Summary
Background and objectives
Compared with non-First Nations, First Nations People with diabetes experience higher rates of kidney failure and death, which may be related to disparities in care. This study examined First Nations and non-First Nations People with diabetes for differences in quality indicators and their association with kidney failure and death.
Design, setting, participants, & measurements
Adults with diabetes and an outpatient creatinine in Alberta from 2005 to 2008 were identified. Logistic regression was used to determine the likelihood of process of care indicators (measurement of urine albumin/creatinine ratio [ACR], LDL, and hemoglobin A1C [A1C]) and surrogate outcome indicators (achievement of LDL and A1C targets). Cox regression was used to determine the association between lack of achievement of indicator targets and each of kidney failure and death.
Results
This study identified 140,709 non-First Nations and 6574 First Nations People with diabetes. There was a significant interaction between First Nations status and CKD for the outcomes (P<0.01); therefore, results are stratified by CKD. Among participants without CKD, First Nations People were less likely to receive process of care indicators and achieve target A1C compared with non-First Nations People. For those with CKD, First Nations People were as likely to receive these indicators (other than LDL) and achieve LDL and A1C targets. Lack of LDL and A1C assessment and achievement of targets were associated with increased risk of kidney failure and death similarly for both groups.
Conclusions
Compared with non-First Nations, First Nations People with diabetes but without CKD experience disparities in assessment of quality indicators and achievement of A1C target.
Introduction
Diabetes affects >6% of the Canadian population (1) and is the leading cause of ESRD (2). The prevalence of diabetes is 3-fold to 4-fold higher in First Nations People (3), and diabetes is the cause of kidney failure in >60% of First Nations ESRD patients (4).
Quality of care indicators are performance measures used to evaluate provision of recommended care (5), and are typically defined by clinical practice guidelines (6–8). Despite the potential importance of high-quality care (9), there is evidence that the quality of care is variable (10), with lack of adherence to quality of care standards contributing to worse clinical outcomes (11,12).
Previous studies suggest First Nations People experience disparities in health care compared with the general population (13,14), including among patients with CKD (15). Although the increased prevalence of diabetes among First Nations People may contribute to their increased rates of ESRD, it is unknown whether the care they receive also plays a role.
We aimed to determine whether there were differences in quality indicators for First Nations and non-First Nations People with diabetes, and whether deficiencies in quality indicators were associated with worse clinical outcomes, including kidney failure and death. We hypothesized that First Nations with diabetes, both with and without CKD, would experience deficiencies in quality indicators and increased rates of adverse outcomes, compared with non-First Nations People.
Materials and Methods
Study Population
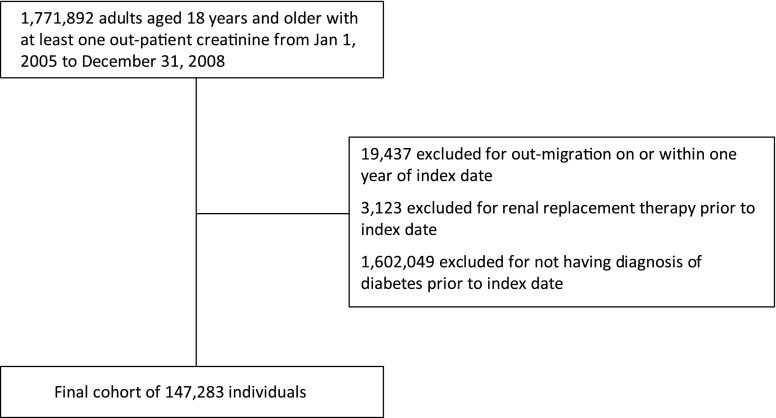
We identified a cohort of adults aged ≥18 years from Alberta, Canada, who had at least one outpatient serum creatinine measurement from January 1, 2005 to December 31, 2008 using our provincial laboratory repository (16). The date of the first creatinine measurement during this period was used to define the index date. Patients with diabetes were identified using Alberta Health and Wellness (AHW) provincial administrative data and a validated diagnostic algorithm (one hospital admission with diabetes or two physician claims for diabetes in a 2-year period) (17). We excluded participants receiving renal replacement therapy (chronic dialysis or kidney transplant) before cohort entry, those relocating outside the province in 1 year of cohort entry (precluding assessment for the outcome), and those without a prior diagnosis of diabetes, for a final study population of 147,283 (Figure 1).
Figure 1.
Flow diagram defining final cohort.
First Nations status was determined from the AHW Registration File, which identifies individuals registered with the Department of Indian and Northern Affairs under the Federal Indian Act. According to the 2006 Canadian census, approximately 81% of the self-identified First Nations population is registered under the Federal Indian Act (18). Median household income and location of residence were determined by linking individual postal codes to 2006 Canadian census data (18).
Hypertension was defined using a validated algorithm (19). The Charlson comorbidity score (excluding renal disease) was calculated as the sum of the weighted comorbidities (20), with comorbidities identified from AHW hospitalization and physician claims files during the 3-year period before the index date. Specialist care was defined as at least one outpatient visit to a nephrologist or general internist in the year before the index date. We estimated GFR (eGFR) using the Modification of Diet in Renal Disease study equation (21), and defined CKD as eGFR <60 ml/min per 1.73 m2.
Outcome Measures
We chose the following three laboratory markers from the 2003 Canadian Diabetes Association guidelines (22) as process of care quality indicators based on their measurement at least once in the year after the index date: urine albumin/creatinine ratio (ACR), fasting LDL, and hemoglobin A1C (A1C). Two surrogate outcome quality indicators were defined as “achieved” based on LDL or A1C of ≤96.7 mg/dl or 7%, respectively (22) (the mean value was used for those with multiple measurements of LDL or A1C during the 1-year observation period). Clinical outcomes were assessed from the index date to the end of study follow-up (December 31, 2009). Kidney failure was defined as a composite renal outcome (doubling of serum creatinine or progression to ESRD), with ESRD defined as the date of registration for chronic dialysis or renal transplantation (23). We determined death from the AHW registry file.
Statistical Analyses
Baseline characteristics are reported as means and SDs, medians and interquartile ranges, or proportions as appropriate. The potential for interaction between First Nations and CKD status was assessed and found to be significant for all laboratory-related outcomes (P<0.01); therefore, results are presented stratified by CKD. The associations between First Nations status and likelihood of measurement of each quality indicator, and between First Nations status and each quality indicator target, were expressed as odds ratios (ORs) with 95% confidence intervals (95% CIs) using multivariable logistic regression models adjusted for sociodemographic characteristics, comorbidities, and specialist (nephrology or internal medicine) care. Age- and sex-adjusted rates of kidney failure and mortality, by First Nations status, were calculated using Poisson regression. Finally, the associations between achievement of each quality indicator target and outcomes of kidney failure and mortality were assessed using Cox proportional hazards models, adjusted as for the logistic regression models, and expressed as hazard ratios (HRs). Ethics approval was obtained from the University of Calgary Conjoint Health Research Ethics Board.
Results
Baseline Characteristics
The study cohort included 6574 (4.5%) First Nations and 140,709 (95.5%) non-First Nations People with diabetes (Table 1). Compared with non-First Nations, First Nations People were younger, more likely to be women, with lower income and living in a rural location of residence. First Nations People also had higher mean index eGFR, were less likely to have hypertension, and were less likely to have seen a nephrologist or internal medicine specialist in the prior year compared with non-First Nations People. Similar baseline characteristics were present when further stratified by CKD status (Supplemental Appendix A).
Table 1.
Baseline characteristics of study population, by First Nations status
| Non-First Nations (n=140,709) | First Nations (n=6574) | P Value | |
|---|---|---|---|
| Age (yr), mean (SD) | 61.7 (15.0) | 53.2 (13.9) | <0.001 |
| Female sex, n (%) | 66,270 (47.1) | 3782 (57.5) | <0.001 |
| eGFR (ml/min per 1.73 m2), mean (SD) | 74.8 (22.6) | 87.9 (27.6) | <0.001 |
| eGFR stage (ml/min per 1.73 m2), n (%) | <0.001 | ||
| ≥60 | 106,946 (76.0) | 5710 (86.9) | |
| <60 | 33,763 (24.0) | 864 (13.1) | |
| Hypertension, n (%) | 88,987 (63.2) | 3399 (51.7) | <0.001 |
| Charlson comorbidity index, median (IQR) | 1 (1–2) | 1 (1–2) | <0.001 |
| Location of residence, n (%) | <0.001 | ||
| Urban | 116,484 (82.8) | 2869 (43.6) | |
| Rural | 23,459 (16.7) | 3692 (56.2) | |
| Unknown | 766 (0.5) | 13 (0.2) | |
| Income quintiles, n (%) | <0.001 | ||
| 1 (lowest) | 29,908 (21.3) | 3415 (52.0) | |
| 2 | 30,519 (21.7) | 928 (14.1) | |
| 3 | 27,510 (19.6) | 754 (11.5) | |
| 4 | 26,097 (18.6) | 521 (7.9) | |
| 5 (highest) | 23,017 (16.4) | 539 (8.2) | |
| Unknown | 3658 (2.6) | 417 (6.3) | |
| Prior specialist care (nephrology or internal medicine), n (%) | 38,450 (27.3) | 1165 (17.7) | <0.001 |
eGFR, estimated GFR; IQR, interquartile range.
Quality Indicators and Targets
Among participants without CKD (eGFR ≥60 ml/min per 1.73 m2), First Nations People were less likely to receive urine ACR (OR, 0.79; 95% CI, 0.74 to 0.85), fasting LDL cholesterol (OR, 0.61; 95% CI, 0.57 to 0.65), and A1C (OR, 0.69; 95% CI, 0.65 to 0.74) measurements compared with non-First Nations People (Table 2 and Supplemental Appendix B). However, among participants with CKD, the likelihood of receiving the quality indicators was similar for First Nations and non-First Nations People, except for measurement of LDL cholesterol (OR, 0.85; 95% CI, 0.73 to 0.98).
Table 2.
Likelihood of assessment of quality indicators for First Nations versus non-First Nations People with diabetes, by CKD status
| Quality Indicator | No CKD (First Nations versus Non-First Nations) | CKD (First Nations versus Non-First Nations) | P Value (Interaction between First Nations and CKD Status) |
|---|---|---|---|
| ACR | 0.79 (0.74 to 0.85) | 1.11 (0.95 to 1.30) | 0.002 |
| LDL | 0.61 (0.57 to 0.65) | 0.85 (0.73 to 0.98) | 0.005 |
| A1C | 0.69 (0.65 to 0.74) | 1.13 (0.97 to 1.31) | <0.001 |
Data are presented as adjusted odds ratios (95% confidence intervals), adjusted for age, sex, hypertension, Charlson score, location of residence, income, and specialist care. ACR, albumin/creatinine ratio; A1C, hemoglobin A1C.
Among participants without CKD (eGFR ≥60 ml/min per 1.73 m2), First Nations People were less likely to achieve the A1C target (OR, 0.83; 95% CI, 0.77 to 0.91) but as likely to achieve the LDL target (OR, 1.06; 95% CI, 0.96 to 1.16) compared with non-First Nations People. Among participants with CKD, the likelihood of achieving the A1C target (OR, 0.93; 95% CI, 0.78 to 1.11) and LDL target (OR, 1.14; 95% CI, 0.91 to 1.41) were similar for First Nations and non-First Nations People.
Clinical Outcomes and Associations with Quality Indicator Targets
First Nations People experienced higher age- and sex-adjusted rates of the composite renal outcome and mortality compared with non-First Nations People in both CKD and non-CKD subgroups (Table 3 and Supplemental Appendix C). Among First Nations People with CKD, rates of the composite renal outcome were almost 4-fold higher compared with non-First Nations People (53.57 per 1000 person-years [95% CI, 44.80 to 62.35], and 15.36 per 1000 person-years [95% CI, 14.52 to 16.19] for First Nations and non-First Nations People, respectively). Mortality rates were approximately 2-fold higher for First Nations People with CKD compared with non-First Nations People with CKD.
Table 3.
Rates of composite renal outcome and mortality per 1000 person-years, by First Nations and CKD status
| Non-First Nations | First Nations | |||
|---|---|---|---|---|
| No CKD (n=106,946) | CKD (n=33,763) | No CKD (n=5710) | CKD (n=864) | |
| Composite renal outcome | 2.64 (2.48 to 2.80) | 15.36 (14.52 to 16.19) | 4.80 (3.85 to 5.74) | 53.57 (44.80 to 62.35) |
| Mortality | 15.37 (14.97 to 15.76) | 24.55 (23.75 to 25.34) | 23.83 (21.45 to 26.21) | 47.44 (41.45 to 53.43) |
Data are presented as the adjusted rate (95% confidence interval), adjusted for age and sex.
The association between achievement of treatment targets and outcomes (kidney failure and death) was similar for First Nations and non-First Nations People, both with and without CKD (Tables 4 and 5). First Nations People without CKD not achieving A1C target had a higher risk of kidney failure (HR, 1.82; 95% CI, 1.02 to 3.25) compared with those achieving the A1C target. A similar increase in the risk of kidney failure was evident among non-First Nations People without CKD not achieving target A1C (HR, 1.62; 95% CI, 1.40 to 1.87). Among First Nations People with CKD, lack of LDL cholesterol measurement was associated with a higher risk of mortality (HR, 1.78; 95% CI, 1.26 to 2.50) compared with those achieving the LDL target, with a similar increase in the risk of mortality among non-First Nations with CKD (HR, 2.04; 95% CI, 1.93 to 2.16) and without CKD (HR, 2.15; 95% CI, 2.03–2.28).
Table 4.
Association between quality indicators, composite renal outcome, and mortality for First Nations with diabetes, by CKD status
| Composite Renal Outcome | Mortality | |||
|---|---|---|---|---|
| No CKD | CKD | No CKD | CKD | |
| LDL target | Reference | Reference | Reference | Reference |
| LDL not target | 0.95 (0.47 to 1.93) | 1.13 (0.69 to 1.85) | 0.52 (0.35 to 0.79) | 0.69 (0.42 to 1.15) |
| LDL not measured | 1.58 (0.89 to 2.83) | 1.29 (0.84 to 1.97) | 1.20 (0.90 to 1.59) | 1.78 (1.26 to 2.50) |
| A1C target | Reference | Reference | Reference | Reference |
| A1C not target | 1.82 (1.02 to 3.25) | 1.13 (0.77 to 1.66) | 1.12 (0.82 to 1.53) | 1.16 (0.83 to 1.62) |
| A1C not measured | 1.10 (0.60 to 2.00) | 0.64 (0.40 to 1.04) | 1.56 (1.16 to 2.09) | 1.31 (0.94 to 1.83) |
Data are presented as adjusted hazard ratios (95% confidence intervals), adjusted for age, sex, hypertension, Charlson score, location of residence, income, specialist care, and other variables in the table. A1C, hemoglobin A1C.
Table 5.
Association between quality indicators, composite renal outcome, and mortality for non-First Nations with diabetes, by CKD status
| Composite Renal Outcome | Mortality | |||
|---|---|---|---|---|
| No CKD | CKD | No CKD | CKD | |
| LDL target | Reference | Reference | Reference | Reference |
| LDL not target | 0.95 (0.80 to 1.14) | 0.93 (0.81 to 1.06) | 0.92 (0.86 to 0.99) | 0.88 (0.82 to 0.95) |
| LDL not measured | 1.76 (1.51 to 2.06) | 1.23 (1.10 to 1.38) | 2.15 (2.03 to 2.28) | 2.04 (1.93 to 2.16) |
| A1C target | Reference | Reference | Reference | Reference |
| A1C not target | 1.62 (1.40 to 1.87) | 1.23 (1.11 to 1.37) | 1.08 (1.02 to 1.14) | 1.02 (0.96 to 1.07) |
| A1C not measured | 0.90 (0.76 to 1.07) | 0.73 (0.63 to 0.83) | 1.28 (1.21 to 1.35) | 1.25 (1.19 to 1.32) |
Data are presented as adjusted hazard ratios (95% confidence intervals), adjusted for age, sex, hypertension, Charlson score, location of residence, income, specialist care, and other variables in the table. A1C, hemoglobin A1C.
Among non-First Nations People with CKD, lack of A1C measurement was associated with a lower risk of kidney failure compared with those achieving the A1C target (HR, 0.73; 95% CI, 0.63 to 0.83). Among non-First Nations People with and without CKD and among First Nations without CKD, those not achieving the LDL target had a lower risk of mortality compared with those who achieved the LDL target.
Discussion
In this population-based cohort of individuals with diabetes, we identified important differences in quality of care and outcomes between First Nations and non-First Nations People. Among the subgroup without CKD, First Nations People were less likely to receive diabetes-specific quality indicators and achieve A1C targets. However among people with CKD (the highest risk group), the likelihood of obtaining these indicators and achieving targets was similar for First Nations and non-First Nations People, other than assessment of LDL. Despite these similarities in measured quality of care, First Nations People with diabetes and CKD continue to experience rates of kidney failure and death that are 2-fold higher than non-First Nations People, suggesting that other aspects of care or disease severity may be contributing to worse outcomes. We are unable to determine the reasons for the differences in care for First Nations People with and without CKD based on our data sources, and can only speculate that it is related to both patient and health care system factors with closer monitoring of disease parameters among patients with more severe disease.
Our results for the subgroup of First Nations with diabetes and without CKD are similar to those reported by Dyck et al. (24). In their study, the authors found that quality indicators and targets were less likely to be achieved in the First Nations People compared with other Saskatchewan residents in a cohort from the province’s two largest health regions. However, in contrast to our results, this disparity was observed in individuals with CKD as well as those without CKD. These differences may be partly explained by the manner in which the outcomes were defined. Whereas we compared the likelihood of achieving an A1C target ≤7% between First Nations and non-First Nations People, Dyck et al. (24) compared the difference in mean A1C between First Nations and non-First Nations People.
Similar to the study by Dyck et al. (24), we reported a >3-fold higher age- and sex-adjusted rate of the composite renal outcome for First Nations compared with non-First Nations People, whereas the rates of earlier stages of CKD were lower for First Nations compared with non-First Nations People. Although there are likely a number of contributing factors, poorer glycemic control at all levels of renal function (24) may contribute to the disparities in these rates. For First Nations People living on reserve, provision of health care services is a federal responsibility. The extent to which delivery of primary care services to First Nations People on reserve may have contributed to these increased rates of the composite renal outcome could not be determined from the data sources available.
One potential explanation for the gap in care for First Nations People with diabetes and without CKD (who comprised the large majority of the current cohort) may be related to rural residence location, where access to health care resources has been reported to be lower compared with urban locations. Rucker et al. has shown an inverse relationship between achieving quality indicators and distance to a nephrologist in patients with CKD (25). Although we did adjust for location of residence, this was broadly categorized as rural or urban. Strategies for improving care to rural First Nations communities specifically have been identified, including emphasis on lifestyle and preventative care, strategies to increase medication compliance, and modifications to environmental factors to enable patients to adopt healthy lifestyles (26).
We chose quality indicators based on laboratory markers of diabetes care. Assessment of these indicators and achievement of targets are associated with reduced morbidity and mortality in patients with diabetes. Both the Diabetes Control and Complications Trial (27) and the UK Prospective Diabetes Study (28) studies demonstrated a reduced risk of proteinuria with intensive glycemic control. Furthermore, long-term follow-up of these studies (29,30), demonstrated that tight glycemic control was associated with a decreased risk of cardiovascular disease and mortality. In the Steno-2 study, adherence to quality indicators and targets (aspirin, angiotensin inhibition, A1C <6.5%, total cholesterol <174 mg/dl, BP goal <130/80 mmHg) led to a reduction in cardiovascular events and mortality (31). However, these quality indicators reflect adherence to clinical practice guidelines; cultural differences between First Nations and non-First Nations People may affect the uptake of these practices and contribute to the differences reported in our study (32).
The disparities in care for First Nations People without CKD reported here are not unique to patients with diabetes. First Nations People in general experience decreased access to both primary generalist care and specialist care, compared with similar geographic and socioeconomic populations (13). Decreased access to specialist care for First Nations People has also been shown in patients with epilepsy and CKD (14,15). However, to our knowledge, our study is the first to correlate disparities in care to clinical outcomes, and to show that such differences vary with the presence of comorbidity such as CKD.
Our results suggest that risk of mortality was lower if LDL was not at target in all non-First Nations People and in First Nations People without CKD. The reasons for this are not clear from the data sources available, but we speculate that it may be related to underlying severity of disease in that patients near death have lower LDL levels (33), and those with higher cardiovascular disease (and higher risk of death) are treated more aggressively to achieve LDL targets (34). Similarly, the reasons for the lower composite renal outcomes for non-First Nations People with CKD and A1C not measured is unclear from the data available, but may be related to better glycemic control and a perceived need for less aggressive monitoring (35,36).
Our study results should be interpreted in light of its limitations. First, administrative data were used to identify both First Nations status and diabetes. Although there is potential for misclassification, the algorithm used to identify diabetes has sensitivity and specificity of 86% and 87%, respectively (17). Furthermore, approximately 81% of the First Nations population are registered under the Federal Indian Act and recorded in the administrative data registry file (18); therefore, any misclassification is likely to be minimal. Second, the number of First Nations People in our study (and in particular the subgroup with CKD) was small, and is consistent with the geographic distribution of First Nations People in Canada (18); therefore, the statistical power to detect differences between First Nations and non-First Nations People may be limited. Third, given the data sources, we were unable to validly determine cause of death; therefore, we were unable to determine the extent to which the increased mortality rates were due to accidental death rather than disease. However, deaths due to accidental causes among First Nations People are more common in younger age groups of male sex; thus, the mortality rates were adjusted for age and sex to account for these differences. Finally, we were only able to access quality indicators available through laboratory data; other important quality indicators for patients with diabetes such as BP control, use of angiotensin inhibitors, as well as access to nonphysician primary care services in First Nations communities, would provide further information regarding quality of care differences but were not available.
Despite these limitations, our study provides important insight into the quality of care delivered to First Nations People with diabetes without CKD. First Nations People with diabetes and CKD do not experience disparities in assessment of most laboratory-based quality indicators or achievement of LDL or A1C targets compared with non-First Nations People. However, disparities in care were observed in the larger group without CKD. Given the overall increased risk for ESRD and mortality, further information is required regarding other processes of care for First Nations People with diabetes that may be contributing to worse outcomes.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by the Canadian Institute of Health Research (CIHR), and a team grant of the Interdisciplinary Chronic Disease Collaboration, Alberta Innovates Health Solutions (AI-HS). V.D. was supported by an AI-HS clinical fellowship. N.J., H.Q., M.T., B.M., and B.R.H. are supported by salary awards from AI-HS. N.J. and M.T. are supported by Government of Canada Research Chairs and B.R.H. is supported by the Roy and Vi Baay Chair in Kidney Disease Research. This study is based in part by data provided by Alberta Health and Alberta Health Services. The interpretation and conclusions are those of the researchers and do not represent the views of the Government of Alberta.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10461012/-/DCSupplemental.
References
- 1.Public Health Agency of Canada: Report from the National Diabetes Surveillance System: Diabetes in Canada, 2009. Available at: http://www.phacaspc.gc.ca/publicat/2009/ndssdic-snsddac-09/index-eng.phpAccessed March 14, 2012
- 2.Canadian Organ Replacement Register: Annual Report 2011, Ottawa, Ontario, Canadian Institute for Health Information, 2011 [Google Scholar]
- 3.Alberta Diabetes Surveillance System: Alberta Diabetes Atlas 2011, Edmonton, Alberta, Canadian Institute of Health Economics, 2011 [Google Scholar]
- 4.Narva AS: The spectrum of kidney disease in American Indians. Kidney Int Suppl 63[83]: S3–S7, 2003 [DOI] [PubMed] [Google Scholar]
- 5.McGlynn EA: Six challenges in measuring the quality of health care. Health Aff (Millwood) 16: 7–21, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Holloway RG, Vickrey BG, Benesch C, Hinchey JA, Bieber J, National Expert Stroke Panel : Development of performance measures for acute ischemic stroke. Stroke 32: 2058–2074, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Spertus JA, Eagle KA, Krumholz HM, Mitchell KR, Normand SL, American College of Cardiology. American Heart Association Task Force on Performance Measures : American College of Cardiology and American Heart Association methodology for the selection and creation of performance measures for quantifying the quality of cardiovascular care. Circulation 111: 1703–1712, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Tu JV, Khalid L, Donovan LR, Ko DT, Canadian Cardiovascular Outcomes Research Team / Canadian Cardiovascular Society Acute Myocardial Infarction Quality Indicator Panel : Indicators of quality of care for patients with acute myocardial infarction. CMAJ 179: 909–915, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lohr KN, Schroeder SA: A strategy for quality assurance in Medicare. N Engl J Med 322: 707–712, 1990 [DOI] [PubMed] [Google Scholar]
- 10.Williams SC, Schmaltz SP, Morton DJ, Koss RG, Loeb JM: Quality of care in U.S. hospitals as reflected by standardized measures, 2002-2004. N Engl J Med 353: 255–264, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Higashi T, Shekelle PG, Adams JL, Kamberg CJ, Roth CP, Solomon DH, Reuben DB, Chiang L, MacLean CH, Chang JT, Young RT, Saliba DM, Wenger NS: Quality of care is associated with survival in vulnerable older patients. Ann Intern Med 143: 274–281, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Peterson ED, Roe MT, Mulgund J, DeLong ER, Lytle BL, Brindis RG, Smith SC, Jr, Pollack CV, Jr, Newby LK, Harrington RA, Gibler WB, Ohman EM: Association between hospital process performance and outcomes among patients with acute coronary syndromes. JAMA 295: 1912–1920, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Shah BR, Gunraj N, Hux JE: Markers of access to and quality of primary care for aboriginal people in Ontario, Canada. Am J Public Health 93: 798–802, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jetté N, Quan H, Faris P, Dean S, Li B, Fong A, Wiebe S: Health resource use in epilepsy: Significant disparities by age, gender, and aboriginal status. Epilepsia 49: 586–593, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Gao S, Manns BJ, Culleton BF, Tonelli M, Quan H, Crowshoe L, Ghali WA, Svenson LW, Ahmed S, Hemmelgarn BR, Alberta Kidney Disease Network : Access to health care among status Aboriginal people with chronic kidney disease. CMAJ 179: 1007–1012, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemmelgarn BR, Clement F, Manns BJ, Klarenbach S, James MT, Ravani P, Pannu N, Ahmed SB, MacRae J, Scott-Douglas N, Jindal K, Quinn R, Culleton BF, Wiebe N, Krause R, Thorlacius L, Tonelli M: Overview of the Alberta Kidney Disease Network. BMC Nephrol 10: 30, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hux JE, Ivis F, Flintoft V, Bica A: Diabetes in Ontario: Determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care 25: 512–516, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Statistics Canada: 2006 Census, Ottawa, Ontario, Canadian Institute for Health Information, 2006 [Google Scholar]
- 19.Quan H, Khan N, Hemmelgarn BR, Tu K, Chen G, Campbell N, Hill MD, Ghali WA, McAlister FA, Hypertension Outcome and Surveillance Team of the Canadian Hypertension Education Programs : Validation of a case definition to define hypertension using administrative data. Hypertension 54: 1423–1428, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA: Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 43: 1130–1139, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee : Canadian Diabetes Association 2003 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes 27[Suppl 2]: S1–S152, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Manns BJ, Mortis GP, Taub KJ, McLaughlin K, Donaldson C, Ghali WA: The Southern Alberta Renal Program database: A prototype for patient management and research initiatives. Clin Invest Med 24: 164–170, 2001 [PubMed] [Google Scholar]
- 24.Dyck RF, Sidhu N, Klomp H, Cascagnette PJ, Teare GF: Differences in glycemic control and survival predict higher ESRD rates in diabetic First Nations adults. Clin Invest Med 33: E390–E397, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Rucker D, Hemmelgarn BR, Lin M, Manns BJ, Klarenbach SW, Ayyalasomayajula B, James MT, Bello A, Gordon D, Jindal KK, Tonelli M: Quality of care and mortality are worse in chronic kidney disease patients living in remote areas. Kidney Int 79: 210–217, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Bhattacharyya OK, Rasooly IR, Naqshbandi M, Estey EA, Esler J, Toth E, Macaulay AC, Harris SB: Challenges to the provision of diabetes care in first nations communities: Results from a national survey of healthcare providers in Canada. BMC Health Serv Res 11: 283, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The Diabetes Control and Complications Trial Research Group : The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329: 977–986, 1993 [DOI] [PubMed] [Google Scholar]
- 28.UK Prospective Diabetes Study (UKPDS) Group : Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352: 837–853, 1998 [PubMed] [Google Scholar]
- 29.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B, Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group : Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 353: 2643–2653, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA: 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 359: 1577–1589, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Gaede P, Lund-Andersen H, Parving HH, Pedersen O: Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 358: 580–591, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Canadian Institutes of Health Research: CIHR Guidelines for Health Research Involving Aboriginal People Ottawa, Ontario, Canadian Institutes of Health Research, 2007
- 33.Roongpisuthipong C, Sobhonslidsuk A, Nantiruj K, Songchitsomboon S: Nutritional assessment in various stages of liver cirrhosis. Nutrition 17: 761–765, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Genest J, Frohlich J, Fodor G, McPherson R, Working Group on Hypercholesterolemia and Other Dyslipidemias : Recommendations for the management of dyslipidemia and the prevention of cardiovascular disease: Summary of the 2003 update. CMAJ 169: 921–924, 2003 [PMC free article] [PubMed] [Google Scholar]
- 35.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee : Canadian Diabetes Association 2008 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes 32[Suppl 1]: S1–S201, 2008 [DOI] [PubMed] [Google Scholar]
- 36.American Diabetes Association : Standards of medical care in diabetes—2007. Diabetes Care 30[Suppl 1]: S4–S41, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.