Summary
Background and objectives
AKI is common and novel biomarkers may help provide earlier diagnosis and prognosis of AKI in the postoperative period.
Design, setting, participants, & measurements
This was a prospective, multicenter cohort study involving 1219 adults and 311 children consecutively enrolled at eight academic medical centers. Performance of two urine biomarkers, kidney injury molecule-1 (KIM-1) and liver fatty acid-binding protein (L-FABP), alone or in combination with other injury biomarkers during the perioperative period was evaluated. AKI was defined as doubling of serum creatinine or need for acute dialysis.
Results
KIM-1 peaked 2 days after surgery in adults and 1 day after surgery in children, whereas L-FABP peaked within 6 hours after surgery in both age groups. In multivariable analyses, the highest quintile of the first postoperative KIM-1 level was associated with AKI compared with the lowest quintile in adults, whereas the first postoperative L-FABP was not associated with AKI. Both KIM-1 and L-FABP were not significantly associated with AKI in adults or children after adjusting for other kidney injury biomarkers (neutrophil gelatinase-associated lipocalin and IL-18). The highest area under the curves achievable for discrimination for AKI were 0.78 in adults using urine KIM-1 from 6 to 12 hours, urine IL-18 from day 2, and plasma neutrophil gelatinase-associated lipocalin from day 2 and 0.78 in children using urine IL-18 from 0 to 6 hours and urine L-FABP from day 2.
Conclusions
Postoperative elevations of KIM-1 associate with AKI and adverse outcmes in adults but were not independent of other AKI biomarkers. A panel of multiple biomarkers provided moderate discrimination for AKI.
Introduction
AKI is a common complication of cardiac surgery in both adults and children and is associated with mortality and poor outcomes (1). Developing therapies for prevention and treatment of AKI has been unsuccessful, likely in part due to dependence on serum creatinine, a nonspecific and late marker of AKI (2). Serum creatinine is a functional marker of glomerular clearance rather than kidney injury and reliance upon creatinine leads to a delay in AKI diagnosis. Proteins that are involved in the pathophysiologic process of kidney injury and renal tubular cell death are likely to be released into the urine promptly after injury. Assessing these urine protein concentrations could assist with early diagnosis of injury and assessment of the burden of injury, key information that serum creatinine alone does not always provide. Urine proteins such as neutrophil gelatinase-associated lipocalin (NGAL), IL-18, kidney injury molecule-1 (KIM-1), and liver fatty acid-binding protein (L-FABP) are promising as biomarkers of AKI based on animal and early human studies (3–6).
We conducted a large, prospective, multicenter cohort study of patients undergoing cardiac surgery to evaluate performance of urinary biomarkers. In the first phase, we reported the results on urine IL-18 and NGAL from these cohorts. Urine IL-18 and NGAL were increased before serum creatinine in adults and children, but only IL-18 offered prognostic information beyond clinical data (7,8). In order to validate additional biomarkers, we examined two other promising urinary proteins, specifically KIM-1 and L-FABP. Both of these proteins have compelling preclinical data as early indicators of AKI and appear promising in initial translation experiments in humans (9–13). In addition, KIM-1 was recently approved by the US Food and Drug Administration as an AKI biomarker for preclinical drug development (14,15) and L-FABP is approved as a diagnostic test for human AKI in Japan. This study represents the first large-scale validation of these two biomarkers and offers comparisons with other novel biomarkers of AKI.
Materials and Methods
Patient Cohorts and Samples
We prospectively enrolled adults undergoing cardiac surgery (coronary artery bypass grafting [CABG] or valve surgery) who were at high risk for AKI (one or more of the following criteria: preexisting renal impairment [baseline serum creatinine >2 mg/dl or 177 mmol/L], ejection fraction <35% or grade 3 or 4 left ventricular dysfunction, age >65 years, diabetes mellitus, concomitant CABG and valve surgery, or repeat revascularization surgery) at eight academic medical centers in North America between July 2007 and December 2009 (7). During the same period, we also enrolled children undergoing surgery for congenital cardiac disorders from three academic centers. All adult participants provided written informed consent and written informed consent was obtained from all parents or legal guardians, along with assent where appropriate for pediatric participants. This trial was registered with Clinicaltrials.gov (NCT00774137).
Each institution’s research ethics board approved the study. Day 1 was the day of surgery and day 2 corresponded to the first day after surgery. We collected urine and plasma specimens preoperatively and daily up to day 5. We collected first postoperative samples soon after admission to the intensive care unit (ICU) and urine samples every 6 hours for the first 24 hours after surgery. We obtained daily blood and urine samples at the time of routine morning blood collection done for clinical care. We stopped specimen collection on day 3 in participants transferred out of the ICU who had no evidence of a 50% increase in serum creatinine. We described the detailed methods of this study, including sample collection and processing, elsewhere (7,8).
Study Variables
The primary outcome was the development of AKI, defined as receiving acute dialysis during the hospital stay or a doubling in serum creatinine from baseline preoperative value consistent with RIFLE-Injury (RIFLE-I) or Acute Kidney Injury Network (AKIN) stage 2 AKI classification (16,17). We also analyzed the outcome of progression of AKI, defined by worsening of AKIN stage (stage 1 to either stage 2 or 3, or from stage 2 to 3). We classified patients treated with acute dialysis at any point during hospitalization as stage 3. Additional clinical outcomes were in-hospital mortality and length of ICU and hospital stay.
We recorded serum creatinine values obtained in routine care for every patient throughout the hospital stay. All preoperative creatinine values were done within 2 months before surgery. The preoperative and postoperative serum creatinine levels were performed in the same clinical laboratory for each patient at all sites. For adults, we estimated preoperative GFR (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (18). For children, we estimated preoperative eGFR using the updated Schwartz equation and determined eGFR percentiles using published normal renal function data (19,20). We collected preoperative characteristics, operative details, and postoperative complications using definitions of the Society of Thoracic Surgeons.
Biomarker Assays
Sekisui Diagnostics LLC developed assays for KIM-1 and L-FABP. Capture antibodies were bound to multi-assay 96-well plates (MesoScale Discovery [MSD], Gaithersburg, MD) and detection antibodies were biotinylated. Signal generation relied on the use of a streptavidin coupled Sulfo-Tag (MSD). The Sulfo-Tag includes ruthenium(II)-tris-bipyridine, which in combination with a triproplyamine buffer generates an electrochemical signal (light) detected by a Sector Imager 2400 (MSD). Sekisui Diagnostics LLC also developed the rabbit anti KIM-1 antibodies (for capture and detection) and recombinant hKIM-1 (for standards and controls). CMIC (Tokyo, Japan) supplied mAbs and recombinant hL-FABP standards. The detection range for KIM-1 is 0.056–60 ng/ml, whereas L-FABP is 0.057–1500 ng/ml. The intra-assay coefficient of variation is ≤10% for both assays.
We measured biomarkers in duplicate and used the average of the two. We blinded the personnel measuring the biomarkers to clinical outcomes.
Statistical Analyses
All analyses were conducted separately for adults and children. We compared continuous variables with a two-sample t test or Wilcoxon rank sum test and dichotomous variables with the chi-squared test or Fisher’s exact test. We divided each population (adults and children separately) into quintiles using the first postoperative value of each biomarker. We assessed unadjusted trends across biomarker quintiles by the Cochran-Armitage test for dichotomous outcomes and the Jonckheere-Terpstra test for continuous outcomes. We assessed adjusted trends using contrasts in linear or logistic regression depending on the outcome. To evaluate the association between each biomarker and AKI, we used mixed logistic regression models with a random intercept for site. The demographic models for both adults and children were adjusted for age (per year), sex, and white race. For adult patients, the clinical model adjusted for demographic covariates, cardiopulmonary bypass (CPB) time >120 minutes, nonelective surgery, preoperative CKD-EPI eGFR, diabetes, hypertension, and site. For children, the clinical model adjusted for demographic covariates, CPB time >120 minutes, nonelective surgery, Risk Adjustment for Congenital Heart Surgery-1 score ≥3, and preoperative eGFR percentile. To determine the ability of the biomarkers to discriminate between patients with and without AKI, we calculated the area under the receiver-operating characteristic curve (AUC) and compared AUCs using the DeLong test. We quantified the improvement in risk prediction after the addition of biomarkers to the clinical model with the categorical net reclassification improvement (NRI) and integrated discrimination index (21). For NRI analyses, risk category definitions were based on previous publications (7,8). For NRI calculations, AKI risk categories were classified as low (<3%), medium (3%–10%), or high (>10%) for adults and as low (<10%), medium (10%–25%), or high (>25%) for children. We used logistic regression models to estimate biomarker combinations and evaluated the discriminatory ability of the combinations with the AUC. To minimize the risk of overfitting, 3-fold cross-validation was used to estimate and evaluate the biomarker combinations. Cross-validation randomly splits the data into subsets of training and testing datasets allowing the evaluation of the biomarker combinations to be completed on a different subset of data than was used in the development of the biomarker combination. We performed all analyses in SAS (version 9.2; SAS Institute, Cary, NC) and R 2.12.1 (R Foundation for Statistical Computing, Vienna, Austria) software.
Results
Cohort Description
Our cohort included 1219 adults and 311 children. Most of the surgeries were elective (79% in adults, 91% in children) and used CPB (90% in adults and 99% in children). AKI occurred at a median 2 days after surgery (interquartile range [IQR], 1, 3) in adults and 1 day after surgery (IQR, 0, 1) in children. Sixty of 1219 adult participants (5%) developed AKI, 18 of whom (1.5%) received acute dialysis. Adult patients who developed AKI were more likely to have a lower preoperative eGFR, a history of congestive heart failure, combined CABG and valve surgery, longer CPB, or longer cross-clamp times and to have received a postoperative intra-aortic balloon pump (Table 1). Fifty-three (17%) children developed AKI, and five (1.6%) required dialysis. Children with AKI were younger and had longer CPB and cross-clamp times (Table 1).
Table 1.
Patient characteristics
| Characteristic | Developed AKI | |||
|---|---|---|---|---|
| Adults (n=1219) | Children (n=311) | |||
| Yes (n=60) | No (n=1159) | Yes (n=53) | No (n=258) | |
| Age at the time of surgery (yr) | 74 (64, 77) | 73 (66, 78) | 0.7 (0.4, 3.7)a | 2.9 (0.5, 5.6) |
| Male sex | 39 (65) | 787 (68) | 27 (51) | 144 (56) |
| White race | 55 (92) | 1086 (94) | 42 (79) | 212 (82) |
| Diabetes | 29 (48) | 482 (42) | — | — |
| Hypertension | 53 (88) | 908 (78) | — | — |
| Congestive heart failure | 25 (42)a | 289 (25) | — | — |
| Status of the procedure | a | |||
| Elective | 40 (67) | 924 (80) | 46 (87) | 237 (92) |
| Urgent or emergentb | 20 (33) | 235 (20) | 7 (13) | 21 (8) |
| Cardiac catheterization in the last 72 h | 6 (10) | 117 (10) | — | — |
| Preoperative renal function | ||||
| Serum creatinine (mg/dl)c | 1.1 (0.9, 1.4)a | 1.0 (0.9, 1.2) | — | — |
| eGFR (ml/min per 1.73 m2), mean (SD) | 63 (23) | 67 (19) | 97 (28)a | 89 (25) |
| eGFR (percentile), mean (SD)d | — | — | 69 (32)a | 49 (33) |
| Operative characteristics | ||||
| Incidence | — | — | ||
| First cardiovascular surgery | 51 (85) | 1013 (87) | — | — |
| Reoperative cardiovascular surgery | 9 (15) | 146 (13) | — | — |
| Surgery | a | — | — | |
| CABG | 20 (33) | 565 (49) | — | — |
| Valve | 16 (27) | 339 (29) | — | — |
| CABG and valve | 24 (40) | 255 (22) | — | — |
| CPB usee | 49 (82)a | 1012 (87) | 52 (98) | 255 (99) |
| Perfusion timef | 151 (114, 249)a | 105 (81, 139) | 132 (95, 203)a | 88 (64, 122) |
| Cross-clamp time (min) | 114 (74, 159)a | 71 (52, 100) | 65 (15, 108)a | 38 (0, 62) |
| Cardioplegia | 55 (92) | 1022 (88) | — | — |
| Vasopressor usage | 16 (27)a | 126 (11) | 2 (4) | 3 (1) |
| Postoperative IABP | 8 (14)a | 49 (4) | — | — |
| RACHS-1 scoreg | a | |||
| 1 | — | — | 0 (0) | 18 (7) |
| 2 | — | — | 30 (57) | 123 (48) |
| 3 | — | — | 16 (30) | 110 (43) |
| 4 | — | — | 7 (13) | 5 (2) |
| Not categorized | — | — | 0 | 2 |
| Oliguria first postoperative dayh | 4 (7)a | 14 (1) | 6 (11)a | 6 (2) |
Data are presented as n (%) or median (interquartile range: 25th percentile, 75th percentile) unless otherwise indicated. AKI is defined by receipt of acute dialysis or a doubling in serum creatinine from the preoperative serum creatinine value. Dashes indicate not applicable. eGFR, estimated GFR; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; IABP, intra-aortic balloon pump; RACHS-1, Risk Adjustment for Congenital Heart Surgery-1.
P<0.05 for comparison of AKI and non-AKI.
No children had emergent surgeries.
To convert serum creatinine values to µmol/L, multiply by 88.4
Percentile eGFR was calculated by quantile regression based on published normal renal function measured by nuclear medicine scan GFR in 651 children (21).
For adults, full CPB utilization has been reported.
Perfusion time is reported for the patients who had full CPB use.
The RACHS-1 consensus-based score system categorizes the complexity of surgery.
In adults, oliguria was defined as a patient who had <125 ml in 6 hours or <500 ml urine output in 24 hours. In children, 24-hour oliguria was defined as a patient who had <0.5 ml/kg per hour.
Postoperative Trend of Urine KIM-1 and L-FABP and Risk of AKI
Preoperative KIM-1 levels were not significantly different between those who did and did not develop AKI (adults: median 1.9 versus 1.2 ng/ml, P=0.06; children: median 0.62 versus 0.54 ng/ml, P=0.42; Supplemental Tables 1 and 2). The first postoperative (0–6 hours) KIM-1 levels were significantly higher in those who developed AKI compared with those who did not (adult: median 1.06 versus 0.42 ng/ml, P<0.001; children: median 0.63 versus 0.39 ng/ml, P=0.001) (Figure 1). The first postoperative levels of KIM-1 in AKI patients were not significantly different from their preoperative levels. KIM-1 levels peaked 2 days after surgery in adults and 1 day after surgery in children. KIM-1 levels remained significantly elevated compared with the non-AKI group until day 5 in the adult AKI group and day 4 in the pediatric AKI group.
Figure 1.
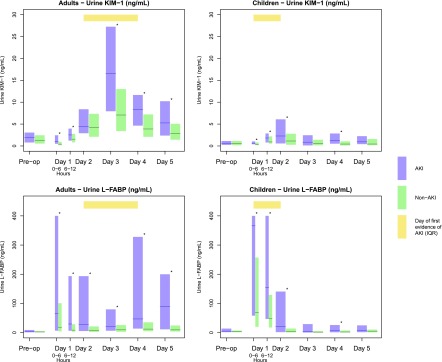
Biomarker distribution over time. AKI defined by receipt of acute dialysis or a doubling in serum creatinine during the hospital stay. Yellow bar indicates the IQR of the day to the first evidence of AKI in patients with AKI. Blue and green bars represent the IQR (25th and 75th percentiles for AKI and non-AKI patients, respectively). The solid lines denote the median values. Day 1 is the day of surgery, with time 0 representing the point when the patient arrived in the postoperative ICU. *P<0.05 for comparison of AKI and non-AKI. KIM-1, kidney injury molecule-1; L-FABP, liver fatty acid-binding protein; IQR, interquartile range.
Preoperative L-FABP levels were not significantly different between individuals who did and did not develop AKI (Supplemental Tables 1 and 2). Median L-FABP levels peaked within 6 hours in both adults and children, and were significantly higher in those with AKI (adults: median 66.3 [IQR, 6.7, 400.0 ng/ml] versus 18.4 [IQR 4.2, 100.9 ng/ml], P<0.001; children: median 366.0 [58.1, 400.0 ng/ml] versus 69.1 [19.9, 257.2 ng/ml], P<0.001). These postoperative levels remained significantly higher in the AKI group compared with the non-AKI group throughout study duration for adults, but were significantly higher in children only through day 2.
The incidence of AKI was greatest in the upper quintile of first postoperative KIM-1 and L-FABP in both adults and children (Tables 2 and 3). After multivariable adjustment, higher quintiles of first postoperative KIM-1 were independently associated with increasing incidence of AKI in both adults and children, although findings were not significant in children (Table 2 and 3). In adults, the fourth and fifth quintiles of urine KIM-1 had 3-fold and 4-fold adjusted odds for the development of AKI, respectively, compared with the lowest quintile. The odds ratio for the upper quintile of L-FABP was not significant after multivariable adjustment in either adults or children (Table 2 and 3). In adults, the association of KIM-1 and L-FABP with AKI was further attenuated and became nonsignificant when the biomarkers were adjusted for the other kidney injury biomarkers, urine NGAL, plasma NGAL, and urine IL-18. Conversely, the effect of KIM-1 and L-FABP on odds ratios for previously published biomarkers (urine IL-18, urine NGAL, plasma NGAL) are presented in Supplemental Table 3. Urine IL-18 and plasma NGAL remained significant after adjustment of other biomarkers. Due to the small sample size, the association of KIM-1 and L-FABP adjusted for other kidney injury markers in children was not estimated.
Table 2.
Association of first postoperative biomarker quintile and outcomes in adults
| Primary Outcome | |||||||
|---|---|---|---|---|---|---|---|
| AKI Adjusted OR (95% CI) | Nonrenal Outcomes | ||||||
| Quintile (Cutpoints)a | AKIb n (%) | Demographicc | Clinical Modeld | Clinical Model and Other Biomarkerse | In-Hospital Death or Dialysis, % | Length of ICU Stay, Median (IQR) | Length of Hospital Stay, Median (IQR) |
| Urine KIM-1 (ng/ml) | |||||||
| Q1 (<0.13) | 4 (1.7) | 1 (referent) | 1 (referent) | 1 (referent) | 1.3 | 2 (1, 3) | 6 (5, 7) |
| Q2 (0.13, 0.31) | 4 (1.7) | 0.9 (0.2 to 3.8) | 0.8 (0.2 to 3.4) | 0.7 (0.1 to 3.1) | 1.2 | 2 (1, 3) | 6 (5, 8) |
| Q3 (0.32, 0.62) | 8 (3.3) | 1.8 (0.5 to 6.3) | 1.6 (0.5 to 5.5) | 1.2 (0.3 to 4.9) | 1.2 | 2 (1, 3) | 7 (5, 8) |
| Q4 (0.62, 1.18) | 16 (6.6) | 3.8 (1.2 to 11.7) | 3.2 (1.0 to 10.0) | 2.2 (0.6 to 8.9) | 2.5 | 2 (1, 3) | 6 (5, 9) |
| Q5 (>1.19) | 24 (10) | 6.2 (2.1 to 18.7) | 4.8 (1.6 to 14.6) | 3.3 (0.8 to 13.1) | 5.4 | 2 (1, 3) | 7 (5, 9) |
| P for trendc | <0.001 | — | — | — | 0.02 | 0.21 | <0.001 |
| Urine L-FABP (ng/ml) | |||||||
| Q1 (<3) | 7 (2.9) | 1 (referent) | 1 (referent) | 1 (referent) | 1.7 | 2 (1, 3) | 6 (5, 8) |
| Q2 (3, 10) | 11 (4.6) | 1.5 (0.6 to 4.1) | 1.5 (0.6 to 4.0) | 1.4 (0.5 to 4.0) | 1.2 | 2 (1, 3) | 6 (5, 8) |
| Q3 (10, 37) | 8 (3.3) | 1.1 (0.4 to 3.2) | 1.0 (0.3 to 2.8) | 0.9 (0.3 to 2.9) | 2.1 | 2 (1, 3) | 6 (5, 8) |
| Q4 (37, 175) | 9 (3.7) | 1.3 (0.5 to 3.5) | 1.0 (0.4 to 2.8) | 0.7 (0.2 to 2.3) | 1.7 | 2 (1, 3) | 6 (5, 8) |
| Q5 (175, 1500) | 21 (8.8) | 2.9 (1.2 to 7.1) | 1.8 (0.7 to 4.6) | 0.6 (0.2 to 2.2) | 5.0 | 2 (1, 4) | 7 (6, 10) |
| P for trendc | 0.32 | — | — | — | 0.19 | 0.02 | <0.001 |
AKI defined by receipt of acute dialysis or a doubling in serum creatinine from the preoperative serum creatinine value. OR, odds ratio; 95% CI, 95% confidence interval; ICU, intensive care unit; IQR, interquartile range; KIM-1, kidney injury molecule-1; L-FABP, liver fatty acid-binding protein; CPB, cardiopulmonary bypass; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated GFR; NGAL, neutrophil gelatinase-associated lipocalin.
Number of patients per quintile: urine KIM-1 Q1 and Q5, n=240; urine KIM-1 Q2, Q3, and Q4, n=241; urine L-FABP Q1 and Q5, n=240; urine L-FABP Q2, Q3, Q4, n=241
Percentage of AKI patients within each quintile.
Adjusted for age(per year), sex, Caucasian race, and site.
Adjusted for age (per year), sex, white race, CPB time >120 minutes, nonelective surgery, preoperative CKD-EPI eGFR, diabetes, hypertension, and center.
Adjusted for age (per year), sex, white race, CPB time >120 minutes, nonelective surgery, preoperative CKD-EPI eGFR, diabetes, hypertension, center, urine IL-18, urine NGAL, plasma NGAL, urine KIM-1, and urine L-FABP. The adjusted relative risks for urine IL-18, urine NGAL, and plasma NGAL from this model are presented in Supplemental Table 3.
Table 3.
Association of first postoperative biomarker quintile and outcomes in children
| Primary Outcome | ||||||
|---|---|---|---|---|---|---|
| AKI Adjusted OR (95% CI) | Nonrenal Outcomes | |||||
| Quintile (cutpoints)a | AKI n (%)b | |||||
| Demographicsc | Clinical Modeld | In-Hospital Death or Dialysis, % | Length of Stay in ICU, Median (IQR) | Length of Stay in Hospital, Median (IQR) | ||
| Urine KIM-1 (ng/ml) | ||||||
| Q1 (<0.15) | 4 (6.7) | 1 (referent) | 1 (referent) | 1.7 | 2 (1, 3) | 4 (3, 5) |
| Q2 (0.15, 0.33) | 6 (9.8) | 1.5 (0.4 to 5.6) | 1 (0.2 to 4) | 3.3 | 2 (1, 3) | 4 (3, 7) |
| Q3 (0.33, 0.59) | 14 (23) | 3.8 (1.2 to 12.5) | 2.7 (0.8 to 9.5) | 4.9 | 2 (1, 4) | 5 (3.5, 8) |
| Q4 (0.60, 0.98) | 12 (19.7) | 3.2 (1 to 10.7) | 3 (0.9 to 10.6) | 3.3 | 2.5 (1, 4.5) | 6 (4, 11) |
| Q5 (>0.99) | 15 (25) | 4 (1.2 to 13.3) | 3 (0.8 to 10.6) | 1.7 | 4 (2, 6) | 7 (4, 13) |
| P for trend | 0.02 | — | — | 0.54 | <0.001 | <0.001 |
| Urine L-FABP (ng/ml) | ||||||
| Q1 (<14) | 6 (10) | 1 (referent) | 1 (referent) | 3.3 | 1 (1, 2) | 4 (3, 5) |
| Q2 (14, 50) | 5 (8.2) | 0.6 (0.2 to 2.3) | 0.4 (0.1 to 1.7) | 1.6 | 2 (1, 3) | 4 (3, 6) |
| Q3 (41, 155) | 5 (8.2) | 0.7 (0.2 to 2.6) | 0.6 (0.2 to 2.3) | 0 | 3 (1, 5) | 5 (3, 12) |
| Q4 (156, 398) | 11 (18.0) | 1.7 (0.6 to 5.2) | 1.1 (0.3 to 3.5) | 1.7 | 2 (2, 4) | 5 (4, 10) |
| Q5 (399, 1500) | 24 (40.0) | 5.1 (1.8 to 14.5) | 3.0 (0.9 to 10.3) | 7.9 | 3.5 (2, 6) | 7 (5, 11) |
| P for trend | 0.01 | — | — | 0.54 | <0.001 | <0.001 |
AKI defined by receipt of acute dialysis or a doubling in serum creatinine from the preoperative serum creatinine value. OR, odds ratio; 95% CI, 95% confidence interval; ICU, intensive care unit; IQR, interquartile range; KIM-1, kidney injury molecule-1; L-FABP, liver fatty acid-binding protein; CPB, cardiopulmonary bypass; RACHS-1, Risk Adjustment for Congenital Heart Surgery-1; eGFR, estimated GFR.
Number of patients per quintile: urine KIM-1 Q1 and Q5, n=60; urine KIM-1 Q2, Q3, Q4, n=61; urine L-FABP, Q1 and Q5, n=60; urine L-FABP Q2, Q3, Q4, n=61.
Percentage of AKI patients within each quintile.
Adjusted for age (per year), sex, Caucasian race, and site.
Adjusted for age (per year), sex, Caucasian race, site, CPB time >120 minutes, nonelective surgery, RACHS ≥3, and preoperative eGFR percentile.
Biomarkers of AKI and Progression
Of 416 adult patients with AKIN stage 1 AKI, 46 (11%) experienced worsening of creatinine progression to a higher severity of AKI (17,22). Neither KIM-1 (median in progressors 2.8 ng/ml versus 3.3 ng/ml in nonprogressors, P=0.2) nor L-FABP (median in progressors 25.9 ng/ml versus 15.5 ng/ml in nonprogressors, P=0.07) measured on the day of clinical AKI was associated with AKI progression.
Biomarkers and Nonrenal Outcomes
Median lengths of ICU stay were 2 days for both adults (IQR, 1, 3) and children (IQR, 1, 4). Median lengths of hospital stay were 6 days in adults (IQR, 5, 8) and 5 days in children (IQR, 4, 8.5). Twenty (1.6%) adult patients and six (2%) children died before discharge. After adjustment for clinical variables, higher KIM-1 levels soon after surgery were incrementally associated with increased risk of hospital death or dialysis in adults but not in children (Tables 2 and 3). L-FABP was not associated with in-hospital death or dialysis in either adults or children. After multivariable adjustment, higher KIM-1 and L-FABP were associated with a longer length of stay in the ICU and in the hospital for both adults and children (adjusted P for trend <0.001 in adults and adjusted P for trend <0.001 in children; Tables 2 and 3).
Evaluating Biomarker Performance
The clinical prediction model with the preoperative and intraoperative variables for AKI had an AUC of 0.69 (SEM 0.04) in adults and 0.78 (SEM 0.03) in children. Addition of KIM-1 as a biomarker to the clinical model marginally improved discrimination and classification in adults (AUC increased to 0.73, P<0.001; NRI, 0.19 [SEM 0.11], P=0.07) (Table 4). In children, addition of KIM-1 did not result in improvement in discrimination and classification over the clinical model (AUC only increased to 0.79, P=0.15; NRI, 0.06 [SEM 0.08], P=0.45) (Table 5). Addition of L-FABP as a biomarker to clinical model did not improve discrimination or classification in adults (AUC 0.72, P=0.19; NRI, 0.06 [SEM 0.07], P=0.42) (Table 4). In children, however, there was marginal benefit as AUC increased to 0.81 (P=0.24) and NRI was 0.19 (SEM 0.08; P=0.02) (Table 5). Normalization of biomarkers by urine creatinine did not change the pattern of the above-described results.
Table 4.
Categorical NRI and IDI of clinical model with first postoperative biomarker in adults
| Biomarker | Categorical NRI | IDI | ||||||
|---|---|---|---|---|---|---|---|---|
| AKI | Non-AKI | Overall NRI (SEM) | P Value | AKI | Non-AKI | Overall IDI (SEM) | P Value | |
| Urine KIM-1 | 0.25 | −0.06 | 0.19 (0.11) | 0.07 | 0.014 | 0.003 | 0.017 (0.01) | 0.01 |
| Urine L-FABP | 0.11 | −0.05 | 0.06 (0.07) | 0.42 | −0.0025 | 0.0026 | 0.001 (0.004) | 0.98 |
| Urine IL-18 (7) | 0.25 | 0 | 0.25 (0.10) | 0.01 | 0.017 | 0.003 | 0.02 (0.0007) | 0.002 |
| Urine NGAL (7) | 0.16 | −0.02 | 0.14 (0.09) | 0.12 | 0.007 | 0.003 | 0.01 (0.01) | 0.08 |
| Plasma NGAL (7) | 0.20 | −0.02 | 0.18 (0.09) | 0.05 | 0.021 | 0.003 | 0.024 (0.007) | <0.001 |
For NRI calculations, AKI risk category definitions were based on a previous publication (7) as low (<3%), medium (3%–10%), or high (>10%). The clinical model is composed of age (per year), sex, white race, CPB time >120 minutes, nonelective surgery, preoperative CKD-EPI eGFR, diabetes, hypertension, and center. NRI, net reclassification improvement; IDI, integrated discrimination index; KIM-1, kidney injury molecule-1; L-FABP, liver fatty acid-binding protein; NGAL, neutrophil gelatinase-associated lipocalin; CPB, cardiopulmonary bypass; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated GFR.
Table 5.
Categorical NRI and IDI of clinical model with first postoperative biomarker in children
| Biomarker | Categorical NRI | IDI | ||||||
|---|---|---|---|---|---|---|---|---|
| AKI | Non-AKI | Overall NRI (SEM) | P Value | AKI | Non-AKI | Overall IDI (SEM) | P Value | |
| Urine KIM-1 | 0.02 | 0.04 | 0.06 (0.08) | 0.45 | 0.02 | 0.01 | 0.03 (0.01) | 0.03 |
| Urine L-FABP | 0.12 | 0.07 | 0.19 (0.08) | 0.02 | 0.05 | 0.01 | 0.06 (0.02) | 0.002 |
| Urine IL-18 (8) | 0.19 | 0.03 | 0.22 (0.09) | 0.02 | 0.04 | 0.01 | 0.05 (0.02) | 0.01 |
| Urine NGAL (8) | 0.15 | 0.03 | 0.18 (0.08) | 0.02 | 0.02 | 0.01 | 0.03 (0.01) | 0.04 |
| Plasma NGAL (8) | 0.12 | 0.02 | 0.14 (0.06) | 0.02 | 0.04 | 0.01 | 0.05 (0.02) | 0.002 |
For NRI calculations, AKI risk category definitions were based on a previous publication (8) as low (<10%), medium (10%–25%), or high (>25%). The clinical model is composed of age (per year), sex, Caucasian race, site, CPB time >120 minutes, nonelective surgery, RACHS ≥3, and preoperative eGFR percentile. NRI, net reclassification improvement; IDI, integrated discrimination index; KIM-1, kidney injury molecule-1; L-FABP, liver fatty acid-binding protein; NGAL, neutrophil gelatinase-associated lipocalin; CPB, cardiopulmonary bypass; RACHS-1, Risk Adjustment for Congenital Heart Surgery-1; eGFR, estimated GFR.
Biomarker Combinations
We evaluated the performance to discriminate for AKI when we combined biomarkers from up to three time points in the first 24 hours after surgery. The combination of urine KIM-1 at 6–12 hours postoperatively with plasma NGAL from day 2 yielded the highest AUC for any two biomarkers (0.76 [SEM 0.04]). When three biomarkers were simultaneously considered, the maximum AUC was 0.78 (SEM 0.04) with the combination of urine KIM-1 from 6 to 12 hours, urine IL-18 from day 2, and plasma NGAL from day 2 (Table 6). In children, the highest AUC achievable was 0.78 (SEM 0.04), which was accomplished with two biomarkers (urine IL-18 from 0 to 6 hours and urine L-FABP from day 2) (Table 7).
Table 6.
Biomarker combinations in adults
| Biomarker Combination | Day 1 | Day 2 | |
|---|---|---|---|
| 0–6 h | 6–12 h | ||
| Individual biomarkers | |||
| Urine KIM-1 (ng/ml) | 0.71 (0.04) | 0.64 (0.04) | 0.56 (0.03) |
| Urine L-FABP (ng/ml) | 0.61 (0.04) | 0.66 (0.04) | 0.67 (0.04) |
| Urine IL-18 (pg/ml) (7) | 0.74 (0.04) | 0.75 (0.03) | 0.71 (0.04) |
| Urine NGAL (ng/ml) (7) | 0.67 (0.04) | 0.70 (0.04) | 0.72 (0.04) |
| Plasma NGAL (ng/ml) (7) | 0.70 (0.04) | NA | 0.73 (0.04) |
| Best combinations across time points | |||
| Two-way combinations | |||
| Urine KIM-1 day 1 (6–12 h) and plasma NGAL day 2 | 0.76 (0.04) | ||
| Three-way combinations | |||
| Urine KIM-1 day 1 6–12 h, urine IL-18 day 2, and plasma NGAL day 2 | 0.78 (0.04) | ||
Data are presented as AUC (SEM). The AUC for the clinical model is 0.69 (SEM 0.04). The clinical model is composed of age (per year), sex, white race, CPB time >120 minutes, nonelective surgery, preoperative CKD-EPI eGFR, diabetes, hypertension, and center. Linear combinations of biomarkers were estimated with logistic regression models. Up to three biomarkers were included in each model. Cross-validation was used to minimize overfitting. The best combination for each category was defined as the highest AUC observed. Each row in the table corresponds to a separate biomarker combination and the biomarkers are listed in no particular order. NA indicates that plasma NGAL values are not available at day 1 (6–12 hours) because blood samples were not collected at this time point. KIM-1, kidney injury molecule-1; L-FABP, liver fatty acid-binding protein; NGAL, neutrophil gelatinase-associated lipocalin; AUC, area under receiver-operating characteristic curves; CPB, cardiopulmonary bypass; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated GFR.
Table 7.
Biomarker combinations in children
| Biomarker Combination | Day 1 | Day 2 | |
|---|---|---|---|
| 0–6 h | 6–12 h | ||
| Individual biomarkers | |||
| Urine KIM-1 (ng/ml) | 0.64 (0.04) | 0.64 (0.04) | 0.63 (0.05) |
| Urine L-FABP (ng/ml) | 0.70 (0.04) | 0.71 (0.04) | 0.66 (0.05) |
| Urine IL-18 (pg/ml) (8) | 0.72 (0.04) | 0.76 (0.04) | 0.60 (0.05) |
| Urine NGAL (ng/ml) (8) | 0.71 (0.04) | 0.69 (0.04) | 0.59 (0.05) |
| Plasma NGAL (ng/ml) (8) | 0.56 (0.05) | NA | 0.57 (0.05) |
| Best combinations across time points | |||
| Two-way combinations | |||
| Urine IL-18 day 1 (0–6 h) and urine L-FABP day 2 | 0.78 (0.04) | ||
| 3-way combinations | |||
| Urine IL-18 day 1 (0–6 h), urine NGAL day 1 (0–6 h), and urine L-FABP day 2 | 0.78 (0.04) | ||
Data are presented as AUC (SEM). The AUC for the clinical model is 0.78 (SEM 0.03). The clinical model is composed of age (per year), sex, Caucasian race, site, CPB time >120 minutes, nonelective surgery, RACHS ≥3, and preoperative eGFR percentile. Linear combinations of biomarkers were estimated with logistic regression models. Up to three biomarkers were included in each model. Cross-validation was used to minimize overfitting. The best combination for each category was defined as the highest AUC observed. Each row in the table corresponds to a separate biomarker combination and the biomarkers are listed in no particular order. NA indicates that plasma NGAL values are not available at day 1 (6–12 hours) because blood samples were not collected at this time point. KIM-1, kidney injury molecule-1; L-FABP, liver fatty acid-binding protein; NGAL, neutrophil gelatinase-associated lipocalin; AUC, area under receiver-operating characteristic curves; CPB, cardiopulmonary bypass; RACHS-1, Risk Adjustment for Congenital Heart Surgery-1; eGFR, estimated GFR.
Discussion
Studies over the past decade have used preclinical models and discovery proteomic techniques to identify several proteins in the urine or serum that signal structural injury to the renal tubule. However, identification of these biomarkers has not been translated to clinical application in human AKI, in part, because of insufficient evidence provided by small single-center studies of variable quality. To guide the efficient translation and validation of these biomarkers for clinical use, we founded the Translational Research Investigating Biomarker End-Points in Acute Kidney Injury (TRIBE-AKI) consortium to conduct prospective multicenter studies for biomarker validation. We focused on cardiac surgery as the optimal human model for validating AKI biomarkers because it allows for precise assessment of baseline renal function, and the knowledge about the timing of ischemic insult that permits biosample collection before clinical diagnosis of AKI. In addition, cardiac surgery is the most popular model for drug development in AKI. One of the fundamental goals of biomarker research is to advance clinical trial designs such that biomarkers may be used as either inclusion criteria to identify high-risk groups, or as intermediate outcomes to identify promising therapies that may warrant further testing. As recommended by the US National Institutes of Health, we enrolled both adults and children, because biomarker performance may differ among these groups due to differences in preexisting CKD and underlying comorbidities. Previously, we reported our findings on urinary NGAL, plasma NGAL, and urinary IL-18 (7,8). Here we report the results of two other promising urine biomarkers for AKI, KIM-1 and L-FABP, and we consider them in the context of the previously biomarkers studied n this cohort.
Similar to IL-18 and plasma NGAL (7), the first postoperative urinary KIM-1 concentration provided additional prognostic information in terms of AKI, death, or dialysis and improved diagnostic accuracy (AUC) upon addition to the clinical model for AKI. In adults, a KIM-1 level of 0.62–1.18 ng/ml soon after surgery denoted a 3-fold adjusted risk of AKI, and a level of >1.18 ng/ml was associated with a 4.7-fold risk of AKI. However, the association between KIM-1 and AKI was not significant after adjusting for other injury biomarkers, NGAL and IL-18. KIM-1 did not yield positive results in children. The AUC of KIM-1 for AKI in our study was toward the lower end of prior reports (0.68–0.95), which used different definitions of AKI and different clinical settings (23).
L-FABP did not provide any additional prognostic information for AKI, death, or dialysis in adults, but it had some benefit in children. In this study, L-FABP levels were much greater in children compared with adults. The AUC of L-FABP was 0.61 in adults and 0.70 in children in our study, which was lower than the AUCs in other published studies, in which L-FABP was found to have an AUC of 0.86 and 0.81 (24,25). Although the reasons remain unclear for the difference in performance of L-FABP between adults and children, it highlights the importance of cross-validation of biomarker study results across these age groups. Potential reasons may include effects of chronic diseases and morbidity in adults but not children and conceivably, developmental and functional differences in biomarker production and metabolism.
KIM-1 is an Ig superfamily transmembrane receptor that is expressed in the tubules in the setting of kidney injury to aid the removal of apoptotic and necrotic bodies (12). KIM-1 was markedly elevated in dedifferentiated proximal tubular cells of ischemic rat kidney and histopathologic scoring correlated with urinary levels of KIM1 (10,11,15). The US Food and Drug Administration approved KIM-1 as a part of urinary biomarker panel for preclinical trials and availability of a dipstick test for urine KIM-1 makes a bedside diagnosis of AKI feasible (10,11,14,26). Fatty acid-binding proteins (L-FABP) bind unsaturated fatty acids and lipid peroxidation products in hypoxia-induced tissue injury. In the human kidney, L-FABP is expressed predominantly in the proximal tubules, which use fatty acids as the major source of energy metabolism. L-FABP is also expressed in ischemic proximal tubule cells and is renoprotective by binding free fatty acids. Systemic upregulation of L-FABP in transgenic mouse models protects against AKI, suggesting a protective effect of L-FABP (13,25). KIM-1 and L-FABP are elevated in various settings of kidney injury and CKD, reflecting tubular injury (12,27–35).
The TRIBE-AKI cohorts have now provided results of four novel urine biomarkers and one plasma biomarker (urine NGAL, IL-18, KIM-1, L-FABP, and plasma NGAL) (7,8). All of the biomarkers peaked in <6 hours except KIM-1, which peaked 2 days after surgery and remained elevated for several days. Our results demonstrate that all of the biomarkers have inter-relationships, but urine IL-18 and plasma NGAL have the strongest signal for capturing tubular injury that will lead to clinical AKI. Of all of the novel biomarkers, only urine IL-18 and plasma NGAL in adults were helpful in predicting progression of AKI when measured on the day of clinical AKI diagnosis (AKIN stage 1) (22). In adults, the AUC for each biomarker we tested increased in magnitude with greater severity of AKI.
Although we did not find any improvement in performance with combination of KIM-1 and L-FABP alone, we did find a strong signal for risk of AKI when five AKI biomarkers were considered in aggregate. We found that combination of three biomarkers in adults from two different time points and combinations of two biomarkers in children from two time points was able to increase the AUC for AKI up to 0.78. We should caution, however, that we only performed linear combination of biomarkers, and that there are more sophisticated techniques of biomarker combination that are being developed. Other limitations of our study included that urine output criteria were not used in the AKI definition. Moreover, biomarkers were measured more frequently in the first 24 hours than serum creatinine, which was measured according to clinical care. In addition, changes in the net fluid balance can affect postoperative serum creatinine levels and assessment of clinical AKI and affect biomarker performance.
Finally, although we utilized a definition of severe AKI as our primary endpoint, to allow for greater specificity, it is still a surrogate outcome that holds questionable association and causality for hard clinical outcomes. More work needs to be done toward assessing the associations between AKI biomarkers and longer-term outcomes such as survival, CKD, and ESRD. In addition, the significance of subclinical AKI (those with increase in biomarkers but no elevation in serum creatinine) needs to be determined. Finally, the nephrology community will need to determine the utility of multiple biomarkers as either enrollment criteria for entry into clinical trials for AKI therapeutics, or as surrogate endpoints to identify potentially efficacious interventions worthy of large-scale testing.
Disclosures
C.L.E. and C.R.P. are named coinventors on the IL-18 patent. P.D. is the coinventor on the NGAL patents.
Supplementary Material
Acknowledgments
Members of the TRIBE-AKI consortium are as follows: Dr. Jai Raman, Dr. Valluvan Jeevanandam, and Dr. Shahab Akhter (University of Chicago); Michael Bennett, Qing Ma, and Ms. Rachel Griffiths (University of Cincinnati Children’s Hospital Medical Center); Ms. Judy Nagy (Danbury Hospital); Dr. Madhav Swaminathan (Duke University); Dr. Michael Chu, Dr. Martin Goldbach, Dr. Lin Ruo Guo, Dr. Neil McKenzie, Dr. Mary Lee Myers, Dr. Richard Novick, Dr. Mac Quantz, Ms. Virginia Schumann, and Ms. Laura Webster (Western University, London, Ontario); Dr. Ana Palijan (Montreal Children’s Hospital); and Dr. Michael Dewar, Dr. Umer Darr, Dr. Sabet Hashim, Dr. John Elefteriades, Dr. Arnar Geirsson, Dr. Susan Garwood, Ms. Rowena Kemp, and Dr. Isabel Butrymowicz (Yale University).
The urine biomarker assays were donated by Abbott Diagnostics (IL-18 and NGAL) and Sekisui Diagnostics LLC (KIM-1 and L-FABP). The research reported in this article was supported by a grant from the National Heart, Lung, and Blood Institute (R01HL-085757 to C.R.P.). The study was also supported by a Clinical and Translational Science Award (UL1 RR024139) from the National Center for Research Resources. S.G.C. has been supported by a Career Development Award from the National Institutes of Health (NIH) (K23DK080132). C.R.P. is also supported by an NIH grant (K24DK090203). S.G.C., A.X.G., P.D., M.Z., and C.R.P. are also members of the NIH-sponsored Assess, Serial Evaluation, and Subsequent Sequelae in Acute Kidney Injury (ASSESS-AKI) Consortium (U01DK082185). The granting agencies, Abbott Diagnostics and Sekisui Diagnostics, Inc., did not participate in the protocol development, analysis, or interpretation of the results.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10971012/-/DCSupplemental.
References
- 1.Rosner MH, Okusa MD: Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 1: 19–32, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Westenfelder C: Earlier diagnosis of acute kidney injury awaits effective therapy. Kidney Int 79: 1159–1161, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Koyner JL, Vaidya VS, Bennett MR, Ma Q, Worcester E, Akhter SA, Raman J, Jeevanandam V, O’Connor MF, Devarajan P, Bonventre JV, Murray PT: Urinary biomarkers in the clinical prognosis and early detection of acute kidney injury. Clin J Am Soc Nephrol 5: 2154–2165, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liangos O, Perianayagam MC, Vaidya VS, Han WK, Wald R, Tighiouart H, MacKinnon RW, Li L, Balakrishnan VS, Pereira BJ, Bonventre JV, Jaber BL: Urinary N-acetyl-beta-(D)-glucosaminidase activity and kidney injury molecule-1 level are associated with adverse outcomes in acute renal failure. J Am Soc Nephrol 18: 904–912, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV: Urinary kidney injury molecule-1: A sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol 290: F517–F529, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Vaidya VS, Waikar SS, Ferguson MA, Collings FB, Sunderland K, Gioules C, Bradwin G, Matsouaka R, Betensky RA, Curhan GC, Bonventre JV: Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin Transl Sci 1: 200–208, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parikh CR, Coca SG, Thiessen-Philbrook H, Shlipak MG, Koyner JL, Wang Z, Edelstein CL, Devarajan P, Patel UD, Zappitelli M, Krawczeski CD, Passik CS, Swaminathan M, Garg AX, TRIBE-AKI Consortium : Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol 22: 1748–1757, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parikh CR, Devarajan P, Zappitelli M, Sint K, Thiessen-Philbrook H, Li S, Kim RW, Koyner JL, Coca SG, Edelstein CL, Shlipak MG, Garg AX, Krawczeski CD, TRIBE-AKI Consortium : Postoperative biomarkers predict acute kidney injury and poor outcomes after pediatric cardiac surgery. J Am Soc Nephrol 22: 1737–1747, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV: Kidney injury molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int 62: 237–244, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M: Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem 273: 4135–4142, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Ichimura T, Asseldonk EJ, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV: Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest 118: 1657–1668, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Timmeren MM, van den Heuvel MC, Bailly V, Bakker SJ, van Goor H, Stegeman CA: Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J Pathol 212: 209–217, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto T, Noiri E, Ono Y, Doi K, Negishi K, Kamijo A, Kimura K, Fujita T, Kinukawa T, Taniguchi H, Nakamura K, Goto M, Shinozaki N, Ohshima S, Sugaya T: Renal L-type fatty acid—binding protein in acute ischemic injury. J Am Soc Nephrol 18: 2894–2902, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Dieterle F, Sistare F, Goodsaid F, Papaluca M, Ozer JS, Webb CP, Baer W, Senagore A, Schipper MJ, Vonderscher J, Sultana S, Gerhold DL, Phillips JA, Maurer G, Carl K, Laurie D, Harpur E, Sonee M, Ennulat D, Holder D, Andrews-Cleavenger D, Gu YZ, Thompson KL, Goering PL, Vidal JM, Abadie E, Maciulaitis R, Jacobson-Kram D, Defelice AF, Hausner EA, Blank M, Thompson A, Harlow P, Throckmorton D, Xiao S, Xu N, Taylor W, Vamvakas S, Flamion B, Lima BS, Kasper P, Pasanen M, Prasad K, Troth S, Bounous D, Robinson-Gravatt D, Betton G, Davis MA, Akunda J, McDuffie JE, Suter L, Obert L, Guffroy M, Pinches M, Jayadev S, Blomme EA, Beushausen SA, Barlow VG, Collins N, Waring J, Honor D, Snook S, Lee J, Rossi P, Walker E, Mattes W: Renal biomarker qualification submission: a dialog between the FDA-EMEA and Predictive Safety Testing Consortium. Nat Biotechnol 28: 455–462, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Rouse RL, Zhang J, Stewart SR, Rosenzweig BA, Espandiari P, Sadrieh NK: Comparative profile of commercially available urinary biomarkers in preclinical drug-induced kidney injury and recovery in rats. Kidney Int 79: 1186–1197, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative workgroup : Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8: R204–R212, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A, Acute Kidney Injury Network : Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piepsz A, Tondeur M, Ham H: Revisiting normal (51)Cr-ethylenediaminetetraacetic acid clearance values in children. Eur J Nucl Med Mol Imaging 33: 1477–1482, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS: Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med 27: 157–172, discussion 207–212, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Koyner JL, Garg AX, Coca SG, Sint K, Thiessen-Philbrook H, Patel UD, Shlipak MG, Parikh CR, TRIBE-AKI Consortium : Biomarkers predict progression of acute kidney injury after cardiac surgery. J Am Soc Nephrol 23: 905–914, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han WK, Wagener G, Zhu Y, Wang S, Lee HT: Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol 4: 873–882, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsui K, Kamijo-Ikemori A, Sugaya T, Yasuda T, Kimura K: Usefulness of urinary biomarkers in early detection of acute kidney injury after cardiac surgery in adults. Circ J 76: 213–220, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Portilla D, Dent C, Sugaya T, Nagothu KK, Kundi I, Moore P, Noiri E, Devarajan P: Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int 73: 465–472, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Vaidya VS, Ford GM, Waikar SS, Wang Y, Clement MB, Ramirez V, Glaab WE, Troth SP, Sistare FD, Prozialeck WC, Edwards JR, Bobadilla NA, Mefferd SC, Bonventre JV: A rapid urine test for early detection of kidney injury. Kidney Int 76: 108–114, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu PC, Zhang JJ, Chen M, Lv JC, Liu G, Zou WZ, Zhang H, Zhao MH: Urinary kidney injury molecule-1 in patients with IgA nephropathy is closely associated with disease severity. Nephrol Dial Transplant 26: 3229–3236, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Jungbauer CG, Birner C, Jung B, Buchner S, Lubnow M, von Bary C, Endemann D, Banas B, Mack M, Böger CA, Riegger G, Luchner A: Kidney injury molecule-1 and N-acetyl-β-D-glucosaminidase in chronic heart failure: possible biomarkers of cardiorenal syndrome. Eur J Heart Fail 13: 1104–1110, 2011 [DOI] [PubMed] [Google Scholar]
- 29.van Timmeren MM, Vaidya VS, van Ree RM, Oterdoom LH, de Vries AP, Gans RO, van Goor H, Stegeman CA, Bonventre JV, Bakker SJ: High urinary excretion of kidney injury molecule-1 is an independent predictor of graft loss in renal transplant recipients. Transplantation 84: 1625–1630, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCullough PA, Chinnaiyan KM, Gallagher MJ, Colar JM, Geddes T, Gold JM, Trivax JE: Changes in renal markers and acute kidney injury after marathon running. Nephrology (Carlton) 16: 194–199, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Doi K, Noiri E, Maeda-Mamiya R, Ishii T, Negishi K, Hamasaki Y, Fujita T, Yahagi N, Koide H, Sugaya T, Nakamura T: Urinary L-type fatty acid-binding protein as a new biomarker of sepsis complicated with acute kidney injury. Crit Care Med 38: 2037–2042, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Nakamura T, Sugaya T, Kawagoe Y, Suzuki T, Ueda Y, Koide H, Inoue T, Node K: Azelnidipine reduces urinary protein excretion and urinary liver-type fatty acid binding protein in patients with hypertensive chronic kidney disease. Am J Med Sci 333: 321–326, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Nakamura T, Inoue T, Sugaya T, Kawagoe Y, Suzuki T, Ueda Y, Koide H, Node K: Beneficial effects of olmesartan and temocapril on urinary liver-type fatty acid-binding protein levels in normotensive patients with immunoglobin A nephropathy. Am J Hypertens 20: 1195–1201, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Nakamura T, Sugaya T, Koide H: Urinary liver-type fatty acid-binding protein in septic shock: effect of polymyxin B-immobilized fiber hemoperfusion. Shock 31: 454–459, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Nielsen SE, Sugaya T, Hovind P, Baba T, Parving HH, Rossing P: Urinary liver-type fatty acid-binding protein predicts progression to nephropathy in type 1 diabetic patients. Diabetes Care 33: 1320–1324, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


