Summary
Background and objectives
Rapid ultrafiltration rate is associated with increased mortality among hemodialysis patients. Ultrafiltration rates are determined by interdialytic weight gain and session length. Although both interdialytic weight gain and session length have been linked to mortality, the relationship of each to mortality, independent of the other, is not adequately defined. This study was designed to evaluate whether shorter session length independent of weight gain and larger weight gain independent of session length are associated with increased mortality.
Design, setting, participants, & measurements
Data were taken from a national cohort of 14,643 prevalent, thrice-weekly, in-center hemodialysis patients dialyzing from 2005 to 2009 (median survival time, 25 months) at a single dialysis organization. Patients with adequate urea clearance and delivered dialysis session ≥240 and <240 minutes were pair-matched on interdialytic weight gain (n=1794), and patients with weight gain ≤3 and >3 kg were pair-matched on session length (n=2114); mortality associations were estimated separately.
Results
Compared with delivered session length ≥240, session length <240 minutes was associated with increased all-cause mortality (adjusted hazard ratio [95% confidence interval], 1.32 [1.03 to 1.69]). Compared with weight gain ≤3, weight gain >3 kg was associated with increased mortality (1.29 [1.01 to 1.65]). The associations were consistent across strata of age, sex, weight, and weight gain and session length. Secondary analyses demonstrated dose-response relationships between both and mortality.
Conclusions
Among patients with adequate urea clearance, shorter dialysis session length and greater interdialytic weight gain are associated with increased mortality; thus, both are viable targets for directed intervention.
Introduction
Hemodialysis patients experience exceptionally poor survival compared with the general population (1,2). Available evidence indicates that rapid fluid removal during dialysis may contribute to all-cause and cardiovascular mortality in this population (3–6). Ultrafiltration rate (UFR) is determined by both the amount of fluid removed, which at steady state is equivalent to interdialytic weight gain (IDWG), and the time over which this fluid is removed (dialysis session length [DSL]). Prior studies have demonstrated that both shorter DSL and larger IDWG (both of which would increase UFR) are associated with increased mortality (3,7–14). However, because DSL and IDWG are highly inter-related, it is unclear whether the association of each with mortality is independent of the other. Although it is plausible that both DSL and IDWG influence mortality, it is also possible that only one is independently associated with mortality and the other only through implied relationship with the first.
Better delineating the independent associations of DSL and IDWG with survival bears important therapeutic consequence. For example, if DSL influences mortality independently of IDWG, extending the length of dialysis treatments would be advisable. Conversely, if DSL does not influence mortality independently of IDWG, extending treatment times would not add benefit, but merely add to cost and potential patient dissatisfaction. Prior studies have attempted to disentangle the independent associations of DSL and IDWG by multivariable adjustment (3,5,14). However, multivariable adjustment may incompletely control for confounding when the confounder (e.g., IDWG) is highly imbalanced across exposure groups (e.g., longer versus shorter DSL) such that limited overlap exists.
Dedicated analytic techniques such as matching, however, do enable disentanglement of independent associations even when exposures are highly correlated. For example, our group recently demonstrated that shorter DSL is associated with increased mortality independently of body weight by matching patients with longer versus shorter prescribed DSL (<240 versus ≥240 minutes) on postdialysis weight (for which there was limited overlap) as well as on age, vascular access type, and sex (14).
We undertook this study to better delineate the association between greater UFR and mortality by testing the hypotheses that shorter DSL is associated with greater mortality independent of IDWG, and that greater IDWG is associated with greater mortality independent of DSL. We used data from a large, nationally representative cohort of prevalent patients who received in-center, thrice-weekly maintenance hemodialysis and who had adequate urea clearance by contemporary standards. To tightly control for confounding, participants were matched on IDWG in analyses of DSL, DSL in analyses of IDWG, and on other key covariates (sex, age, vascular access type, and postdialysis weight) in all analyses.
Materials and Methods
Study Design
This study was approved by the Partners Health Care Institutional Review Board. All study data were obtained from a cohort of 14,643 randomly selected prevalent adult patients receiving thrice-weekly, in-center hemodialysis at one large dialysis organization. Patients entered the cohort between January 1, 2005 and January 16, 2009 and dialyzed at one of 1247 outpatient facilities located in diverse geographic regions across the United States.
We sought to estimate the influence of DSL and IDWG on survival in the context where there was no compelling indication to extend DSL on the basis of urea removal; therefore, we excluded patients with urea reduction ratio (URR) <65%. (URR was chosen over Kt/V for this purpose to limit collinearity with DSL.) Delivered DSL was selected over prescribed DSL as the exposure of interest because it is more directly germane to UFR. To bear greatest relevance to the broader US dialysis population, we excluded patients with delivered DSL <150 or >270 minutes. In analyses of the delivered DSL–mortality association, we further excluded patients dialyzing at facilities without at least one participant in each DSL category, so as to avoid center effect bias derived through deterministic DSL. In analyses of the IDWG–mortality association, we excluded patients with mean IDWG <0 kg, because such patients are not representative of the broader population.
Data Collection
All study data were obtained from the large dialysis organization’s comprehensive electronic medical record and collected according to the organization’s standard clinical protocols. Demographic details were recorded by unit personnel upon admission to one of the organization’s facilities. Treating nephrologists ascertained comorbidity data through patient interview, examination, and medical record review and updated data based on clinical course. Laboratory parameters were measured upon large dialysis organization entry and then on a biweekly or monthly basis. Dialytic session data, including delivered DSL and predialysis and postdialysis weights, were recorded on a session-by-session basis per the organization’s standard clinical protocol. Predialysis and postdialysis weights and BP were measured immediately preceding and after each dialysis treatment; BP was measured in the seated position. IDWG was defined as predialysis weight (in kilograms) – postdialysis weight from the previous session (in kilograms). Date of death and attributed cause of death were recorded in the medical record by dialysis unit staff.
Time Sequence and Designation of Exposures and Outcome
The exposures of interest, delivered DSL and IDWG, were considered as the means of (typically 12) values over the 30-day period after cohort entry. Thirty-day mean delivered DSL demonstrated excellent correlation with 60-day (r=0.95) and 90-day means (r=0.93), and 30-day mean IDWG demonstrated strong correlation with 60-day (r=0.70) and 90-day means (r=0.60). Therefore, a 30-day exposure assessment period was chosen to minimize survivor bias.
Covariate data for demographic characteristics (age, sex, race, vascular access type, and dialytic vintage) and comorbidities (diabetes, coronary artery disease, and congestive heart failure) were considered as of cohort entry. Biochemical covariates (URR, albumin, creatinine, and phosphate) were considered as the last value measured during the 30-day exposure assessment period. Predialysis systolic BP was considered as the mean value over the 30-day exposure window.
The outcome of interest was death from any cause. Cause-specific mortality was not considered due to missingness and untested validity of attributed cause of death data. Patients were considered at-risk for outcome on day 31 after cohort entry (i.e., immediately after the exposure/covariate assessment period) and remained at-risk until death or censoring for the following: transfer of care, transplant, change in dialytic modality, renal function recovery, or end of study (February 21, 2009). Patients failing to maintain large dialysis organization enrollment during the 30-day exposure period were excluded. Given varying cohort entry times, maximal potential follow-up time was 48.7 months.
Statistical Analyses
The delivered DSL–mortality and IDWG–mortality associations were considered in parallel analyses. In the primary analysis, DSL and IDWG were considered as dichotomous variables (<240 versus ≥240 minutes and ≤3 versus >3 kg), because we considered these clinically relevant cut-points. In secondary analyses, IDWG dichotomization cut-points were varied (≤2.5 versus >2.5 kg, ≤3.5 versus >3.5 kg, and ≤4.0 versus >4.0 kg). Baseline participant characteristics were described across exposure groups as counts and proportions for categorical variables and as means and SDs for continuous variables. Bivariable comparisons were made using contingency table methods and chi-squared testing, t tests, or ANOVA, as dictated by data type.
In the DSL–mortality analysis, patients delivered DSL <240 versus ≥240 minutes were matched 1:1 on the basis of age (±2.5 years), sex (identical), vascular access type (identical), postdialysis weight (nearest neighbor with caliper width not exceeding ±1.5 kg), and IDWG (±0.25 kg). Similarly, in the IDWG–mortality analysis, patients with IDWG ≤3 versus >3 kg were matched 1:1 on the basis of age (±2.5 years), sex (identical), vascular access type (identical), postdialysis weight (nearest neighbor with caliper width not exceeding ±1.5 kg), and delivered DSL (rounded to the nearest 5-minute increment). The postdialysis weight caliper width of 1.5 kg was empirically selected based on a balance of minimizing weight differences between matched pair members and achieving adequate successful pair matches for power considerations; this was done without consideration of outcome. All survival analyses were then stratified on matched pair assignment. Adjusted associations were estimated using multivariable Cox proportional hazards models with inclusion of covariate terms for other variables plausibly associated with both exposure and outcome; this included terms for age and postdialysis weight (and in DSL–mortality analyses, IDWG) to account for residual imbalance despite matching. Kt/V was excluded from the primary DSL–mortality analysis because it represented a plausible pathway intermediate, and because it introduced collinearity into the model. Sensitivity analyses with its inclusion yielded near identical estimates (data not shown). Specification of continuous covariates (linear versus categorical) was guided by each covariate’s observed association with outcome as assessed by regression coefficient graphical evaluation, Akaike’s information criterion, and Martingale residual plots. The proportionality assumption for each model was tested graphically and by Schoenfeld residual testing (no variables were in violation).
Effect modification of the DSL–mortality and IDWG–mortality associations on the basis of age, sex, and postdialysis weight and IDWG and delivered DSL was explored through restriction subgroup analysis; for these analyses, age, postdialysis weight, IDWG, and DSL were dichotomized at their medians. Significance of interaction was assessed by likelihood ratio testing of nested models that did and did not include two-way cross product terms (factor by exposure). All analyses were performed using Stata/MP software (release 10; StataCorp LP, College Station, TX).
Results
Baseline Characteristics of Cohorts
Characteristics of patients in the source cohort are presented in Table 1. Compared with patients with DSL ≥240 minutes, those with DSL <240 minutes were older, had lower mean IDWGs and postdialysis weights, were more likely to be women, and were less likely to have diabetes, coronary artery disease, and congestive heart failure. Compared with patients with IDWG ≤3 kg, those with IDWG >3 kg were younger, had higher mean DSLs, postdialysis weights, and predialysis systolic BPs, and were more likely to be men, black, and have diabetes and congestive heart failure.
Table 1.
Comparison of baseline characteristics between delivered DSL <240 versus ≥240 minutes and IDWG ≤3 versus >3 kg within respective (unmatched) source cohorts
| Characteristics | Delivered DSL <240 min (n=7098) | Delivered DSL ≥240 min (n=2327) | P | IDWG ≤3 kg (n=7758) | IDWG >3 kg (n=4076) | P |
|---|---|---|---|---|---|---|
| Delivered DSL (min) | — | <0.001 | ||||
| Mean ± SD | 206.9±20.4 | 246.9±8.3 | 208.4±24.3 | 224.2±22.8 | ||
| Median | 210.0 | 243.3 | 210.0 | 227.4 | ||
| IQR | [190.0, 223.4] | [240.6, 250.8] | [185.5, 228.0] | [209.7, 240.6] | ||
| IDWG (kg) | <0.001 | — | ||||
| Mean ± SD | 2.5±1.0 | 3.2±1.2 | 2.0±0.6 | 3.8±0.7 | ||
| Median | 2.4 | 3.1 | 2.1 | 3.7 | ||
| IQR | [1.8, 3.1] | [2.3, 4.0] | [1.6, 2.5] | [3.3, 4.2] | ||
| IDWG (% of body weight)a | <0.001 | <0.001 | ||||
| Mean ± SD | 3.6±1.5 | 3.8±1.4 | 3.0±1.1 | 4.8±1.2 | ||
| Median | 3.5 | 3.7 | 3.0 | 4.7 | ||
| IQR | [2.6, 4.5] | [2.8, 4.6] | [2.2, 3.7] | [4.0, 5.5] | ||
| Age (yr) | 62.9±15.3 | 60.1±13.5 | <0.001 | 65.4±14.8 | 57.2±13.9 | <0.001 |
| Female sex | 3822 (53.9) | 719 (30.9) | <0.001 | 4325 (55.7) | 1465 (35.9) | <0.001 |
| Access | 0.002 | <0.001 | ||||
| Fistula | 2634 (37.1) | 933 (40.1) | 2544 (32.8) | 1827 (44.8) | ||
| Graft | 2323 (32.7) | 668 (28.7) | 2391 (30.8) | 1390 (34.1) | ||
| Catheter | 2074 (29.2) | 708 (30.4) | 2761 (35.6) | 819 (20.1) | ||
| Missing | 67 (0.9) | 18 (0.8) | 64 (0.8) | 40 (1.0) | ||
| Postdialysis weight (kg) | 71.4±17.3 | 86.2±20.9 | <0.001 | 69.8±16.6 | 83.6±20.3 | <0.001 |
| Race | <0.001 | <0.001 | ||||
| White | 4343 (61.2) | 1275 (54.8) | 4857 (62.6) | 2405 (59.0) | ||
| Black | 2714 (38.2) | 1037 (44.6) | 2852 (36.8) | 1656 (40.6) | ||
| Missing | 42 (0.6) | 15 (0.6) | 51 (0.7) | 15 (0.4) | ||
| Diabetes | 3635 (51.2) | 1355 (58.2) | <0.001 | 3870 (49.9) | 2334 (57.3) | <0.001 |
| Coronary artery disease | 825 (11.6) | 332 (14.3) | 0.001 | 907 (11.7) | 532 (13.1) | 0.03 |
| Congestive heart failure | 2589 (36.5) | 978 (42.0) | <0.001 | 2597 (33.5) | 1833 (45.0) | <0.001 |
| Vintage (yr) | 0.003 | <0.001 | ||||
| <1 | 1787 (25.2) | 531 (22.8) | 2340 (30.2) | 621 (15.2) | ||
| [1–2) | 1046 (14.7) | 324 (13.9) | 1106 (14.3) | 628 (15.4) | ||
| [2–4) | 1780 (25.1) | 589 (25.3) | 1811 (23.3) | 1147 (28.1) | ||
| ≥4 | 2463 (34.7) | 871 (37.4) | 2465 (31.8) | 1672 (41.0) | ||
| Missing | 22 (0.3) | 12 (0.5) | 38 (0.5) | 8 (0.2) | ||
| Predialysis SBP (mmHg) | 0.01 | <0.001 | ||||
| ≤130 | 982 (13.8) | 361 (15.5) | 1240 (16.0) | 491 (12.1) | ||
| 131–150 | 2247 (31.7) | 784 (33.7) | 2604 (33.6) | 1244 (30.5) | ||
| 151–170 | 2550 (35.9) | 749 (32.2) | 2578 (33.2) | 1485 (36.4) | ||
| >170 | 1319 (18.6) | 433 (18.6) | 1338 (17.3) | 856 (21.0) | ||
| Urea reduction ratio (%) | 74.7±5.2 | 74.3±5.2 | <0.001 | 75.1±5.2 | 73.4±5.0 | <0.001 |
| eKt/V | 1.4±0.3 | 1.5±0.3 | <0.001 | 1.4±0.3 | 1.4±0.3 | 0.09 |
| n | 6582 | 2168 | 7190 | 3757 | ||
| Missed sessionsb | <0.001 | <0.001 | ||||
| 0 | 4929 (69.4) | 1751 (75.3) | 5386 (69.4) | 2987 (73.3) | ||
| 1 | 733 (10.3) | 205 (8.8) | 771 (9.9) | 404 (9.9) | ||
| 2 | 620 (8.7) | 184 (7.9) | 725 (9.3) | 311 (7.6) | ||
| 3 | 233 (3.3) | 50 (2.2) | 253 (3.3) | 93 (2.3) | ||
| ≥4 | 583 (8.2) | 137 (5.9) | 625 (8.1) | 281 (6.9) | ||
| Albumin (g/dl) | 0.03 | <0.001 | ||||
| ≤3.0 | 488 (6.9) | 152 (6.5) | 1587 (20.5) | 546 (13.4) | ||
| 3.1–3.5 | 1298 (18.3) | 375 (16.1) | 3424 (44.1) | 1933 (47.4) | ||
| 3.6–4.0 | 3177 (44.8) | 1066 (45.8) | 2060 (26.6) | 1428 (35.0) | ||
| >4.0 | 2106 (29.7) | 716 (30.8) | 33 (0.4) | 26 (0.6) | ||
| Missing | 29 (0.4) | 18 (0.8) | ||||
| Creatinine (quartile) | <0.001 | <0.001 | ||||
| 1 | 1804 (25.4) | 487 (20.9) | 2384 (30.7) | 552 (13.5) | ||
| 2 | 1780 (25.1) | 518 (22.3) | 2046 (26.4) | 857 (21.0) | ||
| 3 | 1708 (24.1) | 588 (25.3) | 1766 (22.8) | 1087 (26.7) | ||
| 4 | 1606 (22.6) | 648 (27.9) | 1331 (17.2) | 1460 (35.8) | ||
| Missing | 200 (2.8) | 86 (3.7) | 233 (3.0) | 120 (2.9) | ||
| Phosphorus (mg/dl) | 0.01 | <0.001 | ||||
| ≤4.0 | 1518 (21.4) | 447 (19.2) | 1937 (25.0) | 517 (12.7) | ||
| 4.1–5.0 | 1861 (26.2) | 598 (25.7) | 2202 (28.4) | 930 (22.8) | ||
| 5.1–6.0 | 1670 (23.5) | 586 (25.2) | 1796 (23.1) | 1008 (24.7) | ||
| >6.0 | 2033 (28.6) | 682 (29.3) | 1801 (23.2) | 1608 (39.5) | ||
| Missing | 16 (0.2) | 14 (0.6) | 24 (0.3) | 13 (0.3) |
Values presented as mean ± SD or n (%) except where indicated. Across delivered session length groups and IDWG groups; determined by t test for continuous variables and chi-squared testing for categorical variables. DSL, dialysis session length; IDWG, interdialytic weight gain; IQR, interquartile range; SBP, systolic BP.
Calculated as IDWG (kg)/postweight (kg).
Number of dialysis sessions missed during the 30-day exposure period.
Primary Analyses
In the primary analysis of delivered DSL–mortality, 897 patients with DSL <240 minutes were pair-matched to 1 control with DSL ≥240 minutes (38.5% of eligible patients with DSL ≥240 minutes were successfully matched). The matched pairs demonstrated excellent balance across all matching factors including IDWG (Table 2 and Supplemental Figure 1).
Table 2.
Comparison of age, sex, vascular access type, postdialysis weights, IDWG, and DSL and other covariates between patients delivered DSL <240 versus ≥240 minutes and IDWG ≤3 versus >3 kg in the matched analytical cohorts
| Characteristics | Delivered DSL <240 min (n=897) | Delivered DSL ≥240 min (n=897) | P | IDWG ≤3 kg (n=1057) | IDWG >3 kg (n=1057) | P |
|---|---|---|---|---|---|---|
| Matching factors and variables derived from these | ||||||
| Age (yr) | — | — | ||||
| Mean ± SD | 63.6±11.7 | 63.6±11.8 | 62.4±11.7 | 62.4±11.7 | ||
| Median [IQR] | 64.0 [56.0, 72.0] | 64.0 [56.0, 73.0] | 63.0 [55.0, 71.0] | 63.0 [54.0, 71.0] | ||
| Min, max | (24, 91) | (24, 90) | (23, 90) | (24, 89) | ||
| Female | 307 (34.2) | 307 (34.2) | — | 404 (38.2) | 404 (38.2) | — |
| Access | — | — | ||||
| Fistula | 406 (45.3) | 406 (45.3) | 500 (47.3) | 500 (47.3) | ||
| Graft | 239 (26.6) | 239 (26.6) | 345 (32.6) | 345 (32.6) | ||
| Catheter | 250 (27.9) | 250 (27.9) | 212 (20.1) | 212 (20.1) | ||
| Missing | 2 (0.2) | 2 (0.2) | 0 | 0 | ||
| Postdialysis weight (kg) | — | — | ||||
| Mean ± SD | 76.5±13.7 | 76.5±13.8 | 75.5±13.3 | 75.5±13.4 | ||
| Median [IQR] | 75.6 [66.3, 85.7] | 75.6 [66.2, 85.6] | 74.0 [66.1, 83.9] | 73.9 [66.0, 84.2] | ||
| Min, max | (45.3, 130.2) | (44.4, 129.4) | (39.5, 129.2) | (40.1, 130.4) | ||
| IDWG (kg) | — | — | ||||
| Mean ± SD | 2.8±0.9 | 2.8±0.9 | 2.1±0.6 | 3.7±0.6 | ||
| Median [IQR] | 2.8 [2.2, 3.4] | 2.8 [2.2, 3.4] | 2.3 [1.8, 2.6] | 3.6 [3.2, 4.1] | ||
| Min, max | (0.4, 5.9) | (0.3, 5.9) | (1.1, 3.0) | (3.0, 6.8) | ||
| IDWG (% of body weight)a | — | — | ||||
| Mean ± SD | 3.7±1.1 | 3.7±1.1 | 2.9±1.0 | 5.1±1.1 | ||
| Median [IQR] | 3.6 [3.0, 4.1] | 3.7 [3.1, 4.3] | 3.0 [2.3, 3.6] | 4.9 [4.3, 5.6] | ||
| Min, max | (0.5, 6.9) | (0.5, 7.1) | (0.0, 5.8) | (2.8, 9.8) | ||
| Delivered DSL (min) | — | |||||
| Mean ± SD | 211.2±19.6 | 244.8±6.8 | 218.1±20.5 | 218.1±20.5 | ||
| Median [IQR] | 211.4 [197.4, 227.1] | 242 [240.3, 245.8] | 215.0 [210.0, 240.0] | 215.0 [210.0, 240.0] | ||
| Min, max | (150, 240) | (240, 270) | (165, 270) | (165, 270) | ||
| Other covariates | ||||||
| Race | 0.005 | 0.03 | ||||
| Nonblack | 567 (63.2) | 506 (56.4) | 630 (59.6) | 690 (65.3) | ||
| Black | 328 (36.6) | 384 (42.8) | 419 (39.6) | 361 (34.2) | ||
| Missing | 2 (0.2) | 7 (0.8) | 8 (0.8) | 6 (0.5) | ||
| Diabetes | 494 (55.1) | 529 (59.0) | 0.10 | 545 (51.6) | 657 (62.2) | <0.001 |
| Coronary artery disease | 101 (11.3) | 137 (15.3) | 0.01 | 131 (12.4) | 150 (14.2) | 0.22 |
| Congestive heart failure | 341 (38.0) | 341 (38.0) | 0.99 | 376 (35.6) | 456 (43.1) | <0.001 |
| Vintage (yr) | 0.13 | <0.001 | ||||
| <1 | 228 (25.4) | 206 (23.0) | 261 (24.7) | 166 (15.7) | ||
| [1–2) | 148 (16.5) | 123 (13.7) | 181 (17.1) | 165 (15.6) | ||
| [2–4) | 212 (23.6) | 220 (24.5) | 249 (23.6) | 305 (28.9) | ||
| ≥4 | 306 (34.1) | 341 (38.0) | 363 (34.3) | 421 (39.8) | ||
| Missing | 3 (0.3) | 7 (0.8) | 3 (0.3) | 0 | ||
| Predialysis SBP (mmHg) | 0.05 | 0.03 | ||||
| ≤130 | 125 (13.9) | 149 (16.6) | 142 (13.4) | 138 (13.1) | ||
| 131–150 | 278 (31.0) | 311 (34.7) | 357 (33.8) | 302 (28.6) | ||
| 151–170 | 323 (36.0) | 278 (31.0) | 372 (35.2) | 392 (37.1) | ||
| >170 | 171 (19.1) | 159 (17.7) | 186 (17.6) | 225 (21.3) | ||
| Urea reduction ratio (%) | 73.6±5.1 | 75.6±5.2 | <0.001 | 74.7±4.8 | 73.9±4.8 | <0.001 |
| eKt/V | 1.4±0.3 | 1.5±0.3 | <0.001 | 1.4±0.3 | 1.4±0.2 | 0.05 |
| n | 829 | 838 | 972 | 975 | ||
| Missed sessionsb | 0.007 | 0.12 | ||||
| 0 | 622 (69.3) | 686 (76.5) | 765 (72.4) | 809 (76.5) | ||
| 1 | 104 (11.6) | 69 (7.7) | 106 (10.0) | 90 (8.5) | ||
| 2 | 71 (7.9) | 68 (7.6) | 87 (8.2) | 78 (7.4) | ||
| 3 | 29 (3.2) | 20 (2.2) | 31 (2.9) | 17 (1.6) | ||
| ≥4 | 71 (7.9) | 54 (6.0) | 68 (6.4) | 63 (6.0) | ||
| Albumin (g/dl) | 0.14 | 0.001 | ||||
| ≤3.0 | 49 (5.5) | 74 (8.3) | 74 (7.0) | 35 (3.3) | ||
| 3.1–3.5 | 147 (16.4) | 133 (14.8) | 179 (16.9) | 155 (14.7) | ||
| 3.6–4.0 | 421 (46.9) | 399 (44.5) | 471 (44.6) | 502 (47.5) | ||
| >4.0 | 275 (30.7) | 284 (31.7) | 330 (31.2) | 362 (34.3) | ||
| Missing | 5 (0.6) | 7 (0.8) | 3 (0.3) | 3 (0.3) | ||
| Creatinine (quartile) | 0.78 | 0.002 | ||||
| 1 | 214 (23.9) | 224 (25.0) | 303 (28.7) | 233 (22.0) | ||
| 2 | 219 (24.4) | 222 (24.8) | 255 (24.1) | 253 (23.9) | ||
| 3 | 211 (23.5) | 222 (24.8) | 239 (22.6) | 267 (25.3) | ||
| 4 | 220 (24.5) | 198 (22.1) | 226 (21.4) | 277 (26.2) | ||
| Missing | 33 (3.7) | 31 (3.5) | 34 (3.2) | 27 (2.6) | ||
| Phosphorus (mg/dl) | 0.08 | <0.001 | ||||
| ≤4.0 | 176 (19.6) | 201 (22.4) | 253 (23.9) | 135 (12.8) | ||
| 4.1–5.0 | 256 (28.5) | 240 (26.8) | 291 (27.5) | 267 (25.3) | ||
| 5.1–6.0 | 200 (22.3) | 230 (25.6) | 281 (26.6) | 280 (26.5) | ||
| >6.0 | 262 (29.2) | 221 (24.6) | 230 (21.8) | 374 (35.4) | ||
| Missing | 3 (0.3) | 5 (0.6) | 2 (0.2) | 1 (0.1) |
Values presented as mean ± SD or n (%) except where indicated. DSL, dialysis session length; IDWG, interdialytic weight gain; IQR, interquartile range; SBP, systolic BP.
Calculated as IDWG (kg)/postweight (kg).
Number of dialysis sessions missed during the 30-day exposure period.
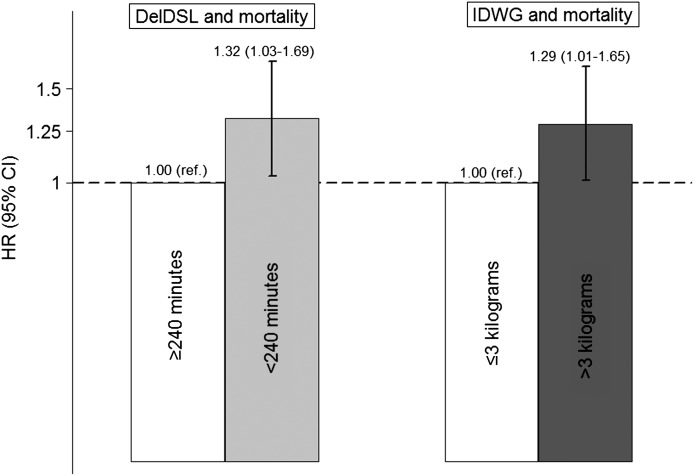
Overall, 567 deaths occurred during 4362 patient-years of at-risk time. The median survival time was 25.0 months. Compared with delivered DSL ≥240 minutes, DSL <240 minutes was significantly associated with increased mortality (adjusted hazard ratio [HR], 1.32; 95% confidence interval [95% CI], 1.03 to 1.69; P=0.03) (Figure 1). The estimates were consistent across strata of age (interaction P=0.92 for <65 versus ≥65 years), sex (interaction P=0.46), postdialysis weight (interaction P=0.28 for <75 versus ≥75 kg), IDWG (interaction P=0.56 for ≤2.8 versus >2.8 kg), and among patients who did and did not miss ≥1 dialysis sessions during the exposure assessment period (interaction P=0.15).
Figure 1.
Adjusted associations between delivered DSL and mortality and IDWG and mortality based on Cox regression models. In the DSL analysis, patients were matched on sex, access type (fistula, graft, catheter), age (±2.5 years), IDWG (±0.25 kg), and postdialysis weight (±1.5 kg). In the IDWG analysis, patients were matched on sex, access type (fistula, graft, catheter), age (±2.5 years), delivered DSL (rounded to 5 minutes), and postdialysis weight (±1.5 kg). Multivariable models were stratified on matched pair assignment, and include covariate adjustment terms for age, race (black, nonblack), postdialysis weight (kg), vintage (<1, 2, 3, ≥4 years), diabetes, coronary artery disease, congestive heart failure, missed dialysis sessions over 30 days (0, 1, 2, 3, ≥4), creatinine (quartiles, mg/dl), albumin (≤3.0, 3.1–3.5, 3.6–4.0, >4.0 g/dl), phosphorus (≤4.0, 4.1–5.0, 5.1–6.0, >6.0 mg/dl), and predialysis systolic BP (≤130, 131–150, 151–170, >170 mmHg); in the DSL analysis, the model was also adjusted for IDWG (kg). DSL, dialysis session length; IDWG, interdialytic weight gain; DelDSL, delivered dialysis session length; HR, hazard ratio; 95% CI, 95% confidence interval; ref, reference.
In the primary analysis of IDWG–mortality, 1057 patients with IDWG >3 kg (25.9% of those eligible) were successfully pair-matched to one control with IDWG ≤3 kg. The matched pairs were well balanced across all matching factors including delivered DSL (Table 2 and Supplemental Figure 2).
Overall, 646 deaths occurred during 5087 patient-years of at-risk time. The median survival time was 25.0 months. Compared with IDWG ≤3 kg, IDWG >3 kg was significantly associated with increased mortality (adjusted HR, 1.29; 95% CI, 1.01 to 1.65; P=0.04) (Figure 1). Similar estimates were observed across strata of age (interaction P=0.33 for <65 versus ≥65 years), sex (interaction P=0.24), postdialysis weight (interaction P=0.86 <75 versus ≥75 kg), delivered DSL (interaction P=0.17 for ≤215 versus >215 minutes), and among patients who did and did not miss ≥1 dialysis sessions during the exposure assessment period (interaction P=0.42).
Secondary Analyses
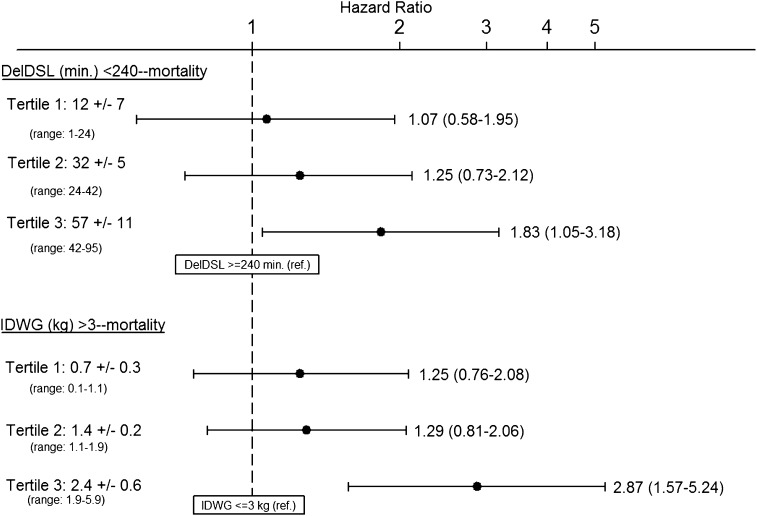
To examine for a dose-response relationship in the delivered DSL–mortality association, we calculated the difference in DSL between matched pair members and categorized pairs by tertile of DSL difference. The association between DSL ≥240 and <240 minutes and mortality was then estimated within each tertile. The magnitude of the association between delivered DSL <240 minutes (referent to DSL ≥240) and mortality was incrementally larger when DSL differences between matched pair members were greater (Figure 2).
Figure 2.
Adjusted associations between delivered DSL <240 minutes (versus delivered DSL ≥240 minutes) and mortality and IDWG >3 kg (versus IDWG ≤3 kg) and mortality among matched pairs in which differences between matched pairs in delivered DSL and IDWG are parsed into tertiles. Results in the top panel indicate the association between DSL <240 minutes (versus DSL ≥240 minutes) among strata of matched pairs in which the difference in DSL (between pair members) was 1–24 minutes (tertile 1), 24–42 minutes (tertile 2), and 42–95 minutes (tertile 3). Results in the bottom panel indicate the associations between IDWG >3 kg (versus IDWG ≤3 kg) among strata of matched pairs in which the difference in IDWG (between pair members) was 0.1–1.1 kg (tertile 1), 1.1–1.9 (tertile 2) and 1.9–5.9 (tertile 3). Patients with DSL <240 versus ≥240 minutes were matched on sex, access type (fistula, graft, catheter), age (±2.5 years), IDWG (±0.25 kg), and postdialysis weight (±1.5 kg). Patients with IDWG >3 versus ≤3 kg were matched on sex, access type (fistula, graft, catheter), age (±2.5 years), delivered DSL (rounded to the nearest 5 minutes), and postdialysis weight (±1.5 kg). Multivariable models were stratified on matched pair assignment and included covariate adjustment terms for age, race (black, nonblack), postdialysis weight (kg), vintage (<1, 2, 3, ≥4 years), diabetes, coronary artery disease, congestive heart failure, missed dialysis sessions over 30 days (0, 1, 2, 3, ≥4), creatinine (quartiles, mg/dl), albumin (≤3.0, 3.1–3.5, 3.6–4.0, >4.0 g/dl), phosphorus (≤4.0, 4.1–5.0, 5.1–6.0, >6.0 mg/dl), and predialysis systolic BP (≤130, 131–150, 151–170, >170 mmHg) in both analyses. In the DSL analysis, the model was also adjusted for IDWG (kg). DSL, dialysis session length; DelDSL, delivered dialysis session length; IDWG, interdialytic weight gain.
An analogous secondary analysis was performed stratifying matched IDWG pairs on the difference in IDWG between pair members. The magnitude of the association between higher (versus lower) IDWG was incrementally larger when differences in IDWG were greater (Figure 2). To further evaluate for a dose-response trend in the IDWG–mortality association, we compared the association between high (versus low) IDWG varying the threshold value used to distinguish low and high IDWG (≤2.5 and >2.5 kg, ≤3.5 and >3.5 kg, and ≤4.0 and >4.0 kg). As the threshold for categorization was increased, the magnitude of the association between high IDWG and mortality was larger (Table 3).
Table 3.
Adjusted associations between high IDWG and mortality using varying thresholds to distinguish between low and high IDWG
| High IDWG (kg) | Low IDWG (kg)a | Matched Pairs (n) | Adjusted HR (95% CI) |
|---|---|---|---|
| >2.5 | ≤2.5 | 1261 | 1.23 (0.98 to 1.53) |
| >3.0 | ≤3.0 | 1057 | 1.29 (1.01 to 1.65) |
| >3.5 | ≤3.5 | 742 | 1.84 (1.36 to 2.50) |
| >4.0 | ≤4 | 436 | 2.85 (1.80 to 4.50) |
In separate analyses, high and low IDWG patients were matched on sex, access type (fistula, graft, catheter), age (±2.5 years), delivered DSL (rounded to 5 minutes), and postdialysis weight (±1.5 kg). Multivariable models were stratified on matched pair assignment, and include covariate adjustment terms for age, race (black, nonblack), postdialysis weight (kg), vintage (<1, 2, 3, ≥4 years), diabetes, coronary artery disease, congestive heart failure, missed dialysis sessions over 30 days (0, 1, 2, 3, ≥4), creatinine (quartiles mg/dl), albumin (≤3.0, 3.1–3.5, 3.6–4.0, >4.0 g/dl), phosphorus (≤4.0, 4.1–5.0, 5.1–6.0, >6.0 mg/dl), and predialysis systolic BP (≤130, 131–150, 151–170, >170 mmHg). IDWG, interdialytic weight gain; DSL, dialysis session length; HR, hazard ratio; 95% CI, 95% confidence interval.
Reference.
Discussion
Prior observational studies have demonstrated positive associations between UFRs and mortality, but no study has fully evaluated the independent roles of DSL and IDWG in mediating this relationship. In this analysis, we demonstrate that among patients receiving thrice-weekly in-center hemodialysis with adequate urea clearance, both DSL and IDWG play important roles in the UFR–mortality relationship, and that these associations are independent of one another. Specifically, shorter delivered DSL is associated with increased mortality independent of IDWG, and larger IDWG is associated with increased mortality independent of delivered DSL. In addition, the data suggest dose-response relationships in both associations, adding evidence in favor of causality. These observations indicate that both DSL extension and IDWG reduction are potentially viable targets for directed clinical intervention.
Prior epidemiologic studies have demonstrated associations between DSL and IDWG and mortality. Four such studies showed an association between shorter session length and decreased survival (3,11,12,14). Of these studies, only two adjusted for a measure of IDWG—one by using ultrafiltration volume as a proxy (3)—and did so through inclusion of a covariate term in the multivariable survival model (3,14). In at least two of these studies, limited overlap of IDWG across DSL groups was observed (3,14), implicitly limiting multivariable modeling’s ability to adequately adjust for confounding.
Despite uncertainty in the empirical clinical data, it remains highly plausible that shorter DSL increases the risk of death. By necessitating more rapid fluid removal in a compressed timeframe, shorter dialysis sessions expose patients to greater fluid shifts with attendant myocardial stunning and ischemia (15), intradialytic hypotension, hemodynamic destabilization (16), and resultant interruptions of end-organ perfusion. The cumulative consequences of these cardiac stresses have been linked to maladaptive changes in left ventricular geometry such as hypertrophy and fibrosis with derivative heart failure and conduction system abnormalities (15,17–22). Shorter dialysis sessions may also detrimentally affect survival through limiting removal of phosphorus, β2-microglobulin, and other middle molecules (23–25).
Prior studies have also demonstrated an association between greater IDWG and increased mortality (7–10). Kalantar-Zadeh et al., for example, demonstrated an adjusted HR of 1.25 (95% CI, 1.12 to 1.39) for IDWG ≥4.0 kg versus referent 1.5–2.0 kg (8). However, none of the prior studies adjusted IDWG–mortality associations for DSL, making it impossible to ascertain whether the associations observed were truly independent of DSL differences. As with the DSL–mortality association, strong biologic basis for deleterious consequences of greater IDWG does exist. Chronic volume overload promotes maladaptive cardiac structural changes (e.g., left ventricular hypertrophy and fibrosis) through direct activation of the mammalian target of rapamycin pathway (26–28) and through upregulation of the sympathetic nervous system and renin-angiotensin-aldosterone pathways (29,30). This, in turn, distorts cardiac conduction pathway architecture and predisposes patients to arrhythmias and sudden cardiac death (18–22).
Delineating the independent associations of IDWG and DSL with mortality has important therapeutic implications. Were only IDWG independently associated with mortality, this would signal the need for strategies aimed at mitigating fluid accumulation and argue against a need for extending treatment times beyond the minimal time required to meet clearance goals. Were only DSL independently associated with mortality, the converse would be true. Our results demonstrate that both IDWG and DSL are independently (of one another) associated with mortality, suggesting that either intervention (or both) could be favorable. Moreover, the absence of statistical interaction between DSL and IDWG suggests that extension of DSL (to ≥240 minutes) would be favorable regardless of ambient IDWG and that mitigation of IDWG (to ≤3 kg) would be favorable regardless of ambient treatment time. One potential therapeutic strategy would be DSL titration on a session-by-session basis (using 240 minutes as a floor) in response to interval IDWG. Such a strategy might actualize the benefit of extended treatment times while also serving as a deterrent for untoward fluid intake. Because longer dialysis and dietary restriction are unappealing to patients (and the latter poorly adhered to), further research is needed to identify and explore the feasibility of alternative therapies that might prove more acceptable to patients.
Despite the growing body of observational data demonstrating the survival benefit of treatment time extension, the mean DSL decreased from 220.8 minutes in 2002 to 216.3 minutes in 2010 in the United States (31). There are several barriers to DSL extension in the United States. These include strict shift schedules, limited physical plant and personnel resources, and financial constraints. In addition, longer sessions are often unappealing to patients. Finally, some practitioners question the validity of the DSL–mortality association given the absence of randomized trial data and are thus reticent to extend DSL in patients with adequate clearance (32).
Strengths of this study include its large, nationally representative cohort, utilization of standardized protocols for dialysis session data collection, and the use of matching to strictly control for the most important confounders. Several limitations of this study bear mention. Uncontrolled confounding or bias is always possible with observational research. To minimize the risk of residual confounding, we matched patients on the factors identified as the most influential confounders, resulting in almost identical balance of age, sex, vascular access type, weight, and IDWG and delivered DSL. In addition, statistical models were adjusted to account for confounding on the basis of a number of additional potential factors. Due to data limitations, we were unable to consider residual renal function, but we did include dialytic vintage as a partial surrogate to residual urine output. We attempted to control for confounding on the basis of treatment nonadherence by adjusting for missed sessions during a 30-day period, BP, and serum phosphorus. Despite these efforts, we cannot exclude the possibility of residual confounding from residual renal function, nonadherence, or other unconsidered factors. Due to the large number of centers studied, we could not directly control for center effect. We attempted to minimize its influence by excluding patients dialyzed at facilities where there was not at least one participant in each delivered DSL category; however, residual confounding could remain. Our use of proportional hazards models with transplant and transfer of care as censoring variables may also have introduced bias because such censoring criteria may not be entirely independent from mortality. In addition, we excluded patients with URR ≤65% in order to study the benefit of DSL extension beyond that required to meet adequacy goals, and patients with delivered DSL <2.5 and >4.5 hours in order to bear relevance to the majority of US patients who dialyze thus. We also excluded patients for whom suitable matches could not be identified in the source cohort, most notably those at the extremes of body weight. Thus, our results should not be extrapolated to patients who are not characteristic of the matched analytical cohorts studied here. In particular, body weight was substantially lower in the high IDWG group of the matched versus source cohort; therefore, these data cannot assess whether large IDWG may be harmful to larger patients. Finally, although our analysis does evaluate the independent associations between DSL and IDWG and mortality, the relative importance of these two phenomena in the UFR–mortality association cannot be inferred from our data, because estimates of associations are expressed on different (nontransferable) scales.
In conclusion, this study demonstrates that among chronic hemodialysis patients with adequate urea clearance, shorter delivered DSL and greater IDWG are each associated with increased all-cause mortality independently of one another. Further prospective studies are needed to confirm and generalize findings and to study the efficacy of targeted interventions.
Disclosures
In the past, S.M.B. has served on advisory boards for Amgen and C.B. Fleet and has received speaking honoraria from Fresenius Medical Care; his spouse is an employee at AstraZeneca. S.M.B. is now an employee at DaVita Clinical Research; however, this affiliation was not present at the time of data analysis. G.C.C. discloses financial involvement in UpToDate, Inc. (author, section editor) and the American Society of Nephrology (CJASN, Editor-in-Chief).
Supplementary Material
Acknowledgments
The authors thank DaVita Clinical Research for providing data for this study. DaVita Clinical Research is committed to advancing the knowledge and practice of kidney care.
This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (DK093159-01 to J.E.F., DK91417 to G.C.C., and DK079056 to S.M.B.). DaVita Inc. provided data for the project. DaVita Clinical Research had no role in the design or implementation of this study, nor on the decision to publish.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.09460912/-/DCSupplemental.
See related editorial, “Oh! What a Tangled Web We Weave,” on pages 1066–1067.
References
- 1.Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ: Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: A pooled analysis of community-based studies. J Am Soc Nephrol 15: 1307–1315, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Foley RN, Parfrey PS, Sarnak MJ: Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32[Suppl 3]: S112–S119, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Saran R, Bragg-Gresham JL, Levin NW, Twardowski ZJ, Wizemann V, Saito A, Kimata N, Gillespie BW, Combe C, Bommer J, Akiba T, Mapes DL, Young EW, Port FK: Longer treatment time and slower ultrafiltration in hemodialysis: Associations with reduced mortality in the DOPPS. Kidney Int 69: 1222–1228, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Movilli E, Gaggia P, Zubani R, Camerini C, Vizzardi V, Parrinello G, Savoldi S, Fischer MS, Londrino F, Cancarini G: Association between high ultrafiltration rates and mortality in uraemic patients on regular haemodialysis. A 5-year prospective observational multicentre study. Nephrol Dial Transplant 22: 3547–3552, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Flythe JE, Kimmel SE, Brunelli SM: Rapid fluid removal during dialysis is associated with cardiovascular morbidity and mortality. Kidney Int 79: 250–257, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curatola G, Bolignano D, Rastelli S, Caridi G, Tripepi R, Tripepi G, Politi R, Catalano F, Delfino D, Ciccarelli M, Mallamaci F, Zoccali C: Ultrafiltration intensification in hemodialysis patients improves hypertension but increases AV fistula complications and cardiovascular events. J Nephrol 24: 465–473, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Kimmel PL, Varela MP, Peterson RA, Weihs KL, Simmens SJ, Alleyne S, Amarashinge A, Mishkin GJ, Cruz I, Veis JH: Interdialytic weight gain and survival in hemodialysis patients: Effects of duration of ESRD and diabetes mellitus. Kidney Int 57: 1141–1151, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Kalantar-Zadeh K, Regidor DL, Kovesdy CP, Van Wyck D, Bunnapradist S, Horwich TB, Fonarow GC: Fluid retention is associated with cardiovascular mortality in patients undergoing long-term hemodialysis. Circulation 119: 671–679, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stegmayr BG, Brannstrom M, Bucht S, Dimeny E, Ekspong A, Granroth B, Grontoft KC, Hadimeri H, Holmberg B, Ingman B, Isaksson B, Johansson G, Lindberger K, Lundberg L, Lundstrom O, Mikaelsson L, Mortzell M, Olausson E, Persson B, Svensson L, Wikdahl AM: Minimized weight gain between hemodialysis contributes to a reduced risk of death. Int J Artif Organs 29: 675–680, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Holmberg B, Stegmayr BG: Cardiovascular conditions in hemodialysis patients may be worsened by extensive interdialytic weight gain. Hemodial Int 13: 27–31, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Brunelli SM, Chertow GM, Ankers ED, Lowrie EG, Thadhani R: Shorter dialysis times are associated with higher mortality among incident hemodialysis patients. Kidney Int 77: 630–636, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marshall MR, Byrne BG, Kerr PG, McDonald SP: Associations of hemodialysis dose and session length with mortality risk in Australian and New Zealand patients. Kidney Int 69: 1229–1236, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Lowrie EG, Li Z, Ofsthun N, Lazarus JM: Measurement of dialyzer clearance, dialysis time, and body size: Death risk relationships among patients. Kidney Int 66: 2077–2084, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Flythe JE, Curhan GC, Brunelli SM: Shorter length dialysis sessions are associated with increased mortality, independent of body weight. Kidney Int 83: 104–113, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burton JO, Jefferies HJ, Selby NM, McIntyre CW: Hemodialysis-induced cardiac injury: Determinants and associated outcomes. Clin J Am Soc Nephrol 4: 914–920, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flythe JE, Kunaparaju S, Dinesh K, Cape K, Feldman HI, Brunelli SM: Factors associated with intradialytic systolic blood pressure variability. Am J Kidney Dis 59: 409–418, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Shivalkar B, Flameng W, Szilard M, Pislaru S, Borgers M, Vanhaecke J: Repeated stunning precedes myocardial hibernation in progressive multiple coronary artery obstruction. J Am Coll Cardiol 34: 2126–2136, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Ritz E, Wanner C: The challenge of sudden death in dialysis patients. Clin J Am Soc Nephrol 3: 920–929, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Törnig J, Amann K, Ritz E, Nichols C, Zeier M, Mall G: Arteriolar wall thickening, capillary rarefaction and interstitial fibrosis in the heart of rats with renal failure: The effects of ramipril, nifedipine and moxonidine. J Am Soc Nephrol 7: 667–675, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Chen PS, Chou CC, Tan AY, Zhou S, Fishbein MC, Hwang C, Karagueuzian HS, Lin SF: The mechanisms of atrial fibrillation. J Cardiovasc Electrophysiol 17[Suppl 3]: S2–S7, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Zoccali C, Benedetto FA, Tripepi G, Mallamaci F: Cardiac consequences of hypertension in hemodialysis patients. Semin Dial 17: 299–303, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Glassock RJ, Pecoits-Filho R, Barberato SH: Left ventricular mass in chronic kidney disease and ESRD. Clin J Am Soc Nephrol 4[Suppl 1]: S79–S91, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Eloot S, Van Biesen W, Dhondt A, Van de Wynkele H, Glorieux G, Verdonck P, Vanholder R: Impact of hemodialysis duration on the removal of uremic retention solutes. Kidney Int 73: 765–770, 2008 [DOI] [PubMed] [Google Scholar]
- 24.McFarlane PA: Nocturnal hemodialysis: Effects on solute clearance, quality of life, and patient survival. Curr Opin Nephrol Hypertens 20: 182–188, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Vaithilingam I, Polkinghorne KR, Atkins RC, Kerr PG: Time and exercise improve phosphate removal in hemodialysis patients. Am J Kidney Dis 43: 85–89, 2004 [DOI] [PubMed] [Google Scholar]
- 26.McMullen JR, Sherwood MC, Tarnavski O, Zhang L, Dorfman AL, Shioi T, Izumo S: Inhibition of mTOR signaling with rapamycin regresses established cardiac hypertrophy induced by pressure overload. Circulation 109: 3050–3055, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Gao XM, Wong G, Wang B, Kiriazis H, Moore XL, Su YD, Dart A, Du XJ: Inhibition of mTOR reduces chronic pressure-overload cardiac hypertrophy and fibrosis. J Hypertens 24: 1663–1670, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Siedlecki AM, Jin X, Muslin AJ: Uremic cardiac hypertrophy is reversed by rapamycin but not by lowering of blood pressure. Kidney Int 75: 800–808, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharpe N: Left ventricular remodeling: Pathophysiology and treatment. Heart Fail Monit 4: 55–61, 2003 [PubMed] [Google Scholar]
- 30.Cotter G, Felker GM, Adams KF, Milo-Cotter O, O’Connor CM: The pathophysiology of acute heart failure—is it all about fluid accumulation? Am Heart J 155: 9–18, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Dialysis Outcomes and Practice Patterns Study: Achieved dialysis session length (minutes), by DOPPS country and cross-section. Available at: http://www.dopps.org/annualreport/html/qdialduration_TAB2010.htm Accessed November 30, 2012
- 32.Daugirdas JT: Is there a minimal amount of time a patient should be dialyzed regardless of measured KT/V? Semin Dial 24: 423–425, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.