kolomeets et al. 2007) has also been reported.
Risk for schizophrenia is associated with a combined effect of multiple susceptibility genes and environmental interactions during development (Stefansson et al 2003; Lewis and Levitt 2002; Meyer and Feldon 2009; Rapoport et al. 2005; Arango et al. 2008). Genetic linkage and genome wide association studies have not identified a clear link between neurotransmitter-associated genes (i.e., for biosynthetic or metabolizing enzymes, receptors) and the etiology of schizophrenia (Harrison and Weinberger 2005). Instead, genetic studies have identified haplotypes and single nucleotide polymorphisms (SNPs) associated with genes regulating neuronal migration, synaptic structure and plasticity (Harrison and Weinberger 2005), and more recently, genome-wide association studies have identified genes encoding proteins regulating neuronal excitability as potential risk factors for cognitive deficits and numerous psychiatric disorders (Weissflog et al. 2012; Franke et al. 2009; Casamassima et al. 2010; Bhat et al. 2012; Meier et al. 2012). Here we will summarize recent findings on some of the most prominent ones and explore their roles in spine dynamics.
A large number of genetic linkage and association studies have suggested that the NRG1 and ERBB4 genes may be risk factors for schizophrenia (Stefansson et al. 2002; Munafo et al. 2008; Munafo et al. 2006; Hall et al. 2006; Silberberg et al. 2006; Law et al. 2007; Tan et al. 2010; Nicodemus et al. 2010) and its endophenotypes (Greenwood et al. 2012; Greenwood et al. 2011). These genes also regulate several biological processes altered in schizophrenia (Buonanno and Fischbach 2001; Mei and Xiong 2008; Buonanno 2010). While many genes have been associated with a risk for schizophrenia and regulate biological process similar to NRG1 and ErbB4, few show the same degree of biological plausibility and reproducible association with the disease. In particular, a functional NRG1 SNP associated with schizophrenia (Walss-Bass et al. 2006) that substitutes a valine in the NRG1 (type III) transmembrane domain necessary for gamma-secretase-dependent cleavage (Chen et al. 2010) constitutes an important “at risk” variant for the disorder. In addition, a second NRG1 polymorphism (SNP8NRG243177) within the original HAPICE “at risk” Icelandic haplotype (Stefansson et al. 2002), a large region of DNA that is hyper-variant in schizophrenia and associated with transcriptional regulation of type III NRG1 (Weickert et al. 2012), is highly associated with risk for developing psychotic symptoms, decreased premorbid IQ and activation of frontal and temporal lobes (Hall et al. 2006). Moreover, studies that have evaluated 94 candidate genes for schizophrenia and ten quantitative endophenotypes, such as pre-pulse inhibition, P50 suppression and the Wisconsin Card Scoring Test, identified NRG1 and ERBB4 as schizophrenia susceptibility genes (Greenwood et al. 2012; Greenwood et al. 2011).
Both NRG1 and ErbB4 regulate synaptic structure and function. ErbB4 is expressed in interneurons, and potentially less abundantly, in cortical pyramidal cells and spines of excitatory neurons (see below). Long-term NRG1 treatment increases pyramidal neuronal spine density and the preponderance of spines with mature phenotypes (Barros et al. 2009). ErbB4 overexpression increases spine density, area, and excitatory synaptic transmission (Li et al. 2007). Conversely, erbB4 knockdown reduces spine density and size (Li et al. 2007). Mice deficient in NRG1 type III show reductions in spine density in hippocampal neurons (Chen et al. 2008). Mice lacking erbB2 and erbB4 in the CNS show reduced spine density in both the hippocampus and cortex (Barros et al. 2009). In both these mice, spine morphological deficits co-occur with schizophrenia-related behavioral phenotypes.
The initial link of the disrupted in schizophrenia 1 (DISC1) gene to schizophrenia was identified in a Scottish pedigree with a disruption of the DISC1 open reading frame. Polymorphisms and frame shift mutations of DISC1 have been linked to schizophrenia in other lineages (Schumacher et al. 2009). As DISC1 has been associated with several psychiatric disorders, including bipolar disorder, depression, and autism, it seems likely that it constitutes a general psychiatric vulnerability gene. DISC1 is highly abundant in spines (Kirkpatrick et al. 2006). In cortical neurons, long-term knockdown reduces spine area (Hayashi-Takagi et al. 2010), whereas its short-term knockdown increases spine density and size. The effects of DISC1 mutations in mice on spine density reflect brain region and developmentally influenced effects. Namely, spine numbers in dentate gyrus granule cells are reduced in a mouse model of disease-associated chromosomal translocation (Kvajo et al. 2008), and dendrite complexity is reduced by early postnatal expression of DISC1 C-terminus in mice (Li et al. 2007). Spine density in cortical pyramidal neurons was increased by prenatal expression of mutant DISC1, while combined prenatal and postnatal expression increased spine density in hippocampal granule cells (Ayhan et al. 2011).
While DISC1 mRNA levels seem unaffected in schizophrenia patients (Dean et al. 2007; Lipska et al. 2006), the expression of DISC1 interacting proteins was reduced in patients carrying high-risk DISC1 SNPs (Lipska et al. 2006), suggesting that DISC1 function might be affected in schizophrenia. Disruption of DISC1's ability to scaffold proteins in spines would be expected to have deleterious consequences on spine morphogenesis. Indeed, DISC1 is known to interact with several well-established regulators of spine morphogenesis, most prominently the RacGEF kalirin-7 (Millar et al. 2003). Recently kalirin-7, via activation of its downstream effector Rac1, was found to directly regulate the effects of DISC1 on spine morphology (Hayashi-Takagi et al. 2010). Interestingly, the expression of kalirin mRNA was reduced in the DLPFC of patients with schizophrenia, irrespective of antipsychotic treatment (Hill et al. 2006). This suggests a potential role for small GTPase pathways in spine pathology in schizophrenia. Indeed, the expression of Cdc42 mRNA was also reduced in postmortem schizophrenic DLPFC (Hill et al. 2006; Ide et al. 2010). Loss of kalirin and Cdc42 strongly correlates with spine loss in layer 3 PFC neurons (Hill et al. 2006). Because of kalirin's important synaptic functions, its interactions with DISC1 and its reduced expression in schizophrenia, recent studies have examined how kalirin loss impacts spines and behavior. Interestingly, kalirin KO mice show severe reductions in spine density and dendrite complexity in the frontal cortex, as well as schizophrenia-related impairments in working memory, sociability, and prepulse inhibition (Cahill et al. 2009; Xie et al. 2010). Remarkably, both spine loss and behavioral dysfunction emerged during adolescence and were absent in juvenile KO mice (Cahill et al. 2009). This is interesting given the onset of schizophrenia symptoms in adolescence in humans, and points to a tight association between the onset of spine loss and the onset of behavioral impairments in these animals.
The 22q11.2 microdeletion syndrome is the most common CNV associated with schizophrenia, accounting for 1-2% of cases (Stark et al. 2008). Primary hippocampal neurons from mice engineered to carry hemizygous deletion of the 1.3-Mb orthologous chromosomal region (Df(16)A+/-) showed reduced spine density and sizes (Mukai et al. 2008). Interestingly, loss of either of two genes within this region (ZDHHC8 and Dgcr8) was sufficient to impair spine and dendrite morphology (Stark et al. 2008; Mukai et al. 2008). ZDHHC8 is a palmitoyl transferase which palmitoylates PSD-95; its loss results in reduced spine density and simpler dendrites, and its replacement into Df(16)A+/- neurons rescued spine and dendrite deficiency (Mukai et al. 2008). Dgcr8 is involved in miRNA processing, and its loss results in smaller spines and simpler dendrites (Stark et al. 2008). Mice modeling the 22q11.2 microdeletion syndrome (Df(16)A+/-) showed reduced hippocampal spine density and sizes (Mukai et al. 2008). Mice deficient in individual genes within this region (ZDHHC8 and Dgcr8) showed simplified dendritic trees and reduced spine density (Mukai et al. 2008), or smaller spines (Stark et al. 2008), respectively.
Abnormalities in inhibitory circuits in SZ
The synchronization of neuronal network activity in the human cortex and hippocampus at gamma frequencies (30-80 Hz) is important for cognition, learning and memory (Engel and Singer 2001) and is altered in schizophrenia (Herrmann and Demiralp 2005; Spencer 2009; Gonzalez-Burgos and Lewis 2008). Gamma oscillations emerge from the synchronized firing of interconnected excitatory glutamatergic and primarily inhibitory fast-spiking GABAergic PV+ interneurons (Cobb et al. 1995), and their power (i.e. amplitude) is modulated by the E/I balance at distinct synaptic sites in the circuit and the intrinsic excitable properties of the neurons (Bartos et al. 2007). Event-related gamma oscillation power is reduced in subjects diagnosed with schizophrenia (Kwon et al. 1999; Wilson et al. 2008), and the regional reaction time phase-lock of oscillations is correlated with either positive or negative symptoms (Spencer et al. 2004). Importantly, GABAergic fast-spiking interneurons and levels of the GABA biosynthetic enzyme GAD67 are reduced in PV+ interneurons in postmortem brains from affected individuals, suggesting that specific neural circuits may be associated with schizophrenia (Akbarian et al. 1995; Woo et al. 1998; Woo et al. 2004) also see (Gonzalez-Burgos and Lewis 2008). Altered functionality of PV+ basket cell GABAergic interneurons, which provide perisomatic inhibition to pyramidal neurons, may account for the observed reduction in neural network oscillations that are important for working memory (Gonzalez-Burgos and Lewis 2012; Spencer 2009).
Determining the cellular and subcellular localization of ErbB4 and its function at pre- and post-synaptic sites is critically important for understanding the role of this signaling pathway in modulating E/I balance and neuronal network activity. ErbB4 mRNA expression patterns have long been known to correspond to GABAergic neurons in the neocortex and hippocampus (Lai and Lemke 1991; Steiner et al. 1999; Gerecke et al. 2001; Fox and Kornblum 2005; Thompson M. et al. 2007), and unlikely to be expressed in glutamatergic neurons as demonstrated by single-cell PCR from electrophysiologically identified neurons (Vullhorst et al. 2009). Recently, using highly specific polyclonal and monoclonal antibodies, ErbB4 receptor protein was detected in the somato-dendritic region of distinct GABAergic neuronal subtypes in the hippocampus (Fisahn et al. 2009; Yau et al. 2003) and the neocortex of rodents and primates (Neddens et al. 2011; Neddens and Buonanno 2011). Of importance, ErbB4 is expressed in approximately 50% hippocampal (Fisahn et al. 2009) and nearly all neocortical PV+ fast-spiking GABAergic interneurons (Neddens and Buonanno 2011). The receptor is also expressed in cholecystokinin (CCK)-positive GABAergic basket cells, another interneuron subtype that contributes to gamma oscillations (Tukker et al. 2007).
Soma targeting basket and axoaxonic AIS targeting chandelier GABAergic interneurons, respectively, can regulate E/I balance and neuronal network activity by regulating the excitability and firing frequency of glutamatergic pyramidal neurons. In contrast to the consistent observation that ErbB4 is expressed in the somatodendritic compartment of interneurons, its presence at presynaptic GABAergic terminals has been more controversial. Activation of ErbB4 by exogenously added NRG1 was reported to promote depolarization-dependent release of GABA purportedly by activation of presynaptic ErbB4 receptors on terminals of basket cells innervating prefrontal cortical pyramidal neurons (Wen et al. 2010); a similar conclusion was reached after analysis of mini inhibitory postsynaptic potentials (mIPSCs) from chandelier GABAergic neurons onto pyramidal neocortical neurons (Fazzari et al. 2010). However, as discussed in more detail below, studies using two highly characterized monoclonal antibodies raised against the extracellular and intracellular domains of ErbB4 (Vullhorst et al. 2009) failed to detect immunoreactivity for the receptor at GABAergic presynaptic terminals innervating either the soma or axon initial segment of pyramidal neurons in the hippocampus or frontal cortex of rodents and primates (Neddens et al. 2011; Neddens and Buonanno 2011). The reasons for these disparate findings may result from the use of commercial polyclonal antisera (Wen et al. 2010; Fazzari et al. 2010) vs. non-commercial monoclonal antibodies (Vullhorst et al. 2009; Neddens and Buonanno 2011; Neddens and Buonanno 2009) or possibly from fixation and unmasking techniques that expose limited amounts of epitope.
An important observation that potentially associates schizophrenia at-risk genes with altered network activity, is the recent demonstration that perfusion of acute hippocampal slices with 1nM NRG1 dramatically increases the power (amplitude) of kainate-induced gamma oscillations (Fisahn et al. 2009). This effect of is blocked by the pan-specific ErbB receptor antagonist PD158780 and totally absent in acute slices prepared from ErbB4 knockout mice. Of importance, the endogenous power of kainate-induced gamma oscillations in slices prepared from ErbB4 null mice was reduced by 60% and coincided with a loss of approximately 50% of hippocampal PV+ GABAergic interneurons (Fisahn et al. 2009).
While some have focused on the potential role of presynaptic ErbB4 receptors on PV+ GABAergic interneurons for regulation of network activity and behavior (Wen et al. 2010; Fazzari et al. 2010), based on studies using monoclonal ErbB4 antibodies and electron microscopy analysis, a hypothesis favored by others (Buonanno 2010; Neddens and Buonanno 2009) is that postsynaptic ErbB4 receptors expressed on dendrites of PV+ GABAergic interneurons, which receive glutamatergic inputs and exhibit the highest levels of ErbB4 immunoreactivity, are a major site for modulation of E/I balance and neuronal network activity. Consistent with the latter hypothesis, targeted ablation of either the AMPA receptor GluR1 or GluR4 subunit at glutamatergic postsynaptic sites of GABAergic interneurons results in the reduction of kainite-induced gamma oscillation power (Fuchs et al. 2007) that are similar to those in ErbB4 null mice (Fisahn et al. 2009). In addition, ablation of the obligatory NMDA receptor NR1 subunit at glutamatergic synapses in approximately 50% of cortical and hippocampal GABAergic interneurons reduces neuronal synchrony and elicits “schizophrenia-like” behaviors in mutant mice (Belforte et al. 2010). Interestingly, mice with either full mutation of ErbB4 or targeted ablation in PV+ interneurons exhibit several behavioral deficits associated with rodent models for schizophrenia (Wen et al. 2010; Shamir et al. 2012). Therefore, regulation of glutamatergic transmission at excitatory synapses on inhibitory neurons is a major site for modulation of neuronal network activity and, as discussed below, NRG/ErbB4 signaling may be a major regulator at glutamatergic synapses driving GABAergic basket cells.
The C-terminal tail of ErbB4 interacts directly with the MAGUK family of postsynaptic proteins including PSD-95 (Garcia et al. 2000; Huang et al. 2000) and accumulates at synaptic puncta on inhibitory neurons (Vullhorst et al. 2009; Fisahn et al. 2009; Longart et al. 2007). Ultrastructural analysis in CA1 interneurons using immunoelectron microscopy revealed abundant ErbB4 expression at, and adjacent to, glutamatergic postsynaptic sites (Vullhorst et al. 2009). By contrast, there is no evidence for presynaptic expression in cultured GAD67-positive hippocampal interneurons and in CA1 basket cell terminals (Vullhorst et al. 2009; Fisahn et al. 2009; Longart et al. 2007). The localization of ErbB4 at excitatory synapses on GABAergic neurons, but not excitatory neurons, identifies these synapses as a primary target of NRG signaling in the hippocampus and indicates that ErbB4 serves as a selective marker for PSDs on GABAergic neurons (Vullhorst et al. 2009). Taken together, these findings strongly support a role for postsynaptic somatodendritic ErbB4 receptors on PV+ GABAergic interneurons in modulating glutamatergic drive onto these cells for regulating gamma oscillation power and “schizophrenia-like” behaviors observed in ErbB4 mutant mice (Buonanno 2010; Vullhorst et al. 2009; Neddens and Buonanno 2009).
Because ErbB4 is expressed in the somatodendritic region of GABAergic interneurons, it was important to investigate if receptor activation acutely regulates the intrinsic excitability and firing properties of ErbB4-positive interneurons. Interneuron output is shaped by the modulation of action potential (AP) waveform and firing rates. Voltage-gated potassium (Kv) and sodium (Nav) channels modulate several aspects of neuronal excitability including AP waveform, duration and firing frequency (Lawrence et al. 2006; Yu et al. 2006; Bean 2007; Milescu et al. 2010). Therefore, regulation of these currents affects AP threshold and neuronal excitability (Matzner and Devor 1992) that can modulate E/I balance and network activity. Two recent studies reported on the effects of NRG/ErbB4 signaling on the intrinsic properties of identified GABAergic interneurons in acute cortical slices from adult mice (Li et al. 2012) and in dissociated hippocampal cultured neurons (Janssen et al. 2012). Li et al. found that acute NRG1 application in cortical slices increased the intrinsic excitability of PV+ interneurons, presumably ErbB4-positive neurons because most cortical PV neurons express the receptor (see (Neddens and Buonanno 2011)), by decreasing AP threshold via Kv1.1 channel blockade; effects on Nav currents were not analyzed (Li et al. 2012). On the other hand, by recording from pharmacologically isolated and labeled ErbB4-positive interneurons in dissociated hippocampal cultures, Janssen et al. demonstrated that NRG1 reduces the excitability of dissociated ErbB4+ interneurons, depolarizes the AP threshold, and decreases maximum Nav channel somatic current. These effects observed only in GABAergic ErbB4-expressing neurons, but not glutamatergic neurons, were totally blocked by the ErbB blocker PD158780. In these experimental conditions no effects of acute NRG1 treatment were observed on macroscopic K+ current or AP duration (Janssen et al. 2012). The apparent discrepancies between these studies may be due to several experimental differences, such as: a) the study by Li et al. was conducted in slices where NRG1-mediated release of dopamine (see below; (Kwon et al. 2008)) or other neuromodulators may affect Kv channels and increase excitability (Govindaiah et al. 2010), while the study by Janssen et al. used dissociated hippocampal cultures that are devoid of afferents; b) The study by Li et al. was restricted to PV+ neurons, whereas the other study recorded from identified ErbB4-positive interneurons that encompass a heterogeneous population of GABAergic neurons (Neddens and Buonanno 2009); and c) the two studies used interneurons of different ages and from distinct brain structures. Additional studies will be necessary to determine if the acute effects of NRG1 on increasing adult interneuron excitability by reducing Kv1.1 result from an intrinsic effect of ErbB4 activation in PV+ GABAergic neurons or from indirect effects of NRG1 produced by augmenting extracellular dopamine (Kwon et al. 2008) and activating D4Rs on this interneuron population (see below, (Andersson et al. 2012)).
Dopamine effects are mediated by D1-type (D1R and D5R) and D2-type (D2R, D3R and D4R) receptors that are positively and negatively coupled to adenylate cyclase, respectively. Most antipsychotics used to date target D2-types receptors. Because mice with reduced levels of NRG1, ErbB4 and NMDA receptor subunits share several “schizophrenia-like” behavioral abnormalities that are reversed or ameliorated by the antipsychotic clozapine (Stefansson et al. 2002; Shamir et al. 2012; Mohn et al. 1999), a possible functional link between NRG1/ErbB signaling and dopamine neurotransmission was investigated. Delivery of NRG1 by reverse-microdialysis in freely moving rats causes a dramatic and rapid accumulation of dopamine and its metabolites in the dorsal hippocampus, and this increase is blocked by PD158780 (Kwon et al. 2008). NRG1-induced increases in dopamine can reverse synaptic potentiation in the hippocampus through activation of D4Rs. Consistent with these findings, the effects of NRG1 on synaptic potentiation are blocked by the D4R-specific antagonist (PD168077) and clozapine, an antipsychotic that preferentially targets D4Rs, and are absent in D4R knockout mice (Kwon et al. 2008). While ErbB4 transcripts are expressed in the ventral tegmental area (Steiner et al. 1999; Gerecke et al. 2001), which sends afferent dopaminergic projections to the hippocampus (Gasbarri et al. 1994; Gasbarri et al. 1997), ErbB4 immunoreactivity was undetectable on dopaminergic axons (Kwon et al. 2008) but can be detected on the cell bodies of mesocortical- and nigrostriatal-projecting TH-positive neurons (Neddens and Buonanno, unpublished). Therefore, the possibility that ErbB4 is present at low levels on dopaminergic axons cannot be excluded presently; alternatively, NRG1 could promote dopamine release in the hippocampus by acting indirectly via GABAergic neurons.
Surprisingly little is known about the effects of dopamine on gamma oscillation activity in the hippocampus and PFC, although it has long been appreciated that dopamine modulates attention, cognitive salience and working memory (Winterer and Weinberger 2004), and that its levels are altered in schizophrenia (see Furth et al., under review). The rodent frontal cortex and hippocampus receive sparse dopaminergic innervation from the VTA that regulates synaptic transmission, plasticity and working memory (Lisman et al. 2008; Andersson et al. 2012; Jay 2003; Lisman and Grace 2005). Based on the aforementioned studies showing that NRG/ErbB4 signaling increases extracellular DA levels and regulates hippocampal synaptic plasticity via D4Rs (Kwon et al. 2008), and that targeted ablation of ErbB4 in PV+ GABAergic interneurons results in “schizophrenia like” behaviors similar to those observed in null ErbB4 mice (Shamir et al. 2012), the effects of dopamine on kainate-induced gamma oscillations in hippocampal slices were investigated (Andersson et al. 2012).
Interestingly, the selective activation of D4Rs, but not of D1/D5Rs and D2/D3Rs, increases gamma oscillation power, and this effect is blocked by a highly specific D4R antagonist (L-745,870). Consistent with the effects of D4R on gamma rhythms, receptor mRNA and protein are expressed in GAD67-positive GABAergic interneurons, but not in glutamatergic hippocampal neurons, and dopamine D4 and ErbB4 receptors are coexpressed in 71% of PV+ basket cells. Of importance, we found that D4R activation is essential for the effects of NRG-1 on gamma oscillation power as the selective D4R anatagonist, as wells as the D4R-preferring atypical antipsychotic clozapine, dramatically reduced the NRG-1-induced increase in gamma oscillation power (Andersson et al. 2012). This study provides a novel link between D4R and ErbB4 signaling on gamma oscillation power, and the coexpression of both receptors in PV+ GABAergic basket cells, suggests a cellular mechanism that may be compromised in different psychiatric disorders affecting cognitive control. These findings suggest potential benefits of D4R modulators for targeting cognitive deficits as the 7-repeat D4R functional variant in humans (DRD4-7R) is associated with alterations in attention, working memory and gamma band activity (Demiralp et al. 2007) and with ADHD (DiMaio et al. 2003).
In summary, the colocalization of D4R and ErbB4 receptors on PV+ interneurons, activity of which is critically important for regulating E/I balance and cognitive functions (Yizhar et al. 2011), perhaps by optimizing “signal-to-noise” ratio of cortical microcircuits (see (Winterer and Weinberger 2004) and Furth et al., in review), makes these receptor systems attractive novel targets for modulating network activities that underlie cognition. These studies identify PV+ GABAergic interneurons as a potential novel cellular target for modulating gamma oscillations and related cognitive functions deficient in psychiatric disorders, and suggest that NRG/ErbB4 and dopamine signaling pathways (and potentially other schizophrenia liability genes) may converge in these cells to modulate the activity of microcircuits altered in psychiatric disorders.
Psychosis in Alzheimer Disease and Excess Vulnerability of Cerebral Cortical Synapses
Synapse pathology in AD has been extensively reviewed in the recent literature. However, psychosis occurring in AD has received much less attention. In a review of 55 studies comprising of 9,749 subjects, the median prevalence of psychosis in subjects with AD (AD+Psychosis, AD+P) was 41% (Ropacki and Jeste 2005). Interestingly, AD+P patients consistently showed faster cognitive decline compared to AD without psychosis (AD-P) patients (Ropacki and Jeste 2005). Nine of nine studies found a significant association between a greater rate of cognitive decline and the presence of AD+P. Recent studies have continued to support the relationship between more rapid cognitive decline and AD+P (Emanuel et al. 2011; Sweet et al. 2012).
Several lines of evidence indicate that psychosis in AD has a specific neurobiology. Perhaps the most compelling is evidence that AD+P risk is transmitted in families (Sweet et al. 2002a), now replicated in two additional cohorts (Hollingworth et al. 2007; Sweet et al. 2010). The heritability of psychosis within AD is estimated as 61% (Bacanu et al. 2005). There are two important implications of these findings. First, and most direct, is that the risk for AD+P is likely to be influenced by genetic variation. Second, is that AD+P results from a distinctive underlying neurobiology. That is, AD+P cannot be seen as arising solely as a non-specific consequence of AD progression. Nor can it arise solely due to a serendipitous accumulation of neurodegenerative lesions in vulnerable “psychosis” brain regions. AD+P has a genetic architecture most consistent with disease modification rather than an independent syndrome. That is, genes which increase the risk for AD itself are found equivalently in AD+P and AD without psychosis, while additional genetic variants which predispose to psychosis are present in AD+P, with some limited overlap of these variants with other psychoses. For example, there is strong evidence against an association of AD+P (in comparison to AD without psychosis) and APOE and TOMM40 (DeMichele-Sweet et al. 2011b; Hollingworth et al. 2011; Chu et al. 2011). Similarly AD+P is not associated with variation in recently identified AD risk genes CLU, PICALM, CR1, BIN1, ABCA7, MS4A, CD2AP, CD33 and EPHA1 (Hollingworth et al. 2011) nor with other genes that may contribute to neurodegeneration risk: APP, BACE1, SORL1, and MAPT (DeMichele-Sweet et al. 2011a). In contrast, the first GWAS of AD+P was recently completed, which identified VSNL1 (the gene encoding Vilip1), and other novel loci (e.g. STK11, RIMBP2) (Hollingworth et al. 2011). This study also, albeit to a lesser extent, found evidence for association of AD+P with a group of SNPs that appear to contribute to risk for schizophrenia (Hollingworth et al. 2011).
It has been appreciated for a number of years that the strongest correlate of cognitive impairment in individuals with AD is loss of synapses across neocortical regions (Terry et al. 1991; Scheff and Price 2003), with excitatory synapses onto dendritic spines particularly affected (Baloyannis et al. 2007; Grutzendler et al. 2007). This has led to the hypothesis that the more rapid cognitive deterioration seen in AD+P reflects greater vulnerability of neocortical synapses than in AD-P (Sweet et al. 2002b). Evidence in support of this hypothesis is described below.
In vivo neuroimaging studies of individuals with AD indicate there is increased disruption of neocortical gray matter is subjects with psychosis. In contrast, findings are largely negative with regard to medial temporal lobe (hippocampal formation) differences between AD+P and AD-P subjects. Delusions were associated with decreased gray matter density in the left frontal lobe and in the right frontoparietal cortex (Bruen et al. 2008). Single photon emission computed tomography (SPECT) studies of regional perfusion in AD+P (in comparison to AD-P) have found lower regional perfusion in bilateral dorsolateral prefrontal cortex (DLPFC), (Mega et al. 2000) left anterior cingulated gyrus (Mega et al. 2000), right frontal (Moran et al. 2008), left frontal (Kotrla et al. 1995), right temporal (Moran et al. 2008), and bilateral temporal cortices (Starkstein et al. 1994). Studies of brain metabolism using 18F-fluorodexoxyglucose imaging have provided further evidence for frontal cortex abnormalities in AD+P, including a relationship between severity of delusions and reduced cerebral metabolism in right DLPFC, right inferior frontal pole, and right lateral orbitofrontal cortex (Sultzer et al. 2003; Mentis et al. 1995).
Using magnetic resonance spectroscopy to examine postmortem brain tissue sample from AD+P and AD-P subjects, significant elevations in concentrations of the phosphodiester membrane breakdown product, glycerol-phosphoethanolamine have been reported (Sweet et al. 2002b). Elevations were present across the neocortex, with DLPFC, superior temporal gyrus (STG), and inferior parietal cortex (IPC) most affected. In contrast, medial temporal cortex (amygdala) and cerebellum were unaffected (Sweet et al. 2002b). These changes were interepreted as evidence of excess synaptic disruption in AD+P, in a pattern consistent with generalized neocortical involvement.
More recently, the dendritic spine associated protein, kalirin, was examined in post-mortem gray matter protein extracts from the dorsolateral prefrontal cortex of subjects with AD+P and AD-P. Kalirin is found in adult human cerebral cortex gray matter as one of four predominant isoforms, kalirin-5, -7, -9, and -12 (Deo et al. 2011). Significant reductions in levels of kalirin-7, -9, and -12 were found in AD+P (Murray et al. 2012). In contrast, levels of the kalirin-5 isoform were unchanged (Murray et al. 2012). Because all four isoforms are found in post-synaptic density fractions (Deo et al. 2011), the absence of reduction in kalirin-5 suggest that the reductions in kalirin-7, -9, and -12 are better interpreted as evidence of synaptic dysfunction than of frank synapse loss (Murray et al. 2012).
Substantial evidence now indicates that aggregation of Aβ into soluble oligomers (dimers→protofibrils) is a primary source of synaptotoxicity in Alzheimer's disease (Selkoe 2002; Selkoe 2008; Walsh and Selkoe 2007; Koffie et al. 2011). This includes evidence from human postmortem tissue that cortical synapse loss is an early pathologic event and that cognitive impairments and synapse loss correlate most strongly with soluble Aβ (Lue et al. 1999), even in subjects with early disease (Naslund et al. 2000).
Studies of the synaptic effects of Aβ are further elucidating a model of how Aβ acts to eliminate dendritic spines. Though reductionistic, the predominant model proposes that Aβ shifts the balance within spines from LTP→LTD, through mechanisms including reduced NMDA receptor dependent Ca++ influx, mGluR activation, and low level caspase-3 activation (Selkoe 2008; Koffie et al. 2011). The final common mechanism for these pathways converge on altered endocytotic recycling of glutamate receptors, resulting in reduced synaptic expression of GluR1 and GluR2 containing AMPA receptors and synaptic NMDAR (Hsieh et al. 2006). The net result of these effects is loss of dendritic spines (Hsieh et al. 2006).
Despite these advances, unresolved questions persist. For example, it is clear that shifting the balance from LTP→LTD leads to net removal of GluR and NMDAR from synapses. However, it is not resolved whether signaling through specific NMDAR subtypes (i.e. NMDAR2A vs NMDAR2B), or signaling through NMDAR in specific locations (synaptic versus extrasynaptic) are associated uniquely with generation of LTP versus of LTD (Fetterolf and Foster 2011). Similarly, although studies clearly indicate phosphoTau is a necessary downstream mediator of Aβ impairments of LTP (Shipton et al. 2011), its dendritic mechanisms are just emerging (Zempel et al. 2010), leaving unclear how it may interact with other LTP and LTD mediators impacted by soluble Aβ. The exact species of soluble Aβ oligomers most relevant for spine toxicity is also not known as species from dimers to protofibrils are typically present in varying degrees. Similarly, soluble Aβ oligomers exist in equilibrium with fibrillar deposits of Aβ in plaques, and thus plaques may serve as reservoirs of soluble Aβ (Walsh and Selkoe 2007; Koffie et al. 2009). Plaques may themselves contribute to spine toxicity through a mechanism that differs from soluble Aβ (see(Bittner et al. 2010)) and serve as a site for inflammatory responses (Spires-Jones et al. 2007). How inflammation contributes to synapse loss is itself an emerging field (Rosen and Stevens 2010).
For AD+P, then, the important residual question is whether the greater cortical synaptic disruption in these subjects reflects enhanced Aβ drive, an increased response of downstream mediators of Aβ-induced synaptotoxicity, or additional independent synaptotoxic pathologies. There is no consistent evidence that AD+P is associated with a higher burden of neocortical deposition of fibrillar Aβ (2000;2000). Similarly, AD+P was not associated with increased neocortical concentrations of soluble Aβ1-42, and the concentrations of soluble Aβ1-40 were reduced in AD+P (Murray et al. 2012). However, the ratio of Aβ1-40 : Aβ1-42 was elevated in the dorsolateral prefrontal cortex in AD+P subjects (Murray et al. 2012). Because Aβ1-40 can inhibit the oligomerization of Aβ1-42 into more toxic species by sequestering it into stable mixed tetramers (Murray et al. 2009), it remains possible there is heightened Aβ drive in AD+P.
In contrast, there is substantial evidence for an association of AD+P with at least one mediator of Aβ effects, neocortical phospho-tau pathology. Neurofibrillary tangle density is increased in AD+P in dorsolateral prefrontal, superior temporal, and inferior parietal cortex, but not in medial temporal lobe. Similarly, increased aggregated tau protein has been found in cortical gray matter extracts from subjects with AD+P (Rakic et al. 1986). More recently, measures of spread of phospho-tau pathology and phospho-tau concentration were evaluated in within dorsolateral prefrontal cortex from subjects with AD+P and AD-P. While only phospho-tau concentration differed significantly between groups, both measures were correlated with degree of cognitive impairment, suggesting they also contribute to greater synapse loss in these subjects (Murray et al., AJGP, abstract).
There is evidence for reduced expression of multiple kalirin isoforms in AD-P, with further reductions in AD+P (Murray et al. 2012). Kalirin plays an integral role in dendritic spine growth, morphogenesis, and activity-dependent plasticity (Cahill et al. 2009; Xie et al. 2007). Importantly, kalirin reduction is known to deplete PSD GluR1, NMDAR2B, and cause spine loss (Xie et al. 2007; Ma et al. 2008), and conversely, increased expression of kalirin-7 and -9 increases spine density (Deo et al. 2011; Ma et al. 2008). An important downstream target of kalirin signaling, p21-activated kinase (PAK), shows reduced activation in response to Aβ in vitro and in vivo, and contributes to dendritic spine loss (Zhao et al. 2006). However, whether kalirin reductions are intermediate between Aβ and these effects of PAK, and therefore contribute to excess dendritic spine loss in AD+P, awaits experimental verification.
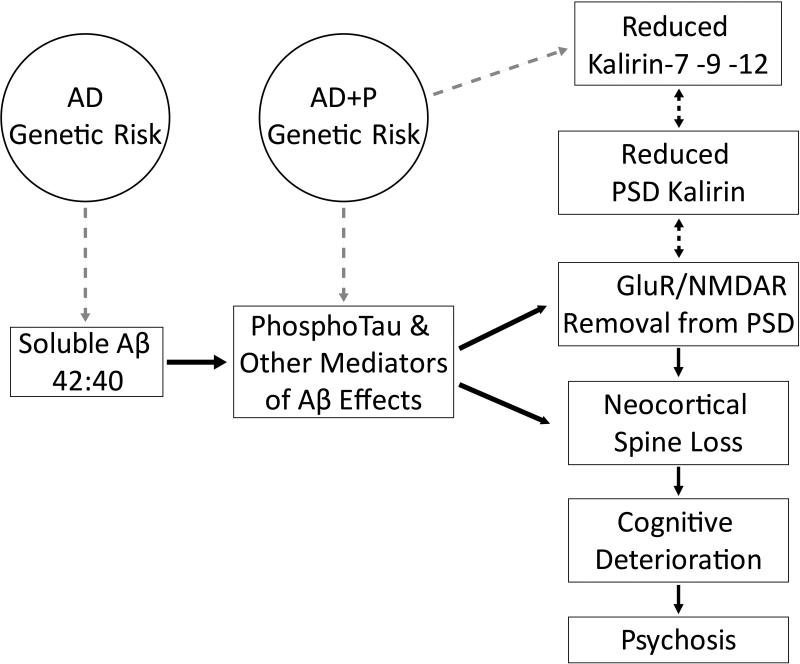
The data reviewed above support a model of AD+P summarized in Figure 3. Importantly the existing imaging and postmortem data suggest that it is neocortex, but not medial temporal cortex, that is most affected in AD+P, with the most consistent findings in the dorsolateral prefrontal cortex. The vulnerability to AD+P, due to underlying genetic factors, may affect the cascade of pathology in AD in any of several ways. The net result of these effects is enhanced drive of the pathologic cascade, increasing pTau, and leading to reductions in kalirin, removal of post-synaptic GluR and NMDAR, and spine loss. These effects are manifest as a greater rate of cognitive deterioration with subsequent emergence of psychotic symptoms.
Figure 3. Summary diagram of synaptic vulnerability in Alzheimer Disease with Psychosis (AD+P).
Effects for which there is existing evidence are shown as unidirectional solid black arrows. Gray arrows indicate hypothesized effects.
Conclusions
Disorders such as ID, ASD, SZ, and AD+P have complex etiologies with heterogeneous symptomatology. An interesting observation that has recently emerged is that a significant overlap exists in the genetic etiology of these disorders. Based on such findings it has been hypothesized that disorders historically considered distinct might share at least partially overlapping pathogenic mechanisms, and differential manifestations of alterations in shared cellular substrates might underlie the phenotypic variability (Burbach and van der Zwaag 2009; Girirajan and Eichler 2010; O'Roak and State 2008; Penzes et al. 2011; Poot et al. 2011). However, there is also a significant phenotypic divergence between these disorders, most notably in the ages of onset spanning infancy, early childhood, adolescence and senescence. How overlapping genetics may lead to such diverse manifestations remains to be identified, although could be conceptualized as resulting from progressively less severe molecular alterations, such that developmental onset is in some cases delayed (e.g. schizophrenia), or requires the presence of an additional neuronal pathology before being manifest (e.g. AD+P). Consistent with this idea, the divergence among these syndromes is already apparent at the cellular level, manifested by differential alterations in dendrites and spines (Penzes et al. 2011; van Spronsen and Hoogenraad 2010). Such findings highlight the importance of finding common final molecular and cellular mechanisms, as well as mechanisms of molecular convergence and divergence. Recent breakthroughs in the characterization of regulators of synaptic circuits have provided opportunities to identify molecular changes that could directly contribute to pathology. The molecular networks that control spines provide a framework for understanding how a large number of genetic perturbations can interact to disrupt synaptic function, neuronal circuit organization, and behavioral output in a disease-specific manner. As the pace of discovery in psychiatric genetics is very rapid, elucidating the functions of newly identified disease-associated genes within synaptic molecular networks is a key step to translating genetic findings into biological understanding and clinical applications. Understanding the causal links between synaptic pathology and disease phenotypes, and the identification of novel drug targets based on these links, will also facilitate the development of effective treatments for these disorders.
Table 1.
Summary of important genes associated with X-linked intellectual disabilities.
| Protein name | Chromosome | Protein function | Phatology |
|---|---|---|---|
| Shank | 22 | scaffold | Phelan-McDremid Syndrome, autism |
| IL1RAPL1 | X | synaptic adhesion | non syndromic XLID |
| Oligophrenin-1 | X | RhoGAP | syndromic XLID |
| TSPAN7 | X | tetraspanin | non syndromic XLID |
Table 2.
Summary of genes discussed in this review associated with schizophrenia.
| Protein name | Chromosome | Protein function |
|---|---|---|
| NRG1 | 8 | neuronal growth and differentiation |
| ErbB4 | 2 | receptor tyrosine kinase |
| DISC1 | 1 | scaffold |
| ZDHHC8 (22q11.2) | 22 | palmitoyl transferase |
| Dgcr8 (22q11.2) | 22 | miRNA processing |
Acknowledgements
This work was supported by NIH grants MH071316 and MH097216 to P.P., Eunice Shriver Kennedy National Institute of Child Health and Human Development Intramural Research Program to A. B., Veterans Health Administration Grant BX000452 and NIH Grants MH071533 and AG027224 to R.A.S. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Veterans Affairs, the National Institutes of Health, or the United States government. We thank Ruoqi Gao for assistance with editing the manuscript. This work was financially supported by Comitato Telethon Fondazione Onlus, grant n. GGP09196 and GGP11095 (to CS), n. GGP12097 and GGP11116B (to MP) Fondazione CARIPLO project number 2009.264, Italian Institute of Technology, Seed Grant, Ministry of Health in the frame of ERA-NET NEURON and PNR-CNR Aging Program 2012-2014 (to CS).
Footnotes
The authors declare no conflicts of interest.
LITERATURE CITED
- Abidi FE, Holinski-Feder E, Rittinger O, Kooy F, Lubs HA, Stevenson RE, Schwartz CE. A novel 2 bp deletion in the TM4SF2 gene is associated with MRX58. J Med Genet. 2002;39:430–433. doi: 10.1136/jmg.39.6.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarian S, Huang HS. Molecular and cellular mechanisms of altered GAD1/GAD67 expression in schizophrenia and related disorders. Brain Res Rev. 2006;52(2):293–304. doi: 10.1016/j.brainresrev.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Akbarian S, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52(4):258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annual Review of Neuroscience. 2007;30:79–97. doi: 10.1146/annurev.neuro.30.051606.094222. [DOI] [PubMed] [Google Scholar]
- Andersson RH, et al. Neuregulin and dopamine modulation of hippocampal gamma oscillations is dependent on dopamine D4 receptors. Proc Natl Acad Sci U S A. 2012;109(32):13118–13123. doi: 10.1073/pnas.1201011109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arango C, et al. Longitudinal brain changes in early-onset psychosis. Schizophr Bull. 2008;34(2):341–353. doi: 10.1093/schbul/sbm157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayhan Y, et al. Differential effects of prenatal and postnatal expressions of mutant human DISC1 on neurobehavioral phenotypes in transgenic mice: evidence for neurodevelopmental origin of major psychiatric disorders. Mol Psychiatry. 2011;16(3):293–306. doi: 10.1038/mp.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacanu SA, Devlin B, Chowdari KV, DeKosky ST, Nimgaonkar VL, Sweet RA. Heritability of psychosis in Alzheimer disease. Am J Geriatr Psychiatry. 2005;13:624–627. doi: 10.1176/appi.ajgp.13.7.624. [DOI] [PubMed] [Google Scholar]
- Baloyannis SJ, Costa V, Mauroudis I, Psaroulis D, Manolides SL, Manolides LS. Dendritic and spinal pathology in the acoustic cortex in Alzheimer's disease: morphological and morphometric estimation by Golgi technique and electron microscopy. Acta Otolaryngol. 2007;127:351–354. doi: 10.1080/00016480601126986. [DOI] [PubMed] [Google Scholar]
- Barros CS, et al. Impaired maturation of dendritic spines without disorganization of cortical cell layers in mice lacking NRG1/ErbB signaling in the central nervous system. Proc Natl Acad Sci U S A. 2009;106(11):4507–4512. doi: 10.1073/pnas.0900355106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barta PE, Pearlson GD, Powers RE, Richards SS, Tune LE. Auditory hallucinations and smaller superior temporal gyral volume in schizophrenia. Am J Psychiatry. 1990;147:1457–1462. doi: 10.1176/ajp.147.11.1457. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8(1):45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Bassani S, Cingolani LA, Valnegri P, Folci A, Zapata J, Gianfelice A, Sala C, Goda Y, Passafaro M. The X-Linked Intellectual Disability Protein TSPAN7 Regulates Excitatory Synapse Development and AMPAR Trafficking. Neuron. 2012;73:1143–1158. doi: 10.1016/j.neuron.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci. 2007;8(6):451–465. doi: 10.1038/nrn2148. [DOI] [PubMed] [Google Scholar]
- Belforte JE, et al. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13(1):76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron R, Coyle JT. NAAG, NMDA receptor and psychosis. Current Medicinal Chemistry. 2012;19(9):1360–1364. doi: 10.2174/092986712799462685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkel S, Marshall CR, Weiss B, Howe J, Roeth R, Moog U, Endris V, Roberts W, Szatmari P, Pinto D, Bonin M, Riess A, Engels H, Sprengel R, Scherer SW, Rappold GA. Mutations in the SHANK2 synaptic scaffolding gene in autism spectrum disorder and mental retardation. Nat Genet. 2010;42:489–491. doi: 10.1038/ng.589. [DOI] [PubMed] [Google Scholar]
- Bhat S, et al. CACNA1C (Ca(v)1.2) in the pathophysiology of psychiatric disease. Prog Neurobiol. 2012;99(1):1–14. doi: 10.1016/j.pneurobio.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat SS, Ladd S, Grass F, Spence JE, Brasington CK, Simensen RJ, Schwartz CE, Dupont BR, Stevenson RE, Srivastava AK. Disruption of the IL1RAPL1 gene associated with a pericentromeric inversion of the X chromosome in a patient with mental retardation and autism. Clin Genet. 2008;73:94–96. doi: 10.1111/j.1399-0004.2007.00920.x. [DOI] [PubMed] [Google Scholar]
- Bittner T, Fuhrmann M, Burgold S, Ochs SM, Hoffmann N, Mitteregger G, Kretzschmar H, LaFerla FM, Herms J. Multiple events lead to dendritic spine loss in triple transgenic Alzheimer's disease mice. PLoS One. 2010;5:e15477. doi: 10.1371/journal.pone.0015477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockers TM, Segger-Junius M, Iglauer P, Bockmann J, Gundelfinger ED, Kreutz MR, Richter D, Kindler S, Kreienkamp HJ. Differential expression and dendritic transcript localization of Shank family members: identification of a dendritic targeting element in the 3′ untranslated region of Shank1 mRNA. Mol Cell Neurosci. 2004;26:182–190. doi: 10.1016/j.mcn.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Bonaglia MC, Giorda R, Borgatti R, Felisari G, Gagliardi C, Selicorni A, Zuffardi O. Disruption of the ProSAP2 gene in a t(12;22)(q24.1;q13.3) is associated with the 22q13.3 deletion syndrome. Am J Hum Genet. 2001;69:261–268. doi: 10.1086/321293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdagi O, Sakurai T, Papapetrou D, Wang X, Dickstein DL, Takahashi N, Kajiwara Y, Yang M, Katz AM, Scattoni ML, Harris MJ, Saxena R, Silverman JL, Crawley JN, Zhou Q, Hof PR, Buxbaum JD. Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol Autism. 2010;1:15. doi: 10.1186/2040-2392-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruen PD, McGeown WJ, Shanks MF, Venneri A. Neuroanatomical correlates of neuropsychiatric symptoms in Alzheimer's disease. Brain. 2008;131:2455–2463. doi: 10.1093/brain/awn151. [DOI] [PubMed] [Google Scholar]
- Buonanno A. The neuregulin signaling pathway and schizophrenia: from genes to synapses and neural circuits. Brain Res Bull. 2010;83(3-4):122–131. doi: 10.1016/j.brainresbull.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonanno A, Fischbach GD. Neuregulin and ErbB receptor signaling pathways in the nervous system. Curr Opin Neurobiol. 2001;11(3):287–296. doi: 10.1016/s0959-4388(00)00210-5. [DOI] [PubMed] [Google Scholar]
- Burbach JP, van der Zwaag B. Contact in the genetics of autism and schizophrenia. Trends Neurosci. 2009;32(2):69–72. doi: 10.1016/j.tins.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Cahill ME, Xie Z, Day M, Photowala H, Barbolina MV, Miller CA, Weiss C, Radulovic J, Sweatt JD, Disterhoft JF, Surmeier DJ, Penzes P. Kalirin regulates cortical spine morphogenesis and disease-related behavioral phenotypes. Proc Natl Acad Sci U S A. 2009;106:13058–13063. doi: 10.1073/pnas.0904636106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrie A, Jun L, Bienvenu T, Vinet MC, McDonell N, Couvert P, Zemni R, Cardona A, Van Buggenhout G, Frints S, Hamel B, Moraine C, Ropers HH, Strom T, Howell GR, Whittaker A, Ross MT, Kahn A, Fryns JP, Beldjord C, Marynen P, Chelly J. A new member of the IL-1 receptor family highly expressed in hippocampus and involved in X-linked mental retardation. Nat Genet. 1999;23:25–31. doi: 10.1038/12623. [DOI] [PubMed] [Google Scholar]
- Casamassima F, et al. L-type calcium channels and psychiatric disorders: A brief review. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(8):1373–1390. doi: 10.1002/ajmg.b.31122. [DOI] [PubMed] [Google Scholar]
- Chattopadhyaya B, Cristo GD. GABAergic circuit dysfunctions in neurodevelopmental disorders. Front Psychiatry. 2012;3:51. doi: 10.3389/fpsyt.2012.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Hancock ML, Role LW, Talmage DA. Intramembranous valine linked to schizophrenia is required for neuregulin 1 regulation of the morphological development of cortical neurons. J Neurosci. 2010;30(27):9199–9208. doi: 10.1523/JNEUROSCI.0605-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YJ, et al. Type III neuregulin-1 is required for normal sensorimotor gating, memory-related behaviors, and corticostriatal circuit components. J Neurosci. 2008;28:6872–6883. doi: 10.1523/JNEUROSCI.1815-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu SH, Roeder K, Ferrell RE, Devlin B, DeMichele-Sweet MA, Kamboh MI, Lopez OL, Sweet RA. TOMM40 poly-T repeat lengths, age of onset and psychosis risk in Alzheimer disease. Neurobiol Aging. 2011;32:2328–2329. doi: 10.1016/j.neurobiolaging.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378(6552):75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- Collins AL, Ma D, Whitehead PL, Martin ER, Wright HH, Abramson RK, Hussman JP, Haines JL, Cuccaro ML, Gilbert JR, Pericak-Vance MA. Investigation of autism and GABA receptor subunit genes in multiple ethnic groups. Neurogenetics. 2006;7(3):167–174. doi: 10.1007/s10048-006-0045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean B, Keriakous D, Scarr E, Thomas EA. Gene expression profiling in Brodmann's area 46 from subjects with schizophrenia. Aust N Z J Psychiatry. 2007;41:308–320. doi: 10.1080/00048670701213245. [DOI] [PubMed] [Google Scholar]
- Delahaye A, Toutain A, Aboura A, Dupont C, Tabet AC, Benzacken B, Elion J, Verloes A, Pipiras E, Drunat S. Chromosome 22q13.3 deletion syndrome with a de novo interstitial 22q13.3 cryptic deletion disrupting SHANK3. Eur J Med Genet. 2009;52:328–332. doi: 10.1016/j.ejmg.2009.05.004. [DOI] [PubMed] [Google Scholar]
- DeMichele-Sweet MA, Klei L, Devlin B, Ferrell RE, Weamer EA, Emanuel JE, Lopez OL, Sweet RA. No association of psychosis in Alzheimer disease with neurodegenerative pathway genes. Neurobiol Aging. 2011a;32:555–11. doi: 10.1016/j.neurobiolaging.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMichele-Sweet MA, Lopez OL, Sweet RA. Psychosis in Alzheimer's disease in the national Alzheimer's disease coordinating center uniform data set: clinical correlates and association with apolipoprotein e1. Int J Alzheimers Dis. 2011b;2011:926597. doi: 10.4061/2011/926597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demiralp T, et al. DRD4 and DAT1 Polymorphisms Modulate Human Gamma Band Responses. Cereb. Cortex. 2007;17(5):1007–1019. doi: 10.1093/cercor/bhl011. [DOI] [PubMed] [Google Scholar]
- Deo AJ, Cahill ME, Li S, Goldszer I, Henteleff R, Vanleeuwen JE, Rafalovich I, Gao R, Stachowski EK, Sampson AR, Lewis DA, Penzes P, Sweet RA. Increased expression of Kalirin-9 in the auditory cortex of schizophrenia subjects: Its role in dendritic pathology. Neurobiol. Dis. 2011;45(2):796–803. doi: 10.1016/j.nbd.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMaio S, Grizenko N, Joober R. Dopamine genes and attention-deficit hyperactivity disorder: a review. J Psychiatry Neurosci. 2003;28(1):27–38. [PMC free article] [PubMed] [Google Scholar]
- Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, Fauchereau F, Nygren G, Rastam M, Gillberg IC, Anckarsater H, Sponheim E, Goubran-Botros H, Delorme R, Chabane N, Mouren-Simeoni MC, de Mas P, Bieth E, Roge B, Heron D, Burglen L, Gillberg C, Leboyer M, Bourgeron T. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet. 2007;39(1):25–27. doi: 10.1038/ng1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuel JE, Lopez OL, Houck PR, Becker JT, Weamer EA, DeMichele-Sweet MA, Kuller L, Sweet RA. Trajectory of cognitive decline as a predictor of psychosis in early Alzheimer disease in the cardiovascular health study. Am J Geriatr Psychiatry. 2011;19:160–168. doi: 10.1097/JGP.0b013e3181e446c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Singer W. Temporal binding and the neural correlates of sensory awareness. Trends Cogn Sci. 2001;5(1):16–25. doi: 10.1016/s1364-6613(00)01568-0. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Halt AR, Stary JM, Kanodia R, Schulz SC, Realmuto GR. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biol Psychiatry. 2002;52(8):805–810. doi: 10.1016/s0006-3223(02)01430-0. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Thuras PD. GABA(A) receptor downregulation in brains of subjects with autism. J Autism Dev Disord. 2009;39(2):223–230. doi: 10.1007/s10803-008-0646-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazzari P, et al. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signaling. Nature. 2010;464(7293):1376–1380. doi: 10.1038/nature08928. [DOI] [PubMed] [Google Scholar]
- Fetterolf F, Foster KA. Regulation of long-term plasticity induction by the channel and C-terminal domains of GluN2 subunits. Mol Neurobiol. 2011;44:71–82. doi: 10.1007/s12035-011-8190-4. [DOI] [PubMed] [Google Scholar]
- Fisahn A, Neddens J, Yan L, Buonanno A. Neuregulin-1 modulates hippocampal gamma oscillations: implications for schizophrenia. Cereb Cortex. 2009;19(3):612–618. doi: 10.1093/cercor/bhn107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox IJ, Kornblum HI. Developmental profile of ErbB receptors in murine central nervous system: implications for functional interactions. J Neurosci Res. 2005;79(5):584–597. doi: 10.1002/jnr.20381. [DOI] [PubMed] [Google Scholar]
- Franek KJ, Butler J, Johnson J, Simensen R, Friez MJ, Bartel F, Moss T, DuPont B, Berry K, Bauman M, Skinner C, Stevenson RE, Schwartz CE. Deletion of the immunoglobulin domain of IL1RAPL1 results in nonsyndromic X-linked intellectual disability associated with behavioral problems and mild dysmorphism. Am J Med Genet A. 2011;155A:1109–1114. doi: 10.1002/ajmg.a.33833. [DOI] [PubMed] [Google Scholar]
- Franke B, Neale BM, Faraone SV. Genome-wide association studies in ADHD. Human Genetics. 2009;126(1):13–50. doi: 10.1007/s00439-009-0663-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs EC, et al. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007;53(4):591–604. doi: 10.1016/j.neuron.2007.01.031. [DOI] [PubMed] [Google Scholar]
- Gai X, Xie HM, Perin JC, Takahashi N, Murphy K, Wenocur AS, D'Arcy M, O'Hara RJ, Goldmuntz E, Grice DE, Shaikh TH, Hakonarson H, Buxbaum JD, Elia J, White PS. Rare structural variation of synapse and neurotransmission genes in autism. Mol Psychiatry. 2012;17(4):402–411. doi: 10.1038/mp.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia RA, Vasudevan K, Buonanno A. The neuregulin receptor ErbB-4 interacts with PDZ-containing proteins at neuronal synapses. Proc Natl Acad Sci U S A. 2000;97(7):3596–3601. doi: 10.1073/pnas.070042497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasbarri A, Packard MG, Campana E, Pacitti C. Anterograde and retrograde tracing of projections from the ventral tegmental area to the hippocampal formation in the rat. Brain Res Bull. 1994;33(4):445–452. doi: 10.1016/0361-9230(94)90288-7. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Sulli A, Packard MG. The dopaminergic mesencephalic projections to the hippocampal formation in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21(1):1–22. doi: 10.1016/s0278-5846(96)00157-1. [DOI] [PubMed] [Google Scholar]
- Gauthier J, Spiegelman D, Piton A, Lafreniere RG, Laurent S, St-Onge J, Lapointe L, Hamdan FF, Cossette P, Mottron L, Fombonne E, Joober R, Marineau C, Drapeau P, Rouleau GA. Novel de novo SHANK3 mutation in autistic patients. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:421–424. doi: 10.1002/ajmg.b.30822. [DOI] [PubMed] [Google Scholar]
- Gerecke KM, Wyss JM, Karavanova I, Buonanno A, Carroll SL. ErbB transmembrane tyrosine kinase receptors are differentially expressed throughout the adult rat central nervous system. J Comp Neurol. 2001;433(1):86–100. doi: 10.1002/cne.1127. [DOI] [PubMed] [Google Scholar]
- Gilman SR, Iossifov I, Levy D, Ronemus M, Wigler M, Vitkup D. Rare de novo variants associated with autism implicate a large functional network of genes involved in formation and function of synapses. Neuron. 2011;70(5):898–907. doi: 10.1016/j.neuron.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girirajan S, Eichler EE. Phenotypic variability and genetic susceptibility to genomic disorders. Hum Mol Genet. 2010;19(R2):R176–187. doi: 10.1093/hmg/ddq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57(1):65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Leblanc JJ, Quast KB, Sudhof TC, Fagiolini M, Hensch TK. Common circuit defect of excitatory-inhibitory balance in mouse models of autism. J Neurodev Disord. 2009;1(2):172–181. doi: 10.1007/s11689-009-9023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull. 2008;34(5):944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA. NMDA receptor hypofunction, parvalbumin-positive neurons, and cortical gamma oscillations in schizophrenia. Schizophr Bull. 2012;38(5):950–957. doi: 10.1093/schbul/sbs010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govek EE, Newey SE, Akerman CJ, Cross JR, Van der Veken L, Van Aelst L. The X-linked mental retardation protein oligophrenin-1 is required for dendritic spine morphogenesis. Nat Neurosci. 2004;7:364–372. doi: 10.1038/nn1210. [DOI] [PubMed] [Google Scholar]
- Govindaiah G, Wang Y, Cox CL. Dopamine enhances the excitability of somatosensory thalamocortical neurons. Neuroscience. 2010;170(4):981–991. doi: 10.1016/j.neuroscience.2010.08.043. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, et al. Analysis of 94 candidate genes and 12 endophenotypes for schizophrenia from the Consortium on the Genetics of Schizophrenia. The American Journal of Psychiatry. 2011;168(9):930–946. doi: 10.1176/appi.ajp.2011.10050723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood TA, Light GA, Swerdlow NR, Radant AD, Braff DL. Association analysis of 94 candidate genes and schizophrenia-related endophenotypes. PloS ONE. 2012;7(1):e29630. doi: 10.1371/journal.pone.0029630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grutzendler J, Helmin K, Tsai J, Gan WB. Various dendritic abnormalities are associated with fibrillar amyloid deposits in Alzheimer's disease. Ann N Y Acad Sci. 2007;1097:30–39. doi: 10.1196/annals.1379.003. [DOI] [PubMed] [Google Scholar]
- Guilmatre A, Dubourg C, Mosca AL, Legallic S, Goldenberg A, Drouin-Garraud V, Layet V, Rosier A, Briault S, Bonnet-Brilhault F, Laumonnier F, Odent S, Le Vacon G, Joly-Helas G, David V, Bendavid C, Pinoit JM, Henry C, Impallomeni C, Germano E, Tortorella G, Di Rosa G, Barthelemy C, Andres C, Faivre L, Frebourg T, Saugier Veber P, Campion D. Recurrent rearrangements in synaptic and neurodevelopmental genes and shared biologic pathways in schizophrenia, autism, and mental retardation. Arch Gen Psychiatry. 2009;66(9):947–956. doi: 10.1001/archgenpsychiatry.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guptill JT, Booker AB, Gibbs TT, Kemper TL, Bauman ML, Blatt GJ. [3H]-flunitrazepam-labeled benzodiazepine binding sites in the hippocampal formation in autism: a multiple concentration autoradiographic study. J Autism Dev Disord. 2007;37(5):911–920. doi: 10.1007/s10803-006-0226-7. [DOI] [PubMed] [Google Scholar]
- Hall J, et al. A neuregulin 1 variant associated with abnormal cortical function and psychotic symptoms. Nat Neurosci. 2006;9(12):1477–1478. doi: 10.1038/nn1795. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10(1):40–68. doi: 10.1038/sj.mp.4001558. image 45. [DOI] [PubMed] [Google Scholar]
- Hayashi-Takagi A, et al. Disrupted-in-Schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nat Neurosci. 2010;13(3):327–332. doi: 10.1038/nn.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann CS, Demiralp T. Human EEG gamma oscillations in neuropsychiatric disorders. Clin Neurophysiol. 2005;116(12):2719–2733. doi: 10.1016/j.clinph.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Hill JJ, Hashimoto T, Lewis DA. Molecular mechanisms contributing to dendritic spine alterations in the prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2006;11:557–566. doi: 10.1038/sj.mp.4001792. [DOI] [PubMed] [Google Scholar]
- Hollingworth P, Hamshere ML, Holmans PA, O'Donovan MC, Sims R, Powell J, Lovestone S, Myers A, Devrieze FW, Hardy J, Goate A, Owen M, Williams J. Increased familial risk and genomewide significant linkage for Alzheimer's disease with psychosis. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:841–848. doi: 10.1002/ajmg.b.30515. [DOI] [PubMed] [Google Scholar]
- Hollingworth P, Sweet R, Sims R, Harold D, Russo G, Abraham R, Stretton A, Jones N, Gerrish A, Chapman J, Ivanov D, Moskvina V, Lovestone S, Priotsi P, Lupton M, Brayne C, Gill M, Lawlor B, Lynch A, Craig D, McGuinness B, Johnston J, Holmes C, Livingston G, Bass NJ, Gurling H, McQuillin A, Holmans P, Jones L, Devlin B, Klei L, Barmada MM, Demirci FY, DeKosky ST, Lopez OL, Passmore P, Owen MJ, O'Donovan MC, Mayeux R, Kamboh MI, Williams J. Genome-wide association study of Alzheimer's disease with psychotic symptoms. Mol Psychiatry. 2011;17(12):1316–1327. doi: 10.1038/mp.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nature Reviews. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- Hsieh H, et al. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YZ, et al. Regulation of neuregulin signaling by PSD-95 interacting with ErbB4 at CNS synapses. Neuron. 2000;26(2):443–455. doi: 10.1016/s0896-6273(00)81176-9. [DOI] [PubMed] [Google Scholar]
- Hung AY, Futai K, Sala C, Valtschanoff JG, Ryu J, Woodworth MA, Kidd FL, Sung CC, Miyakawa T, Bear MF, Weinberg RJ, Sheng M. Smaller dendritic spines, weaker synaptic transmission, but enhanced spatial learning in mice lacking Shank1. J Neurosci. 2008;28:1697–1708. doi: 10.1523/JNEUROSCI.3032-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide M, Lewis DA. Altered Cortical CDC42 Signaling Pathways in Schizophrenia: Implications for Dendritic Spine Deficits. Biol Psychiatry. 2010;68(1):25–32. doi: 10.1016/j.biopsych.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen MJ, Leiva-Salcedo E, Buonanno A. Neuregulin directly decreases voltage-gated sodium current in hippocampal ErbB4-expressing interneurons. J Neurosci. 2012;32(40):13889–13895. doi: 10.1523/JNEUROSCI.1420-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay TM. Dopamine: a potential substrate for synaptic plasticity and memory mechanisms. Prog Neurobiol. 2003;69(6):375–390. doi: 10.1016/s0301-0082(03)00085-6. [DOI] [PubMed] [Google Scholar]
- Kalus P, Bondzio J, Federspiel A, Muller TJ, Zuschratter W. Cell-type specific alterations of cortical interneurons in schizophrenic patients. Neuroreport. 2002;13(5):713–717. doi: 10.1097/00001756-200204160-00035. [DOI] [PubMed] [Google Scholar]
- Kantrowitz JT, Javitt DC. N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain Res Bull. 2010;83(3-4):108–121. doi: 10.1016/j.brainresbull.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, et al. DISC1 immunoreactivity at the light and ultrastructural level in the human neocortex. J Comp Neurol. 2006;497:436–450. doi: 10.1002/cne.21007. [DOI] [PubMed] [Google Scholar]
- Kirov G, Pocklington AJ, Holmans P, Ivanov D, Ikeda M, Ruderfer D, Moran J, Chambert K, Toncheva D, Georgieva L, Grozeva D, Fjodorova M, Wollerton R, Rees E, Nikolov I, van de Lagemaat LN, Bayes A, Fernandez E, Olason PI, Bottcher Y, Komiyama NH, Collins MO, Choudhary J, Stefansson K, Stefansson H, Grant SG, Purcell S, Sklar P, O'Donovan MC, Owen MJ. De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Mol Psychiatry. 2012;17(2):142–153. doi: 10.1038/mp.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffie RM, Hyman BT, Spires-Jones TL. Alzheimer's disease: synapses gone cold. Mol Neurodegener. 2011;6:63. doi: 10.1186/1750-1326-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffie RM, Meyer-Luehmann M, Hashimoto T, Adams KW, Mielke ML, Garcia-Alloza M, Micheva KD, Smith SJ, Kim ML, Lee VM, Hyman BT, Spires-Jones TL. Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci U S A. 2009;106:4012–4017. doi: 10.1073/pnas.0811698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolomeets NS, Orlovskaya DD, Rachmanova VI, Uranova NA. Ultrastructural alterations in hippocampal mossy fiber synapses in schizophrenia: a postmortem morphometric study. Synapse. 2005;57:47–55. doi: 10.1002/syn.20153. [DOI] [PubMed] [Google Scholar]
- Kolomeets NS, Orlovskaya DD, Uranova NA. Decreased numerical density of CA3 hippocampal mossy fiber synapses in schizophrenia. Synapse. 2007;61:615–621. doi: 10.1002/syn.20405. [DOI] [PubMed] [Google Scholar]
- Kotrla KJ, Chacko RC, Harper RG, Jhingran S, Doody R. SPECT findings on psychosis in Alzheimer's disease. Am J Psychiatry. 1995;152:1470–1475. doi: 10.1176/ajp.152.10.1470. [DOI] [PubMed] [Google Scholar]
- Kwon JS, et al. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56(11):1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon OB, et al. Neuregulin-1 regulates LTP at CA1 hippocampal synapses through activation of dopamine D4 receptors. Proc Natl Acad Sci U S A. 2008;105(40):15587–15592. doi: 10.1073/pnas.0805722105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvajo M, et al. A mutation in mouse Disc1 that models a schizophrenia risk allele leads to specific alterations in neuronal architecture and cognition. Proc Natl Acad Sci U S A. 2008;105:7076–7081. doi: 10.1073/pnas.0802615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C, Lemke G. An extended family of protein-tyrosine kinase genes differentially expressed in the vertebrate nervous system. Neuron. 1991;6(5):691–704. doi: 10.1016/0896-6273(91)90167-x. [DOI] [PubMed] [Google Scholar]
- Laumonnier F, Cuthbert PC, Grant SG. The role of neuronal complexes in human X-linked brain diseases. Am J Hum Genet. 2007;80:205–220. doi: 10.1086/511441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law AJ, Kleinman JE, Weinberger DR, Weickert CS. Disease-associated intronic variants in the ErbB4 gene are related to altered ErbB4 splice-variant expression in the brain in schizophrenia. Hum Mol Genet. 2007;16(2):129–141. doi: 10.1093/hmg/ddl449. [DOI] [PubMed] [Google Scholar]
- Law AJ, Weickert CS, Hyde TM, Kleinman JE, Harrison PJ. Reduced spinophilin but not microtubule-associated protein 2 expression in the hippocampal formation in schizophrenia and mood disorders: molecular evidence for a pathology of dendritic spines. Am J Psychiatry. 2004;161:1848–1855. doi: 10.1176/ajp.161.10.1848. [DOI] [PubMed] [Google Scholar]
- Lawrence JJ, et al. Somatodendritic Kv7/KCNQ/M channels control interspike interval in hippocampal interneurons. J Neurosci. 2006;26(47):12325–12338. doi: 10.1523/JNEUROSCI.3521-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Gonzalez-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33(1):141–165. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6(4):312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- Li B, Woo RS, Mei L, Malinow R. The neuregulin-1 receptor erbB4 controls glutamatergic synapse maturation and plasticity. Neuron. 2007;54:583–597. doi: 10.1016/j.neuron.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li KX, et al. Neuregulin 1 regulates excitability of fast-spiking neurons through Kv1.1 and acts in epilepsy. Nat Neurosci. 2012;15(2):267–273. doi: 10.1038/nn.3006. [DOI] [PubMed] [Google Scholar]
- Lipska BK, et al. Expression of DISC1 binding partners is reduced in schizophrenia and associated with DISC1 SNPs. Hum Mol Genet. 2006;15:1245–1258. doi: 10.1093/hmg/ddl040. [DOI] [PubMed] [Google Scholar]
- Lisman JE, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31(5):234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46(5):703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Longart M, Chatani-Hinze M, Gonzalez CM, Vullhorst D, Buonanno A. Regulation of ErbB-4 endocytosis by neuregulin in GABAergic hippocampal interneurons. Brain Res Bull. 2007;73(4-6):210–219. doi: 10.1016/j.brainresbull.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer's disease. Am J Pathol. 1999;155:853–862. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Kiraly DD, Gaier ED, Wang Y, Kim EJ, Levine ES, Eipper BA, Mains RE. Kalirin-7 is required for synaptic structure and function. J Neurosci. 2008;28:12368–12382. doi: 10.1523/JNEUROSCI.4269-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzner O, Devor M. Na+ conductance and the threshold for repetitive neuronal firing. Brain Res. 1992;597(1):92–98. doi: 10.1016/0006-8993(92)91509-d. [DOI] [PubMed] [Google Scholar]
- Mega MS, Lee L, Dinov ID, Mishkin F, Toga AW, Cummings JL. Cerebral correlates of psychotic symptoms in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2000;69:167–171. doi: 10.1136/jnnp.69.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9(6):437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier S, et al. SCN1A affects brain structure and the neural activity of the aging brain. Biological Psychiatry. 2012;72(8):677–683. doi: 10.1016/j.biopsych.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Mentis MJ, Weinstein EA, Horwitz B, McIntosh AR, Pietrini P, Alexander GE, Furey M, Murphy DG. Abnormal brain glucose metabolism in the delusional misidentification syndromes: a positron emission tomography study in Alzheimer disease. Biol Psychiatry. 1995;38:438–449. doi: 10.1016/0006-3223(94)00326-x. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J. Neural basis of psychosis-related behaviour in the infection model of schizophrenia. Behav Brain Res. 2009;204(2):322–334. doi: 10.1016/j.bbr.2008.12.022. [DOI] [PubMed] [Google Scholar]
- Milescu LS, Yamanishi T, Ptak K, Smith JC. Kinetic properties and functional dynamics of sodium channels during repetitive spiking in a slow pacemaker neuron. J Neurosci. 2010;30(36):12113–12127. doi: 10.1523/JNEUROSCI.0445-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar JK, Christie S, Porteous DJ. Yeast two-hybrid screens implicate DISC1 in brain development and function. Biochem Biophys Res Commun. 2003;311:1019–1025. doi: 10.1016/j.bbrc.2003.10.101. [DOI] [PubMed] [Google Scholar]
- Moessner R, Marshall CR, Sutcliffe JS, Skaug J, Pinto D, Vincent J, Zwaigenbaum L, Fernandez B, Roberts W, Szatmari P, Scherer SW. Contribution of SHANK3 mutations to autism spectrum disorder. Am J Hum Genet. 2007;81(6):1289–1297. doi: 10.1086/522590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98(4):427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- Moran EK, Becker JA, Satlin A, Lyoo IK, Fischman AJ, Johnson KA. Psychosis of Alzheimer's disease: Gender differences in regional perfusion. Neurobiol Aging. 2008;29:1218–1225. doi: 10.1016/j.neurobiolaging.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Mukai J, et al. Palmitoylation-dependent neurodevelopmental deficits in a mouse model of 22q11 microdeletion. Nat Neurosci. 2008;11:1302–1310. doi: 10.1038/nn.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo MR, Attwood AS, Flint J. Neuregulin 1 genotype and schizophrenia. Schizophr Bull. 2008;34(1):9–12. doi: 10.1093/schbul/sbm129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo MR, Thiselton DL, Clark TG, Flint J. Association of the NRG1 gene and schizophrenia: a meta-analysis. Mol Psychiatry. 2006;11(6):539–546. doi: 10.1038/sj.mp.4001817. [DOI] [PubMed] [Google Scholar]
- Murray MM, Bernstein SL, Nyugen V, Condron MM, Teplow DB, Bowers MT. Amyloid beta protein: Abeta40 inhibits Abeta42 oligomerization. J Am Chem Soc. 2009;131:6316–6317. doi: 10.1021/ja8092604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PS, Kirkwood CM, Gray MC, Ikonomovic MD, Paljug WR, Abrahamson EE, Henteleff RA, Hamilton RL, Kofler JK, Klunk WE, Lopez OL, Penzes P, Sweet RA. beta-Amyloid 42/40 ratio and kalirin expression in Alzheimer disease with psychosis. Neurobiol Aging. 2012;33(12):2807–2816. doi: 10.1016/j.neurobiolaging.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadif Kasri N, Van Aelst L. Rho-linked genes and neurological disorders. Pflugers Arch. 2008;455:787–797. doi: 10.1007/s00424-007-0385-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslund J, Haroutunian V, Mohs R, Davis KL, Davies P, Greengard P, Buxbaum JD. Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. JAMA. 2000;283:1571–1577. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- Neddens J, Buonanno A. Selective populations of hippocampal interneurons express ErbB4 and their number and distribution is altered in ErbB4 knockout mice. Hippocampus. 2009;20(6):724–44. doi: 10.1002/hipo.20675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neddens J, Buonanno A. Expression of the neuregulin receptor ErbB4 in the brain of the rhesus monkey (Macaca mulatta). PloS ONE. 2011;6(11):e27337. doi: 10.1371/journal.pone.0027337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neddens J, et al. Conserved interneuron-specific ErbB4 expression in frontal cortex of rodents, monkeys, and humans: implications for schizophrenia. Biological Psychiatry. 2011;70(7):636–645. doi: 10.1016/j.biopsych.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicodemus KK, et al. Biological validation of increased schizophrenia risk with NRG1, ERBB4, and AKT1 epistasis via functional neuroimaging in healthy controls. Arch Gen Psychiatry. 2010;67(10):991–1001. doi: 10.1001/archgenpsychiatry.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Roak BJ, State MW. Autism genetics: strategies, challenges, and opportunities. Autism Res. 2008;1(1):4–17. doi: 10.1002/aur.3. [DOI] [PubMed] [Google Scholar]
- Oblak AL, Gibbs TT, Blatt GJ. Decreased GABA(B) receptors in the cingulate cortex and fusiform gyrus in autism. J Neurochem. 2010;114(5):1414–1423. doi: 10.1111/j.1471-4159.2010.06858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen MJ, Craddock N, O'Donovan MC. Schizophrenia: genes at last? Trends Genet. 2005;21(9):518–525. doi: 10.1016/j.tig.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Pavlowsky A, Gianfelice A, Pallotto M, Zanchi A, Vara H, Khelfaoui M, Valnegri P, Rezai X, Bassani S, Brambilla D, Kumpost J, Blahos J, Roux MJ, Humeau Y, Chelly J, Passafaro M, Giustetto M, Billuart P, Sala C. A postsynaptic signaling pathway that may account for the cognitive defect due to IL1RAPL1 mutation. Curr Biol. 2010;20:103–115. doi: 10.1016/j.cub.2009.12.030. [DOI] [PubMed] [Google Scholar]
- Peça J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN, Lascola CD, Fu Z, Feng G. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472(7344):437–42. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci. 2011;14(3):285–293. doi: 10.1038/nn.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Cahill ME, Jones KA, Srivastava DP. Convergent CaMK and RacGEF signals control dendritic structure and function. Trends in Cell Biology. 2008;18:405–413. doi: 10.1016/j.tcb.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Penzes P, Jones KA. Dendritic spine dynamics--a key role for kalirin-7. Trends Neurosci. 2008;31:419–427. doi: 10.1016/j.tins.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D, Pagnamenta AT, et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature. 2010;466:368–372. doi: 10.1038/nature09146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piton A, Michaud JL, Peng H, Aradhya S, Gauthier J, Mottron L, Champagne N, Lafrenière RG, Hamdan FF, Joober R, Fombonne E, Marineau C, Cossette P, Dubé MP, Haghighi P, Drapeau P, Barker PA, Carbonetto S, Rouleau GA, team SD Mutations in the calcium-related gene IL1RAPL1 are associated with autism. Hum Mol Genet. 2008;17:3965–3974. doi: 10.1093/hmg/ddn300. [DOI] [PubMed] [Google Scholar]
- Poot M, van der Smagt JJ, Brilstra EH, Bourgeron T. Disentangling the myriad genomics of complex disorders, specifically focusing on autism, epilepsy, and schizophrenia. Cytogenet Genome Res. 2011;135(3-4):228–240. doi: 10.1159/000334064. [DOI] [PubMed] [Google Scholar]
- Powell AD, Gill KK, Saintot PP, Jiruska P, Chelly J, Billuart P, Jefferys JG. Rapid reversal of impaired inhibitory and excitatory transmission but not spine dysgenesis in a mouse model of mental retardation. J Physiol. 2012;590:763–776. doi: 10.1113/jphysiol.2011.219907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldman-Rakic PS. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232:232–235. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Addington AM, Frangou S, Psych MR. The neurodevelopmental model of schizophrenia: update 2005. Mol Psychiatry. 2005;10(5):434–449. doi: 10.1038/sj.mp.4001642. [DOI] [PubMed] [Google Scholar]
- Rauch A, Hoyer J, Guth S, Zweier C, Kraus C, Becker C, Zenker M, Hüffmeier U, Thiel C, Rüschendorf F, Nürnberg P, Reis A, Trautmann U. Diagnostic yield of various genetic approaches in patients with unexplained developmental delay or mental retardation. Am J Med Genet A. 2006;140:2063–2074. doi: 10.1002/ajmg.a.31416. [DOI] [PubMed] [Google Scholar]
- Ropacki SA, Jeste DV. Epidemiology of and risk factors for psychosis of Alzheimer's disease: a review of 55 studies published from 1990 to 2003. Am J Psychiatry. 2005;162:2022–2030. doi: 10.1176/appi.ajp.162.11.2022. [DOI] [PubMed] [Google Scholar]
- Ropers HH, Hamel BC. X-linked mental retardation. Nat Rev Genet. 2005;6:46–57. doi: 10.1038/nrg1501. [DOI] [PubMed] [Google Scholar]
- Rosen AM, Stevens B. The role of the classical complement cascade in synapse loss during development and glaucoma. Adv Exp Med Biol. 2010;703:75–93. doi: 10.1007/978-1-4419-5635-4_6. [DOI] [PubMed] [Google Scholar]
- Sato D, Lionel AC, et al. SHANK1 Deletions in Males with Autism Spectrum Disorder. Am J Hum Genet. 2012;90:879–887. doi: 10.1016/j.ajhg.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff SW, Price DA. Synaptic pathology in Alzheimer's disease: a review of ultrastructural studies. Neurobiol Aging. 2003;24:1029–1046. doi: 10.1016/j.neurobiolaging.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Schmeisser MJ, Ey E, et al. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature. 2012;486:256–260. doi: 10.1038/nature11015. [DOI] [PubMed] [Google Scholar]
- Schumacher J, et al. The DISC locus and schizophrenia: evidence from an association study in a central European sample and from a meta-analysis across different European populations. Hum Mol Genet. 2009;18:2719–2727. doi: 10.1093/hmg/ddp204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P. Glutamate and dopamine components in schizophrenia. J Psychiatry Neurosci. 2009;34(2):143–149. [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res. 2008;192:106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamir A, et al. The importance of the NRG-1/ErbB4 pathway for synaptic plasticity and behaviors associated with psychiatric disorders. J Neurosci. 2012;32(9):2988–2997. doi: 10.1523/JNEUROSCI.1899-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipton OA, Leitz JR, Dworzak J, Acton CE, Tunbridge EM, Denk F, Dawson HN, Vitek MP, Wade-Martins R, Paulsen O, Vargas-Caballero M. Tau protein is required for amyloid {beta}-induced impairment of hippocampal long-term potentiation. J Neurosci. 2011;31:1688–1692. doi: 10.1523/JNEUROSCI.2610-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberberg G, Darvasi A, Pinkas-Kramarski R, Navon R. The involvement of ErbB4 with schizophrenia: association and expression studies. American journal of medical genetics. Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2006;141B(2):142–148. doi: 10.1002/ajmg.b.30275. [DOI] [PubMed] [Google Scholar]
- Spencer KM. The functional consequences of cortical circuit abnormalities on gamma oscillations in schizophrenia: insights from computational modeling. Front Hum Neurosci. 2009;3:33. doi: 10.3389/neuro.09.033.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, et al. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci U S A. 2004;101(49):17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires-Jones TL, Meyer-Luehmann M, Osetek JD, Jones PB, Stern EA, Bacskai BJ, Hyman BT. Impaired spine stability underlies plaque-related spine loss in an Alzheimer's disease mouse model. Am J Pathol. 2007;171:1304–1311. doi: 10.2353/ajpath.2007.070055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark KL, et al. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat Genet. 2008;40:751–760. doi: 10.1038/ng.138. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Vazquez S, Petracca G, Sabe L, Migliorelli R, Teson A, Leiguarda R. A SPECT study of delusions in Alzheimer's disease. Neurology. 1994;44:2055–2059. doi: 10.1212/wnl.44.11.2055. [DOI] [PubMed] [Google Scholar]
- Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 2006;188:510–518. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- Stefansson H, et al. Neuregulin 1 and Susceptibility to Schizophrenia. Am J Hum Genet. 2002;71(4) doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Thorgeirsson TE, Gulcher JR, Stefansson K. Neuregulin 1 in schizophrenia: out of Iceland. Mol Psychiatry. 2003;8(7):639–640. doi: 10.1038/sj.mp.4001384. [DOI] [PubMed] [Google Scholar]
- Steiner H, Blum M, Kitai ST, Fedi P. Differential expression of ErbB3 and ErbB4 neuregulin receptors in dopamine neurons and forebrain areas of the adult rat. Exp Neurol. 1999;159(2):494–503. doi: 10.1006/exnr.1999.7163. [DOI] [PubMed] [Google Scholar]
- Sultzer DL, Brown CV, Mandelkern MA, Mahler ME, Mendez MF, Chen ST, Cummings JL. Delusional thoughts and regional frontal/temporal cortex metabolism in Alzheimer's disease. Am J Psychiatry. 2003;160:341–349. doi: 10.1176/appi.ajp.160.2.341. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Bennett DA, Graff-Radford NR, Mayeux R. Assessment and familial aggregation of psychosis in Alzheimer's disease from the National Institute on Aging Late Onset Alzheimer's Disease Family Study. Brain. 2010;133:1155–1162. doi: 10.1093/brain/awq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet RA, Henteleff RA, Zhang W, Sampson AR, Lewis DA. Reduced dendritic spine density in auditory cortex of subjects with schizophrenia. Neuropsychopharmacology. 2009;34:374–389. doi: 10.1038/npp.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet RA, Nimgaonkar VL, Devlin B, Lopez OL, DeKosky ST. Increased familial risk of the psychotic phenotype of Alzheimer disease. Neurology. 2002a;58:907–911. doi: 10.1212/wnl.58.6.907. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Panchalingam K, Pettegrew JW, McClure RJ, Hamilton RL, Lopez OL, Kaufer DI, DeKosky ST, Klunk WE. Psychosis in Alzheimer disease: postmortem magnetic resonance spectroscopy evidence of excess neuronal and membrane phospholipid pathology. Neurobiol Aging. 2002b;23:547–553. doi: 10.1016/s0197-4580(02)00009-x. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Seltman H, Emanuel JE, Lopez OL, Becker JT, Bis JC, Weamer EA, DeMichele-Sweet MA, Kuller LH. Effect of Alzheimer's disease risk genes on trajectories of cognitive function in the Cardiovascular Health Study. Am J Psychiatry. 2012;169:954–962. doi: 10.1176/appi.ajp.2012.11121815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T, Sheng M. Molecular mechanisms of dendritic spine morphogenesis. Curr Opin Neurobiol. 2006;16:95–101. doi: 10.1016/j.conb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Tan W, Dean M, Law AJ. Molecular cloning and characterization of the human ErbB4 gene: identification of novel splice isoforms in the developing and adult brain. PloS ONE. 2010;5(9):e12924. doi: 10.1371/journal.pone.0012924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: Synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Thompson M, et al. Widespread expression of ErbB2, ErbB3 and ErbB4 in non-human primate brain. Brain Res. 2007;1139:95–109. doi: 10.1016/j.brainres.2006.11.047. [DOI] [PubMed] [Google Scholar]
- Tukker JJ, Fuentealba P, Hartwich K, Somogyi P, Klausberger T. Cell type-specific tuning of hippocampal interneuron firing during gamma oscillations in vivo. J Neurosci. 2007;27(31):8184–8189. doi: 10.1523/JNEUROSCI.1685-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valnegri P, Khelfaoui M, Dorseuil O, Bassani S, Lagneaux C, Gianfelice A, Benfante R, Chelly J, Billuart P, Sala C, Passafaro M. A circadian clock in hippocampus is regulated by interaction between oligophrenin-1 and Rev-erbα. Nat Neurosci. 2011a;14(10):1293–301. doi: 10.1038/nn.2911. [DOI] [PubMed] [Google Scholar]
- Valnegri P, Montrasio C, Brambilla D, Ko JW, Passafaro M, Sala C. The X-linked intellectual disability protein IL1RAPL1 regulates excitatory synapse formation by binding PTPδ and RhoGAP2. Hum Mol Genet. 2011b;20(24):4797–4809. doi: 10.1093/hmg/ddr418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Spronsen M, Hoogenraad CC. Synapse pathology in psychiatric and neurologic disease. Curr Neurol Neurosci Rep. 2010;10(3):207–214. doi: 10.1007/s11910-010-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verpelli C, Dvoretskova E, Vicidomini C, Rossi F, Chiappalone M, Schoen M, Di Stefano B, Mantegazza R, Broccoli V, Boeckers TM, Dityatev A, Sala C. Importance of shank3 in regulating metabotropic glutamate receptor 5 (mGluR5) expression and signaling at synapses. J Biol Chem. 2011;286(40):34839–50. doi: 10.1074/jbc.M111.258384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vullhorst D, et al. Selective expression of ErbB4 in interneurons, but not pyramidal cells, of the rodent hippocampus. J Neurosci. 2009;29(39):12255–12264. doi: 10.1523/JNEUROSCI.2454-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. A beta oligomers - a decade of discovery. J Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- Walss-Bass C, et al. A novel missense mutation in the transmembrane domain of neuregulin 1 is associated with schizophrenia. Biological Psychiatry. 2006;60(6):548–553. doi: 10.1016/j.biopsych.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Wang X, McCoy PA, Rodriguiz RM, Pan Y, Je HS, Roberts AC, Kim CJ, Berrios J, Colvin JS, Bousquet-Moore D, Lorenzo I, Wu G, Weinberg RJ, Ehlers MD, Philpot BD, Beaudet AL, Wetsel WC, Jiang YH. Synaptic dysfunction and abnormal behaviors in mice lacking major isoforms of Shank3. Hum Mol Genet. 2011;20(15):3093–108. doi: 10.1093/hmg/ddr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weickert CS, Tiwari Y, Schofield PR, Mowry BJ, Fullerton JM. Schizophrenia-associated HapICE haplotype is associated with increased NRG1 type III expression and high nucleotide diversity. Translational Psychiatry. 2012;2:e104. doi: 10.1038/tp.2012.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissflog L, et al. KCNIP4 as a candidate gene for personality disorders and adult ADHD. European neuropsychopharmacology. The Journal of the European College of Neuropsychopharmacology S0924. 2012;977X(12):00214–3. doi: 10.1016/j.euroneuro.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Wen L, et al. Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons. Proc Natl Acad Sci U S A. 2010;107(3):1211–1216. doi: 10.1073/pnas.0910302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson HL, Wong AC, Shaw SR, Tse WY, Stapleton GA, Phelan MC, Hu S, Marshall J, McDermid HE. Molecular characterisation of the 22q13 deletion syndrome supports the role of haploinsufficiency of SHANK3/PROSAP2 in the major neurological symptoms. J Med Genet. 2003;40:575–584. doi: 10.1136/jmg.40.8.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, et al. Cortical gamma generators suggest abnormal auditory circuitry in early-onset psychosis. Cereb Cortex. 2008;18(2):371–378. doi: 10.1093/cercor/bhm062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G, Weinberger DR. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci. 2004;27(11):683–690. doi: 10.1016/j.tins.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Wöhr M, Roullet FI, Hung AY, Sheng M, Crawley JN. Communication impairments in mice lacking Shank1: reduced levels of ultrasonic vocalizations and scent marking behavior. PLoS ONE. 2011;6:e20631. doi: 10.1371/journal.pone.0020631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won H, Lee HR, Gee HY, Mah W, Kim JI, Lee J, Ha S, Chung C, Jung ES, Cho YS, Park SG, Lee JS, Lee K, Kim D, Bae YC, Kaang BK, Lee MG, Kim E. Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature. 2012;486:261–265. doi: 10.1038/nature11208. [DOI] [PubMed] [Google Scholar]
- Woo TU, Walsh JP, Benes FM. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2004;61(7):649–657. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]
- Woo TU, Whitehead RE, Melchitzky DS, Lewis DA. A subclass of prefrontal gamma-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proc Natl Acad Sci U S A. 1998;95(9):5341–5346. doi: 10.1073/pnas.95.9.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Cahill ME, Penzes P. Kalirin loss results in cortical morphological alterations. Mol Cell Neurosci. 2010;43:81–89. doi: 10.1016/j.mcn.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Srivastava DP, Photowala H, Kai L, Cahill ME, Woolfrey KM, Shum CY, Surmeier DJ, Penzes P. Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron. 2007;56:640–656. doi: 10.1016/j.neuron.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau HJ, Wang HF, Lai C, Liu FC. Neural development of the neuregulin receptor ErbB4 in the cerebral cortex and the hippocampus: preferential expression by interneurons tangentially migrating from the ganglionic eminences. Cereb. Cortex. 2003;13(3):252–264. doi: 10.1093/cercor/13.3.252. [DOI] [PubMed] [Google Scholar]
- Yip J, Soghomonian JJ, Blatt GJ. Decreased GAD67 mRNA levels in cerebellar Purkinje cells in autism: pathophysiological implications. Acta Neuropathol. 2007;113(5):559–568. doi: 10.1007/s00401-006-0176-3. [DOI] [PubMed] [Google Scholar]
- Yizhar O, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477(7363):171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Yasumura M, Uemura T, Lee SJ, Ra M, Taguchi R, Iwakura Y, Mishina M. IL-1 Receptor Accessory Protein-Like 1 Associated with Mental Retardation and Autism Mediates Synapse Formation by Trans-Synaptic Interaction with Protein Tyrosine Phosphatase {delta}. J Neurosci. 2011;31:13485–13499. doi: 10.1523/JNEUROSCI.2136-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FH, et al. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat Neurosci. 2006;9(9):1142–1149. doi: 10.1038/nn1754. [DOI] [PubMed] [Google Scholar]
- Zemni R, Bienvenu T, et al. A new gene involved in X-linked mental retardation identified by analysis of an X;2 balanced translocation. Nat Genet. 2000;24:167–170. doi: 10.1038/72829. [DOI] [PubMed] [Google Scholar]
- Zempel H, Thies E, Mandelkow E, Mandelkow EM. Abeta oligomers cause localized Ca(2+) elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation, and destruction of microtubules and spines. J Neurosci. 2010;30:11938–11950. doi: 10.1523/JNEUROSCI.2357-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Ma QL, Calon F, Harris-White ME, Yang F, Lim GP, Morihara T, Ubeda OJ, Ambegaokar S, Hansen JE, Weisbart RH, Teter B, Frautschy SA, Cole GM. Role of p21-activated kinase pathway defects in the cognitive deficits of Alzheimer disease. Nat Neurosci. 2006;9:234–242. doi: 10.1038/nn1630. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Lin A, Chang P, Gan WB. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron. 2005;46:181–189. doi: 10.1016/j.neuron.2005.04.001. [DOI] [PubMed] [Google Scholar]