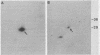
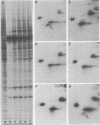
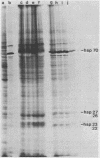
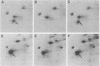
Abstract
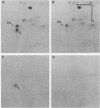
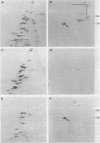
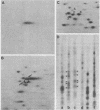
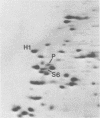
A basic ribosomal phosphoprotein of 30,000 molecular weight was rapidly dephosphorylated in cultured Drosophila melanogaster cells heat shocked at 37 degrees C. The protein was associated with the 40S ribosomal subunit and had an electrophoretic mobility similar to that of purified rat liver protein S6 on basic two-dimensional polyacrylamide gels as well as a similar partial proteolysis peptide map. In logarithmically growing cultures, this D. melanogaster S6 protein appeared to have a single phosphorylated species consisting of 30 to 40% of the total cellular S6. Thus, the nearly complete dephosphorylation of this protein observed in heat shock involves a large fraction of the cellular S6. The significance of this dephosphorylation in the expression of the heat shock response was investigated by examining the phosphorylation status of S6 in recovery from heat shock and in response to chemical inducers of the heat shock response. During recovery from a 30-min heat shock, the recovery of normal protein synthesis was almost complete in 2 to 4 hr, whereas there was no significant rephosphorylation of S6 for 8 h. Two chemical inducers of the heat shock response, canavanine and sodium arsenite, induced the synthesis of heat shock proteins in D. melanogaster cells. Sodium arsenite also caused an inhibition of normal protein synthesis similar to that observed in heat shock. Neither agent, however, caused significant dephosphorylation of S6. These results suggest that the dephosphorylation of S6, although invariably observed in heat-shocked cells, may in some cases be dissociated from both the induction of heat shock protein synthesis and the turnoff of normal protein synthesis which occur in a heat shock response.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Ballinger D. G., Hunt T. Fertilization of sea urchin eggs is accompanied by 40 S ribosomal subunit phosphorylation. Dev Biol. 1981 Oct 30;87(2):277–285. doi: 10.1016/0012-1606(81)90151-2. [DOI] [PubMed] [Google Scholar]
- Berger E. The ribosomes of Drosophila. I. Subunit and protein composition. Mol Gen Genet. 1974;128(1):1–9. doi: 10.1007/BF00267290. [DOI] [PubMed] [Google Scholar]
- Burdon R. H., Slater A., McMahon M., Cato A. C. Hyperthermia and the heat-shock proteins of HeLa cells. Br J Cancer. 1982 Jun;45(6):953–963. doi: 10.1038/bjc.1982.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chooi W. Y., Sabatini L. M., Macklin M., Fraser D. Group fractionation and determination of the number of ribosomal subunit proteins from Drosophila melanogaster embryos. Biochemistry. 1980 Apr 1;19(7):1425–1433. doi: 10.1021/bi00548a025. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Echalier G., Ohanessian A. In vitro culture of Drosophila melanogaster embryonic cells. In Vitro. 1970 Nov-Dec;6(3):162–172. doi: 10.1007/BF02617759. [DOI] [PubMed] [Google Scholar]
- Glover C. V. Heat shock induces rapid dephosphorylation of a ribosomal protein in Drosophila. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1781–1785. doi: 10.1073/pnas.79.6.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover C. V., Vavra K. J., Guttman S. D., Gorovsky M. A. Heat shock and deciliation induce phosphorylation of histone H1 in T. pyriformis. Cell. 1981 Jan;23(1):73–77. doi: 10.1016/0092-8674(81)90271-3. [DOI] [PubMed] [Google Scholar]
- Groppi V. E., Jr, Browning E. T. Norepinephrine-dependent protein phosphorylation in intact C-6 glioma cells. Analysis by two-dimensional gel electrophoresis. Mol Pharmacol. 1980 Nov;18(3):427–437. [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. The effect of amino acid analogues and heat shock on gene expression in chicken embryo fibroblasts. Cell. 1978 Dec;15(4):1277–1286. doi: 10.1016/0092-8674(78)90053-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lastick S. M., Nielsen P. J., McConkey E. H. Phosphorylation of ribosomal protein S6 in suspension cultured HeLa cells. Mol Gen Genet. 1977 Apr 29;152(3):223–230. doi: 10.1007/BF00693074. [DOI] [PubMed] [Google Scholar]
- McKenzie S. L., Henikoff S., Meselson M. Localization of RNA from heat-induced polysomes at puff sites in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1117–1121. doi: 10.1073/pnas.72.3.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirault M. E., Goldschmidt-Clermont M., Moran L., Arrigo A. P., Tissières A. The effect of heat shock on gene expression in Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):819–827. doi: 10.1101/sqb.1978.042.01.082. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Olsen A. S., Breckenridge B. M., Sanders M. M. Identification of cyclic guanosine monophosphate-binding proteins in Drosophila by direct photoaffinity labeling. Anal Biochem. 1982 Nov 1;126(2):306–311. doi: 10.1016/0003-2697(82)90520-6. [DOI] [PubMed] [Google Scholar]
- Petersen N. S., Mitchell H. K. Recovery of protein synthesis after heat shock: prior heat treatment affects the ability of cells to translate mRNA. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1708–1711. doi: 10.1073/pnas.78.3.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders M. M., Groppi V. E., Jr, Browning E. T. Resolution of basic cellular proteins including histone variants by two-dimensional gel electrophoresis: evaluation of lysine to arginine ratios and phosphorylation. Anal Biochem. 1980 Mar 15;103(1):157–165. doi: 10.1016/0003-2697(80)90250-x. [DOI] [PubMed] [Google Scholar]
- Sanders M. M. Identification of histone H2b as a heat-shock protein in Drosophila. J Cell Biol. 1981 Nov;91(2 Pt 1):579–583. doi: 10.1083/jcb.91.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf K. D., Nover L. Heat-shock-induced alterations of ribosomal protein phosphorylation in plant cell cultures. Cell. 1982 Sep;30(2):427–437. doi: 10.1016/0092-8674(82)90240-9. [DOI] [PubMed] [Google Scholar]
- Scott M. P., Pardue M. L. Translational control in lysates of Drosophila melanogaster cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3353–3357. doi: 10.1073/pnas.78.6.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherton C. C., Wool I. G. Two-dimensional polyacrylamide gel electrophoresis of eukaryotic ribosomal proteins. Methods Enzymol. 1974;30:506–526. doi: 10.1016/0076-6879(74)30051-1. [DOI] [PubMed] [Google Scholar]
- Spradling A., Pardue M. L., Penman S. Messenger RNA in heat-shocked Drosophila cells. J Mol Biol. 1977 Feb 5;109(4):559–587. doi: 10.1016/s0022-2836(77)80091-0. [DOI] [PubMed] [Google Scholar]
- Storti R. V., Scott M. P., Rich A., Pardue M. L. Translational control of protein synthesis in response to heat shock in D. melanogaster cells. Cell. 1980 Dec;22(3):825–834. doi: 10.1016/0092-8674(80)90559-0. [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Ogata K. Ribosomal proteins cross-linked to natural mRNA by UV irradiation of rat liver polysomes. J Biochem. 1981 Nov;90(5):1549–1552. doi: 10.1093/oxfordjournals.jbchem.a133624. [DOI] [PubMed] [Google Scholar]
- Terao K., Ogata K. Proteins of small subunits of rat liver ribosomes that interact with poly(U). II. Cross-links between poly(U) and ribosomal proteins in 40 S subunits induced by UV irradiation. J Biochem. 1979 Sep;86(3):605–617. doi: 10.1093/oxfordjournals.jbchem.a132564. [DOI] [PubMed] [Google Scholar]
- Thomas G., Siegmann M., Kubler A. M., Gordon J., Jimenez de Asua L. Regulation of 40S ribosomal protein S6 phosphorylation in Swiss mouse 3T3 cells. Cell. 1980 Apr;19(4):1015–1023. doi: 10.1016/0092-8674(80)90092-6. [DOI] [PubMed] [Google Scholar]
- Wool I. G. The structure and function of eukaryotic ribosomes. Annu Rev Biochem. 1979;48:719–754. doi: 10.1146/annurev.bi.48.070179.003443. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]