Summary
Background and objective
Calcific uremic arteriolopathy (CUA) is an often fatal condition with no effective treatment. Multiple case reports and case series have described intravenous sodium thiosulfate (STS) administration in CUA, but no studies have systematically evaluated this treatment.
Design, setting, participants, & measurements
This study included 172 patients undergoing maintenance hemodialysis who had CUA and were treated with STS between August 2006 and June 2009 at Fresenius Medical Care North America. Of these, 85% completed STS therapy. Clinical, laboratory, and mortality data were abstracted from clinical information systems. Responses to survey questionnaires sent to treating physicians regarding patient-level outcomes were available for 53 patients. Effect on CUA lesions and mortality were summarized as CUA outcomes. Relevant laboratory measures, weight (using pairwise comparisons of values before, during, and after STS), and adverse events were summarized as safety parameters.
Results
Mean age of the cohort was 55 years, and 74% of patients were women. Median STS dose was 25 g, and median number of doses was 38. Among surveyed patients, CUA completely resolved in 26.4%, markedly improved in 18.9%, improved in 28.3%, and did not improve in 5.7%; in the remaining patients (20.8%), the response was unknown. One-year mortality in patients treated with STS was 35%. Adverse events, laboratory abnormalities, and weight-related changes were mild. Significant reductions in serum phosphorous (P=0.02) and parathyroid hormone (P=0.01) were noted during STS treatment in patients who completed the therapy.
Conclusions
Although conclusive evidence regarding its efficacy is lacking, a majority of patients who received STS demonstrated clinical improvement in this study.
Introduction
Calcific uremic arteriolopathy (CUA), also referred to as calciphylaxis, is a thrombotic disorder of skin and subcutaneous tissue (1,2). Although calciphylaxis has been occasionally described in patients with normal renal function (3,4), most CUA is seen in ESRD, where it is estimated to affect 1%–4% of patients undergoing maintenance hemodialysis (HD) (5,6). CUA typically presents with painful purpuric plaques and nodules that progress to necrotic ulcers that frequently become superinfected (7). CUA has a dismal prognosis, with 1-year mortality between 45% and 80% (8,9), and a significant morbidity burden. The exact pathobiology of CUA remains obscure, and there is no effective treatment (10).
Because calcification of arterioles within the dermis is a key histologic feature of CUA, strategies that target calcification (e.g., lowering calcium-phosphorous product by calcium-free phosphate binders, controlling secondary hyperparathyroidism by cinacalcet or selective vitamin D analogues) have been suggested; however, their effectiveness has not been confirmed (11–14). Cicone et al. first reported that administration of sodium thiosulfate (STS) may be useful in treating CUA (15). Although the exact mechanism of action is unknown (16,17), several case reports and small case series have been published on STS administration for CUA (17). However, published data on STS have been mixed, with some demonstrating beneficial effects (18–20) and others showing no benefit (21,22). Adding complexity to the issue is the possibility of publication bias. Furthermore, safety concerns related to STS have been raised, including metabolic acidosis and sodium load (each 25 g of STS in 100 ml normal saline conveys approximately 4.8 g sodium) (23,24). Although STS is now routinely used (off label) in CUA management, to date no randomized controlled trial has examined its efficacy and safety; considering the rarity and complexity of CUA, such a trial is unlikely.
In the absence of a randomized controlled trial, systematically collected extensive observational data offer the best strategy to examine any intervention. To this end, we designed the present study to objectively assess the outcomes of patients with CUA from a large national dialysis services provider who were treated with STS.
Materials and Methods
Study Design and Patients
Data were obtained from all patients who underwent maintenance HD at any Fresenius Medical Care North America (FMCNA) facility and received STS between August 2006 and June 2009. At FMCNA, STS is distributed from a central formulary and requires approval by the Pharmacy and Therapeutics Committee; study patients were identified from records of this committee. Facility staff caring for these patients were contacted to confirm that STS was used to treat CUA. The study was deemed exempt from institutional review board approval, and we adhered to Declaration of Helsinki guidance.
STS-treated patients who were no longer receiving STS at the time of data abstraction were considered to have “completed” STS therapy. This group included patients who died while receiving STS therapy. Patients who were receiving STS at the time of data abstraction were considered to be receiving “ongoing” therapy. A two-part survey was also sent to each patient’s attending nephrologist. Patients for whom survey data were available (from the “completed” and “ongoing” therapy groups) were analyzed as “surveyed” patients.
Study Data
Study data were obtained from the FMCNA clinical information system and included age, sex, race, diabetes mellitus, vascular access, relevant laboratory measures, postdialysis weight, interdialytic weight gain, STS-related data (dose, timing in relation to HD session, and duration of administration), and mortality during study period. Laboratory and weight-related data were collected for the 90 days before STS initiation (before), during STS therapy (during), and the 90 days immediately after cessation of STS (after); mean values over each period were analyzed as before, during, and after STS, respectively. Mortality data were confirmed by discharge diagnosis reports from the individual dialysis centers.
The initial part of the two-part survey that was sent to each patient’s nephrologist included questions on (1) confirmation of CUA diagnosis; (2) method of diagnosis; (3) additional treatments given before and during STS treatment; (4) location, number, and appearance of CUA lesions; and (5) dose, timing in relation to HD session, and duration of administration of STS (Supplemental Material). Part 2 of the survey was sent to each patient’s dialysis facility between June 2010 and September 2010 and contained questions regarding STS therapy status (ongoing, completed, or terminated), skin lesions (resolution, improvement, or deterioration), adverse events during STS, and living status (Supplemental Material). Facility staff caring for these patients were also contacted (by phone) to obtain follow-up data. Data on cause of death (from CUA complications or from STS complications) were reviewed.
Study Outcomes
Outcome of CUA was determined by survey responses and mortality data. Survey responses were categorized into five groups: complete resolution, marked improvement, improvement, no improvement, and unknown response. Mortality data were assessed at 1 year from STS initiation and also during the entire study follow-up.
Safety of STS was evaluated by recording adverse events symptoms, postdialysis weight, and interdialytic weight gain and by monitoring changes in serum bicarbonate, anion gap, calcium, phosphorous, and parathyroid hormone (PTH).
Statistical Analyses
Categorical variables were summarized by frequency. Mean and SD values were reported for normally distributed continuous variables. Medians and interquartile ranges (IQRs) were reported for non-normally distributed data. Categorical variables were compared between surveyed and nonsurveyed patients using a chi-squared test. Normally distributed continuous variables were compared using a t test, and non-normally distributed variables were compared using a Wilcoxon test. Cox regression analyses were performed to compute unadjusted and adjusted (for case mix and quality indicators) hazard ratios (HRs) and 95% confidence intervals (CIs) to compare mortality between surveyed and nonsurveyed patients. A paired t test (or Wilcoxon signed-rank test for non-normally distributed data) was used to compare laboratory measures and weight-related data before, during, and after STS.
For the laboratory and weight-related data, analyses were first conducted for all patients who completed STS therapy. Subgroup analyses were then conducted for (1) patients who completed therapy and had complete laboratory and weight-related data available at all three time points, (2) surveyed patients who completed therapy, and (3) surveyed patients who completed therapy and who also had complete laboratory and weight-related data available at all three time points.
All analyses were performed using SAS software, version 9.2 (Cary, NC). Statistical significance was set as a two-sided P value < 0.05.
Results
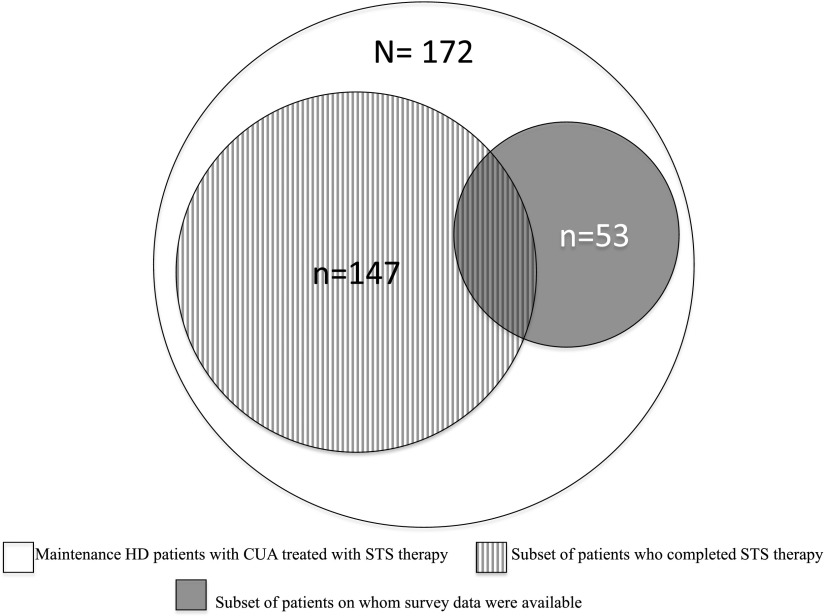
During the study period, we identified 172 maintenance HD patients who received intravenous STS for CUA. Figure 1 summarizes the distribution of these patients as those having “completed” STS (n=147) and “surveyed” patients (n=53). Of all patients who completed STS therapy, postdialysis weight data were available at all three time periods (before, during, and after STS) in 96 patients; availability of results for individual laboratory tests over the three time periods varied. Of surveyed patients, 43 patients completed STS, and postdialysis weights at all three time periods were available for 36 of these patients. Again, availability of laboratory data varied. Postdialysis weight was used as a proxy for having a dialysis treatment during each of the periods.
Figure 1.
Summary of distribution of study patients.
Baseline Characteristics
The average age of the entire study cohort was 55 years, 74% of patients were women, and 56% of patients were white. Fifty-five percent had diabetes mellitus, and 59% were receiving HD via an arteriovenous fistula or a graft. Median HD vintage was 3.1 years (IQR, 1.2, 6.2 years), the mean eKt/V was 1.43, the mean albumin level was 3.5 g/dl, and the mean hemoglobin level was 11.0 g/dl. Patients with available survey data were significantly younger (52 versus 57 years; P=0.02); however, no other baseline characteristics differed between surveyed and nonsurveyed patients (Table 1). Among the surveyed patients, diagnosis of CUA was confirmed by skin biopsy in 47% of cases and was based on clinical impression in the remaining cases. The majority of the CUA lesions involved the legs (60%), abdomen (23%), and buttocks (9%). Distal lesions, such as those involving the feet and hands, were present in approximately 7% of patients.
Table 1.
Baseline characteristics of study patients
| Characteristic | All Patients (n=172) | Nonsurveyed Patients (n=119) | Surveyed Patients (n=53) | P Value |
|---|---|---|---|---|
| Age (yr) | 55±13 | 57±13 | 52±12 | 0.02a |
| Women (%) | 73.8 | 74.8 | 71.7 | 0.67 |
| White patients (%) | 56.4 | 53.8 | 62.3 | 0.36 |
| Diabetes mellitus (%) | 55.2 | 55.5 | 54.7 | 0.93 |
| AVF or AVG access (%) | 59.3 | 58.8 | 60.4 | 0.72 |
| HD vintage (yr) | 3.1 (1.2, 6.2) | 3.4 (1.2, 6.4) | 2.8 (1.0, 5.9) | 0.64 |
| Serum albumin (g/dl) | 3.5±0.5 | 3.5±0.5 | 3.5±0.5 | 0.96 |
| Hemoglobin (%) | 11.0±1.3 | 11.1±1.4 | 10.9±1.2 | 0.30 |
| eKt/V | 1.4±0.3 | 1.4±0.3 | 1.5±0.4 | 0.22 |
| Serum sodium (mmol/L) | 138.0±3.0 | 137.7±3.1 | 138.7±2.9 | 0.08 |
| Serum calcium (mg/dl) | 8.7±0.8 | 8.7±0.8 | 8.7±0.7 | 0.63 |
| Serum phosphorous (mg/dl) | 5.8±1.5 | 5.8±1.6 | 5.8±1.3 | 0.95 |
| Serum PTH (pg/ml) | 332 (170, 654) | 324 (196, 682) | 345 (137, 542) | 0.25 |
| Serum bicarbonate (mmol/L) | 22.8±3.0 | 22.7±3.0 | 23.0±2.9 | 0.61 |
| Serum anion gap (mmol/L) | 13.7±23.7 | 12.0±26.3 | 17.4±16.4 | 0.17 |
| Postdialysis weight (kg) | 91.4±28.6 | 89.7±26.7 | 95.1±32.2 | 0.27 |
| IDWG (kg) | 3.0±1.3 | 3.0±1.3 | 3.0±1.3 | 0.98 |
Unless otherwise noted, values are mean ± SD. HD vintage and serum PTH are expressed as median and interquartile range. P value compares surveyed patients with nonsurveyed patients. AVF, arteriovenous fistula; AVG, arteriovenous graft; HD, hemodialysis; PTH, parathyroid hormone; IDWG, interdialytic weight gain.
Statistically significant.
Twenty-five patients in our study were receiving ongoing STS at the time of data abstraction. Compared with the completed subgroup (n=147), the ongoing subgroup was younger (mean age, 51 versus 56 years; P=0.03), had fewer women (56% versus 77%; P=0.03), and had higher albumin levels (3.6±0.3 versus 3.5±0.5 g/dl). Although serum phosphorous and PTH levels were higher in the ongoing subgroup than in the completed subgroup, these differences did not reach statistical significance.
STS Therapy
The median dose of STS treatment was 25 g administered intravenously in 100 ml of normal saline given over the last half-hour of each HD session. The median number of STS treatments was 38 for all patients (IQR, 17, 79), 39 for nonsurveyed patients (IQR, 14, 78), and 37 for surveyed patients (IQR, 20, 90) (Wilcoxon rank-sum P=0.36). The median number of STS treatments was 96 (IQR, 78, 127) in the ongoing STS therapy subgroup. The median duration of treatments was 92 days for all patients, 91 for nonsurveyed patients, and 94 for surveyed patients (Wilcoxon rank-sum P=0.89). The average total treatment duration for the ongoing subgroup was 229 days. Frequencies of additional treatments that were undertaken before or during STS therapy are summarized in Table 2.
Table 2.
Additional treatment modalities that were undertaken before or during sodium thiosulfate therapy
| Treatment Modality | Cases (%) |
|---|---|
| Initiation/increased dose of non–calcium-based phosphorous binder | 59 |
| Initiation of cinacalcet | 57 |
| Wound care | 34 |
| Discontinuation of vitamin D compounds | 30 |
| Increased frequency of hemodialysis sessions | 15 |
| Surgical parathyroidectomy | 15 |
| Lowering of dialysate calcium | 15 |
| Initiation of corticosteroids | 9 |
| Switching from nonselective vitamin D analogue to selective analogue | 8 |
| Discontinuation of warfarin | 6 |
| Discontinuation of calcium-based phosphate binders | 4 |
Outcome of CUA
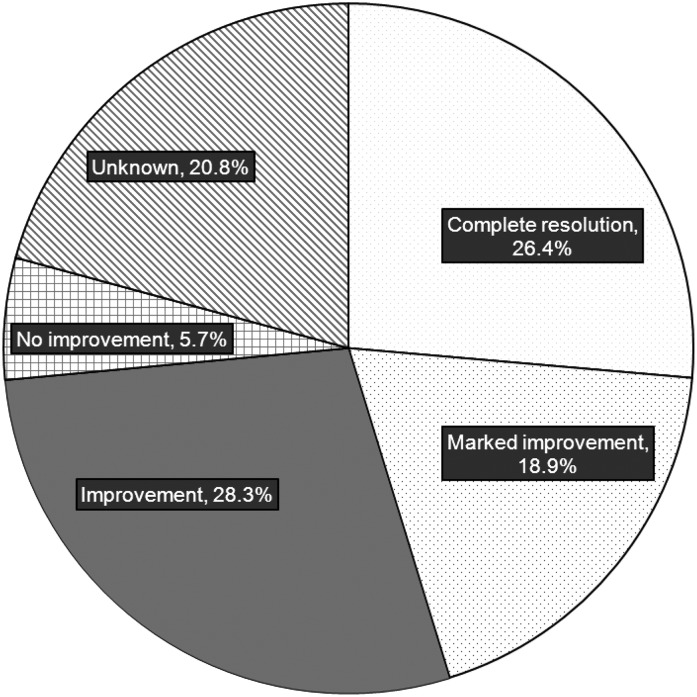
Among surveyed patients, CUA completely resolved in 26.4% of patients. CUA markedly improved in 18.9% of patients, improved in 28.3%, and did not improve in 5.7%; in the remaining patients (20.8%), the response was unknown (Figure 2). Among surveyed patients who completed STS therapy (n=43), CUA completely resolved in 30.2% of patients, markedly improved in 18.6%, improved in 18.6%, and did not improve in 7%; the response was unknown in the remaining patients (25.6%).
Figure 2.
Outcome of calcific uremic arteriolopathy in surveyed patients treated with sodium thiosulfate.
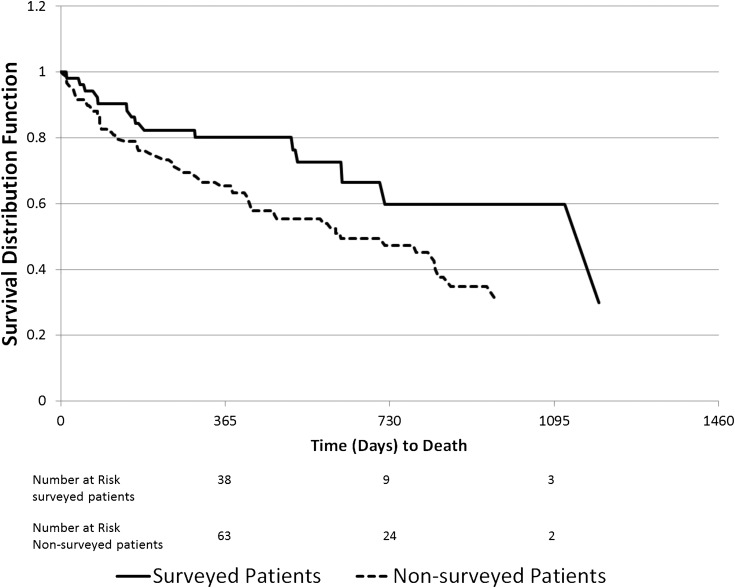
Overall mortality during the study follow-up was 42%, and 1-year mortality in all patients treated with STS was 35%. The 1-year mortality rate is lower compared with historical published data in patients with CUA not treated with STS, in whom the 1-year mortality rate has been reported at 55% (25). As shown in Figure 3, surveyed patients had lower overall mortality than nonsurveyed patients: The unadjusted HR was 0.51 (95% CI, 0.29–0.90). Upon adjustment for case mix and quality indicators, the HR was unchanged, but statistical significance was lost: 0.51 (95% CI, 0.25–1.05).
Figure 3.
Kaplan-Meier survival curves comparing surveyed and nonsurveyed patients treated with sodium thiosulfate.
Safety of STS
During STS therapy, 19% of patients experienced nausea and 15% had vomiting (data available on surveyed patients only). These events were self-limited in all but one patient, in whom STS was discontinued because of intractable nausea. Bad taste with periorbital tingling, fatigue, hypotension, and decreased hearing were reported but were rare (each <2%). None of the deaths during the study period were attributed to STS complications.
Table 3 summarizes trends in laboratory and weight-related measures during STS therapy for all patients who completed STS. Compared with pretreatment levels, serum sodium levels and anion gap were significantly higher and phosphorus, bicarbonate, and postdialysis weight were significantly lower during STS therapy. Serum calcium and PTH levels trended toward being lower, but this difference did not achieve statistical significance. Upon cessation of STS, all measures rebounded toward pretreatment levels except for PTH and postdialysis weight, which remained stable. When consideration was limited to patients who had available data at all three time points, we observed similar evolution in serum sodium, bicarbonate, anion gap, and postdialysis weight. The reduction in serum calcium upon STS treatment achieved statistical significance, whereas changes in phosphorous and PTH were lost.
Table 3.
Laboratory and weight-related measures for patients who completed sodium thiosulfate therapy
| Variable | Before | During | After | P Value Before versus During | P Value During versus After | P Value Before versus After |
|---|---|---|---|---|---|---|
| All patients who completed STS therapy (n=147) | ||||||
| Serum sodium (mmol/L) | 138.0±3.1 (n=120) | 139.0±3.3 (n=117) | 137.6±3.0 (n=74) | <0.01a | <0.001a | 0.16 |
| Serum calcium (mg/dl) | 8.7±0.8 (n=138) | 8.5±0.9 (n=136) | 8.4±0.9 (n=92) | 0.09 | 0.02a | <0.01a |
| Serum phosphorous (mg/dl) | 5.8±1.5 (n=138) | 5.4±1.3 (n=134) | 5.7±1.7 (n=92) | 0.02a | 0.13 | 0.66 |
| Serum PTH (pg/ml) | 309 (169, 555) (n=136) | 270 (121, 478) (n=130) | 262 (130, 500) (n=81) | 0.11 | 0.54 | 0.27 |
| Serum bicarbonate (mmol/L) | 22.7±3.0 (n=138) | 21.9±3.1 (n=135) | 22.9±2.7 (n=89) | 0.02a | <0.01a | 0.67 |
| Serum anion gap (mmol/L) | 14.0±24.4 (n=107) | 17.9±22.9 (n=106) | 11.6±23.1 (n=68) | 0.02a | 0.02a | 0.35 |
| Postdialysis weight (kg) | 90.7±28.5 (n=138) | 88.2±27.3 (n=145) | 88.4±29.6 (n=102) | <0.01a | 0.04a | <0.01a |
| IDWG (kg) | 2.9±1.3 (n=136) | 3.0±1.2 (n=144) | 3.0±1.2 (n=103) | 0.59 | 0.51 | 0.67 |
| Patients who completed STS therapy and had data available from all 3 time periods | ||||||
| Serum sodium (mmol/L) | 138.3±3.1 (n=65) | 139.0±3.2 (n=65) | 137.8±3.0 (n=65) | 0.10 | <0.001a | 0.15 |
| Serum calcium (mg/dl) | 8.7±0.8 (n=83) | 8.6±0.9 (n=83) | 8.4±0.9 (n=83) | 0.37 | 0.01a | 0.01a |
| Serum phosphorous (mg/dl) | 5.7±1.5 (n=83) | 5.5±1.2 (n=83) | 5.7±1.6 (n=83) | 0.25 | 0.24 | 0.91 |
| Serum PTH (pg/ml) | 312 (168, 542) (n=74) | 310 (134, 484) (n=74) | 265 (130, 493) (n=74) | 0.75 | 0.55 | 0.28 |
| Serum bicarbonate (mmol/L) | 22.8±3.1 (n=81) | 21.9±2.9 (n=81) | 23.0±2.8 (n=81) | 0.03a | <0.01a | 0.75 |
| Serum anion gap (mmol/L) | 15.4±21.0 (n=58) | 16.5±21.7 (n=58) | 13.7±21.1 (n=58) | 0.48 | <0.01a | 0.31 |
| Postdialysis weight (kg) | 91.6±31.4 (n=96) | 89.9±30.0 (n=96) | 88.8±30.0 (n=96) | <0.01a | 0.05a | <0.01a |
| IDWG (kg) | 3.1±1.1 (n=95) | 3.1±1.1 (n=95) | 3.0±1.3 (n=95) | 0.95 | 0.55 | 0.71 |
Values with normal distribution are the mean ± SD, and values not normally distributed are the median (interquartile range). The n values in parentheses are number of patients for whom the variable information was available. STS, sodium thiosulfate; PTH, parathyroid hormone; IDWG, interdialytic weight gain.
Statistically significant.
Table 4 summarizes trends in laboratory and weight-related variables during STS therapy for surveyed patients who completed STS therapy. In these patients the trends in laboratory variables were similar, but statistical significance was often not achieved, probably because of limited statistical power.
Table 4.
Laboratory and weight-related variables for surveyed patients who completed sodium thiosulfate therapy
| Variable | Before | During | After | P Value Before versus During | P Value During versus After | P Value Before versus After |
|---|---|---|---|---|---|---|
| All surveyed patients who completed STS therapy (n=53) | ||||||
| Serum sodium ( mmol/L) | 139.0±2.9 (n=36) | 139.7±3.2 (n=35) | 138.3±2.2 (n=28) | 0.21 | 0.12 | 0.37 |
| Serum calcium ( mg/dl) | 8.6±0.7 (n=41) | 8.5±0.8 (n=40) | 8.3±0.9 (n=33) | 0.18 | <0.01a | <0.01a |
| Serum phosphorous (mg/dl) | 5.9±1.3 (n=41) | 5.6±1.2 (n=40) | 5.8±1.6 (n=33) | 0.34 | 0.51 | 0.44 |
| Serum PTH (pg/ml) | 196 (129, 522) (n=38) | 290 (136, 456) (n=40) | 288 (159, 787) (n=30) | 0.70 | 0.15 | 0.18 |
| Serum bicarbonate (mmol/L) | 22.7±2.9 (n=41) | 22.3±2.5 (n=40) | 22.9±2.4 (n=32) | 0.38 | 0.20 | 0.66 |
| Serum anion gap (mmol/L) | 17.6±17.5 (n=34) | 20.5±20.3 (n=34) | 13.9±22.9 (n=26) | 0.03a | <0.01a | 0.53 |
| Postdialysis weight (kg) | 94.2±33.3 (n=41) | 92.2±31.9 (n=42) | 93.0±33.5 (n=37) | 0.06 | 0.52 | 0.18 |
| IDWG (kg) | 2.9±1.4 (n=39) | 3.0±1.2 (n=42) | 3.2±1.2 (n=37) | 0.66 | 0.39 | 0.65 |
| Surveyed patients who completed STS therapy and had data available from all 3 time periods | ||||||
| Serum sodium (mmol/L) | 138.9±2.7 (n=26) | 139.2±2.5 (n=26) | 138.4±2.1 (n=26) | 0.58 | 0.16 | 0.43 |
| Serum calcium (mg/dl) | 8.7±0.7 (n=31) | 8.6±0.9 (n=31) | 8.3±0.8 (n=31) | 0.26 | 0.01a | <0.01a |
| Serum phosphorous (mg/dl) | 6.0±1.4 (n=31) | 5.7±1.2 (n=31) | 5.9±1.6 (n=31) | 0.23 | 0.42 | 0.59 |
| Serum PTH (pg/ml) | 196 (130, 532) (n=28) | 374 (136, 520) (n=28) | 302 (163, 844) (n=28) | 0.45 | 0.13 | 0.10 |
| Serum bicarbonate (mmol/L) | 22.8±2.8 (n=30) | 22.2±2.4 (n=30) | 23.0±2.4 (n=30) | 0.36 | 0.15 | 0.77 |
| Serum anion gap (mmol/L) | 18.0±19.9 (n=24) | 21.2±16.5 (n=24) | 16.8±20.9 (n=24) | 0.13 | 0.05a | 0.42 |
| Postdialysis weight (kg) | 95.8±34.6 (n=36) | 94.2±33.1 (n=36) | 93.4±33.9 (n=36) | 0.07 | 0.49 | 0.18 |
| IDWG (kg) | 3.1±1.3 (n=34) | 3.1±1.2 (n=34) | 3.2±1.2 (n=34) | 0.95 | 0.51 | 0.65 |
Values with normal distribution are the mean ± SD, and values not normally distributed are the median (interquartile range). The n values in parentheses are number of patients for whom the variable information was available. STS, sodium thiosulfate; PTH, parathyroid hormone; IDWG, interdialytic weight gain.
Statistically significant.
Discussion
STS, a reducing agent that forms water-soluble complexes with many metals, was described as having a role in the treatment of calcium urolithiasis by Yatzidis in the 1980s (26). These and other investigators subsequently described improvement in soft-tissue calcifications with STS in small ESRD cohorts (27,28). Given the successful use of STS in these settings, Cicone et al. attempted intravenous STS for CUA and published the first case report outlining STS as a potential treatment for this highly fatal condition (15). Although many case reports and case series have been published on this topic since then, systematic evaluation of STS is lacking and prior attempts at such evaluation have been limited by small sample sizes, with the largest series having 14 patients (29,30). The exact mechanism of action of STS remains elusive; recent investigations question the previously believed calcium-chelating properties (31,32 ) and instead point toward direct extracellular effects of inhibiting vascular calcification that are independent of calcium binding and occur selectively in injured blood vessels (31). Additional antioxidant and vasodilatory properties have been proposed but remain speculative (17,32).
We adopted a systematic approach to identify a large number of CUA cases treated with STS from a large national-level dialysis service provider. This study, the largest to date on this topic, notes that STS is generally safe and well tolerated. Although efficacy of STS cannot be conclusively determined from this study, one quarter of patients who received STS demonstrated clinical improvement (complete resolution, marked improvement, or improvement). Laboratory and weight-related abnormalities that are commonly considered in assessing risk versus benefits of STS were mostly mild and temporary, and most resolved upon therapy completion. The weight loss that was noted with STS could be related to water removal because serum albumin levels remained stable during STS therapy and were higher after STS therapy than before STS therapy (data not shown), indicating that worsening malnutrition during STS therapy is less likely. Given this relatively “mild” adverse effect profile, combined with lack of any other proven therapy for CUA, we suggest that STS can be considered in the care of patients with CUA despite the inconclusive evidence of efficacy.
In many respects, our data are concordant with the previously published data on CUA: (1) CUA is predominantly seen in women (8), (2) most of the lesions are located on proximal areas of body (e.g., abdomen and thighs) (9), (3) patients with CUA tend to have long HD vintage and have concurrent hypoalbuminemia (33), (4) skin biopsy, although the gold standard for CUA diagnosis, is not frequently performed (8,10), and (5) multiple treatment modalities are simultaneously used in CUA management, and many doses of STS are frequently administered (34,35). This consistency with prior data and national scope of our large investigation indicates that our results are representative of “real world” patients and treatment practices. By adopting prospective and systematic data collection, our study also reduces the bias inherent in case reports and case series.
Mortality rates were lower in surveyed patients than nonsurveyed patients. This may point to a systematic nonresponse bias (36). For this reason, laboratory data were reported separately for surveyed patients and for the greater cohort of patients who completed STS therapy. Reassuringly, patterns of laboratory variable evolution were similar. Of note, selective censoring of patients who died during STS therapy may have affected intra-STS and post-STS estimates. It was not possible to assess disease progression in nonsurveyed patients. Patients with CUA not treated with STS could not be identified among the source cohort because these patients were not tracked by the central formulary; hence, we could not directly compare mortality rates between patients treated and not treated with STS. Compared with historical published data from patients not treated with STS (25), the 1-year mortality rate in our cohort appeared to be lower. However, this lower mortality may not necessarily be due to STS treatment because other factors, such as temporal changes in practice patterns (e.g., availability of calcimimetic agents) and differences in lesion distribution (e.g., higher proportion of lesions on trunk or thighs in prior studies), may have contributed to differences in mortality.
Additional limitations of this study include lack of data on CUA risk factors (e.g., warfarin), suboptimal survey response rate, missing laboratory and weight-related data (because of reasons such as deaths, discharge from FMCNA, hospitalizations, or ongoing STS therapy), possibility of recall bias in answering survey questionnaire, and multiple co-treatments, which make attributing effectiveness to STS difficult. Low co-treatment rates (e.g., wound care in 34%) could have been due to recall bias or to survey physicians possibly misinterpreting “wound care” as consisting only of aggressive wound care that involves debridement or vacuum-assisted closure dressing. Survey response rates, although suboptimal, are representative of studies of this nature (33,37). Consistency of results regarding laboratory and weight-related variables among surveyed patients and among surveyed patients with data available at all three study points suggests that selection bias from missing data are less likely. Only a prospective randomized trial can definitively address such bias, but the rarity and complexity of CUA make a randomized trial very challenging. The dose and frequency of STS administration were chosen by the treating providers; standardized pharmacokinetic simulations that consider the frequency and length of HD sessions were not applied (38). This may have introduced suboptimal STS dosing for some patients. However, our study patients were stable outpatients who received HD three times a week, making intensive pharmacokinetic simulations less necessary. Our study also does not address possible adverse effects that may be seen years after completion of STS, such as long-term effects on bone. CUA is an extremely painful condition, and prior reports suggest that STS may improve pain in these patients. However, assessment of pain is subjective, and our survey data were based on clinicians’ perceptions; thus, we maintained the focus on more objective assessments, such as lesion characteristics, laboratory measures, and weight changes.
In conclusion, this study suggests that STS is reasonably safe in the treatment of CUA in maintenance HD patients. The majority of patients who received STS demonstrated clinical improvement in this observational study. Although a randomized trial in CUA would be challenging, exploration toward the design of a collaborative randomized trial evaluating STS in HD patients is needed to better understand the effects of STS on vascular calcification and mortality. Future prospective observational studies on this topic should aim for better physician survey response rates and should consider evaluating longer-term follow-up after STS completion.
Disclosures
S.B. is now a full-time employee of DaVita Clinical Research. He has received prior speaking honoraria from Fresenius Medical Care North America. He previously served on Advisory Boards for CB Fleet and Amgen. His spouse is employed by AstraZeneca. D.M., W.W., J.H., and E.L. are full-time employees of Fresenius Medical Care North America.
Supplementary Material
Acknowledgments
We thank Cindy Premo and Melanie Cousins for their assistance in collecting the survey data used in this manuscript. Parts of these data were presented as poster abstracts during 2008 annual American Society of Nephrology meeting in Philadelphia, Pennsylvania (SA-PO2775) and during the 2010 annual American Society of Nephrology meeting in Denver, Colorado (TH-PO459).
S.N. is supported by the Clinical Scientist in Nephrology award from the American Kidney Fund.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.09880912/-/DCSupplemental.
See related editorial, “Sodium Thiosulfate: Mythical Treatment for a Mysterious Disease?,” on pages 1068–1069.
References
- 1.Wilmer WA, Magro CM: Calciphylaxis: Emerging concepts in prevention, diagnosis, and treatment. Semin Dial 15: 172–186, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Hayden MR, Goldsmith D, Sowers JR, Khanna R: Calciphylaxis: Calcific uremic arteriolopathy and the emerging role of sodium thiosulfate. Int Urol Nephrol 40: 443–451, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Nigwekar SU, Wolf M, Sterns RH, Hix JK: Calciphylaxis from nonuremic causes: A systematic review. Clin J Am Soc Nephrol 3: 1139–1143, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nigwekar SU: An unusual case of nonhealing leg ulcer in a diabetic patient. South Med J 100: 851–852, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Budisavljevic MN, Cheek D, Ploth DW: Calciphylaxis in chronic renal failure. J Am Soc Nephrol 7: 978–982, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Angelis M, Wong LL, Myers SA, Wong LM: Calciphylaxis in patients on hemodialysis: A prevalence study. Surgery 122: 1083–1089, discussion 1089–1090, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Bazari H, Jaff MR, Mannstadt M, Yan S: Case records of the Massachusetts General Hospital. Case 7-2007. A 59-year-old woman with diabetic renal disease and nonhealing skin ulcers. N Engl J Med 356: 1049–1057, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Fine A, Zacharias J: Calciphylaxis is usually non-ulcerating: Risk factors, outcome and therapy. Kidney Int 61: 2210–2217, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Weenig RH, Sewell LD, Davis MD, McCarthy JT, Pittelkow MR: Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol 56: 569–579, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Brandenburg VM, Cozzolino M, Ketteler M: Calciphylaxis: A still unmet challenge. J Nephrol 24: 142–148, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Mohammed IA, Sekar V, Bubtana AJ, Mitra S, Hutchison AJ: Proximal calciphylaxis treated with calcimimetic ‘Cinacalcet’. Nephrol Dial Transplant 23: 387–389, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Russell R, Brookshire MA, Zekonis M, Moe SM: Distal calcific uremic arteriolopathy in a hemodialysis patient responds to lowering of Ca x P product and aggressive wound care. Clin Nephrol 58: 238–243, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Kakagia D, Kriki P, Thodis E, Roumeliotis A, Vargemezis V: Calcific uremic arteriolopathy treated with cinacalcet, paricalcitol, and autologous growth factors. J Cutan Med Surg 15: 121–124, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Parra E, Martin-Cleary C, Martin J, Ortiz A: Calcific uremic arteriolopathy while on cinacalcet. J Postgrad Med 57: 51–52, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Cicone JS, Petronis JB, Embert CD, Spector DA: Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis 43: 1104–1108, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Farese S, Stauffer E, Kalicki R, Hildebrandt T, Frey BM, Frey FJ, Uehlinger DE, Pasch A: Sodium thiosulfate pharmacokinetics in hemodialysis patients and healthy volunteers. Clin J Am Soc Nephrol 6: 1447–1455, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ross EA: Evolution of treatment strategies for calciphylaxis. Am J Nephrol 34: 460–467, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Auriemma M, Carbone A, Di Liberato L, Cupaiolo A, Caponio C, De Simone C, Tulli A, Bonomini M, Amerio P: Treatment of cutaneous calciphylaxis with sodium thiosulfate: Two case reports and a review of the literature. Am J Clin Dermatol 12: 339–346, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Meissner M, Bauer R, Beier C, Betz C, Wolter M, Kaufmann R, Gille J: Sodium thiosulphate as a promising therapeutic option to treat calciphylaxis. Dermatology 212: 373–376, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Musso CG, Enz P, Vidal F, Gelman R, Lizarraga A, Giuseppe LD, Kowalczuk A, Garfi L, Galimberti R, Algranati L: Use of sodium thiosulfate in the treatment of calciphylaxis. Saudi J Kidney Dis Transpl 20: 1065–1068, 2009 [PubMed] [Google Scholar]
- 21.Miceli S, Milio G, La Placa S, Di Raimondo D, Tuttolomondo A, Li Vecchi M, Licata G, Pinto A: Sodium thiosulfate not always resolves calciphylaxis: An ambiguous response. Ren Fail 33: 84–87, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Malabu UH, Manickam V, Kan G, Doherty SL, Sangla KS: Calcific uremic arteriolopathy on multimodal combination therapy: Still unmet goal. Int J Nephrol 2012:390768, 2012 [DOI] [PMC free article] [PubMed]
- 23.Selk N, Rodby RA: Unexpectedly severe metabolic acidosis associated with sodium thiosulfate therapy in a patient with calcific uremic arteriolopathy. Semin Dial 24: 85–88, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Schlieper G, Brandenburg V, Ketteler M, Floege J: Sodium thiosulfate in the treatment of calcific uremic arteriolopathy. Nat Rev Nephrol 5: 539–543, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Mazhar AR, Johnson RJ, Gillen D, Stivelman JC, Ryan MJ, Davis CL, Stehman-Breen CO: Risk factors and mortality associated with calciphylaxis in end-stage renal disease. Kidney Int 60: 324–332, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Yatzidis H: Successful sodium thiosulphate treatment for recurrent calcium urolithiasis. Clin Nephrol 23: 63–67, 1985 [PubMed] [Google Scholar]
- 27.Kyriakopoulos G, Kontogianni K: Sodium thiosulfate treatment of tumoral calcinosis in patients with end-stage renal disease. Ren Fail 12: 213–219, 1990 [DOI] [PubMed] [Google Scholar]
- 28.Papadakis JT, Patrikarea A, Digenis GE, Stamatelou K, Ntaountaki I, Athanasopoulos V, Tamvakis N: Sodium thiosulfate in the treatment of tumoral calcifications in a hemodialysis patient without hyperparathyroidism. Nephron 72: 308–312, 1996 [DOI] [PubMed] [Google Scholar]
- 29.Sood AR, Wazny LD, Raymond CB, Leung K, Komenda P, Reslerova M, Verrelli M, Rigatto C, Sood MM: Sodium thiosulfate-based treatment in calcific uremic arteriolopathy: A consecutive case series. Clin Nephrol 75: 8–15, 2011 [PubMed] [Google Scholar]
- 30.Noureddine L, Landis M, Patel N, Moe SM: Efficacy of sodium thiosulfate for the treatment for calciphylaxis. Clin Nephrol 75: 485–490, 2011 [DOI] [PubMed] [Google Scholar]
- 31.O’Neill WC, Hardcastle KI: The chemistry of thiosulfate and vascular calcification. Nephrol Dial Transplant 27: 521–526, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pasch A, Schaffner T, Huynh-Do U, Frey BM, Frey FJ, Farese S: Sodium thiosulfate prevents vascular calcifications in uremic rats. Kidney Int 74: 1444–1453, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Hayashi M, Takamatsu I, Kanno Y, Yoshida T, Abe T, Sato Y, Japanese Calciphylaxis Study Group : A case-control study of calciphylaxis in Japanese end-stage renal disease patients. Nephrol Dial Transplant 27: 1580–1584, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Baldwin C, Farah M, Leung M, Taylor P, Werb R, Kiaii M, Levin A: Multi-intervention management of calciphylaxis: A report of 7 cases. Am J Kidney Dis 58: 988–991, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Brucculeri M, Cheigh J, Bauer G, Serur D: Long-term intravenous sodium thiosulfate in the treatment of a patient with calciphylaxis. Semin Dial 18: 431–434, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Jones J: The effects of non-response on statistical inference. J Health Soc Policy 8: 49–62, 1996 [DOI] [PubMed] [Google Scholar]
- 37.Boulware LE, Troll MU, Jaar BG, Myers DI, Powe NR: Identification and referral of patients with progressive CKD: A national study. Am J Kidney Dis 48: 192–204, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Singh RP, Derendorf H, Ross EA: Simulation-based sodium thiosulfate dosing strategies for the treatment of calciphylaxis. Clin J Am Soc Nephrol 6: 1155–1159, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.