Summary
Background and objectives
Higher morning serum phosphorus has been associated with cardiovascular disease (CVD) in patients with or without CKD. In patients with CKD and a phosphorous level >4.6 mg/dl, the Kidney Disease Improving Global Outcomes guidelines recommend dietary phosphorus restriction. However, whether phosphorus restriction influences serum phosphorus concentrations and whether dietary phosphorus is itself associated with CVD or death are uncertain.
Design, setting, participants, & measurements
Among 880 patients with stable CVD and normal kidney function to moderate CKD, 24-hour urine phosphorus excretion (UPE) and serum phosphorus were measured at baseline. Participants were followed for a median of 7.4 years for CVD events and all-cause mortality.
Results
Mean ± SD age was 67±11 years, estimated GFR (eGFR) was 71±22 ml/min per 1.73 m2, and serum phosphorus was 3.7±0.6 mg/dl. Median UPE was 632 (interquartile range, 439, 853) mg/d. In models adjusted for demographic characteristics and eGFR, UPE was weakly and nonsignificantly associated with serum phosphorus (0.03 mg/dl higher phosphorus per 300 mg higher UPE; P=0.07). When adjusted for demographics, eGFR, and CVD risk factors, each 300-mg higher UPE was associated with 17% lower risk of CVD events. The association of UPE with all-cause mortality was not statistically significant (hazard ratio, 0.93; 95% confidence interval, 0.82 to 1.05). Results were similar irrespective of CKD status (P interactions > 0.87).
Conclusions
Among outpatients with stable CVD, the magnitude of the association of UPE with morning serum phosphorus is modest. Greater UPE is associated with lower risk for CVD events. The association was similar for all-cause mortality but was not statistically significant.
Introduction
The association of serum phosphorus concentrations with cardiovascular disease (CVD) events and death in ESRD is well established (1). Similarly, in individuals with kidney function ranging from normal to moderate CKD, higher fasting morning serum phosphorus concentrations have been associated with arterial calcification (2,3), arterial stiffness (4–6), and incident CVD events in some prior studies (7–9). In 2009, the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines recommended that serum phosphorus concentrations should be targeted to <4.6 mg/dl in patients with CKD stage 3–5. In patients with higher serum phosphorus, dietary phosphorus restriction or use of oral phosphorus binders should be considered. These recommendations were based largely on expert opinion (10).
Serum phosphorus concentrations increase in patients with ESRD who have greater dietary phosphorus intake (1,11–13); however, whether a similar relationship extends to persons with earlier stages of CKD or to those with normal kidney function is uncertain. The residual kidney function may allow such individuals to respond to dietary phosphorus intake by excreting more phosphorus through the urine without significantly influencing the serum phosphorus concentration. Regulatory hormones may keep phosphorus concentrations at a specific serum concentration, independent of dietary intake. Studies published after the KDIGO recommendations support this hypothesis. Oral phosphorus binders markedly decrease urine phosphorus excretion (UPE), suggesting marked decreases in intestinal absorption, but have had little effect on serum phosphorus concentrations in CKD stage 3–4 (14–17). Several large epidemiologic studies have detected weak or absent associations of phosphorus intake estimated by food-frequency questionnaires with serum phosphorus concentrations (4,6,18); however, the accuracy of these questionnaires for estimating phosphorus intake has been questioned (19). To our knowledge, no study has evaluated whether greater dietary phosphorus intake is itself associated with CVD events or all-cause mortality.
Twenty-four-hour UPE provides a reliable estimate of intestinal phosphorus absorption and has been used as the preferred method to determine the effects of interventions on intestinal absorption in prior studies (14,15,20). Here, we evaluated a relatively large cohort of community-living individuals with stable CVD and kidney ranging from normal function to moderate CKD who all provided 24-hour urine collections at baseline. We determined the relationship of 24-hour UPE with fasting morning serum phosphorus, calcium, parathyroid hormone (PTH), and fibroblast growth factor-23 (FGF-23) concentrations at baseline. Subsequently, we determined the relationships of 24-hour UPE with all-cause mortality and CVD events during 7.4 years of follow-up. A priori, we hypothesized that the relationship of 24-hour UPE with fasting morning serum phosphorus concentrations would be weak or absent and that 24-hour UPE would not be associated with mortality or CVD events.
Materials and Methods
Study Participants
The Heart and Soul Study is an observational study designed to investigate the relationship of psychosocial factors on the progression of CVD. Methods have been previously described (21). Participants were recruited from outpatient clinics in the San Francisco Bay area in California if they met at least one of the following inclusion criteria: history of myocardial infarction (MI), angiographic evidence of >50% stenosis in one or more coronary vessels, evidence of exercise-induced ischemia with treadmill or nuclear testing, history of coronary revascularization, or documented diagnosis of coronary artery disease by an internist or cardiologist. Participants were excluded if they were unable to walk 1 block, had experienced an MI within the last 6 months, or were likely to relocate out of the area within 3 years. The study protocol was approved by the institutional review boards of participating institutions, and all participants provided written informed consent.
From September 2000 to December 2002, 1024 participants enrolled. The baseline visit included a medical history, physical examination, comprehensive health status questionnaire, 24-hour urine collection, and fasting (12-hour) morning venous blood samples. Participants were followed up for CVD events and death through May 2, 2012. For the present analysis, we excluded 122 (13%) participants who did not have baseline urine samples for 24-hour UPE measurements, 13 (1%) for whom covariate data were missing, and 9 (1%) without available follow-up data after the baseline examination, resulting in a final sample size of 880 participants.
Measurements
Urine Phosphorus Excretion.
The protocol used for timed urine collections has been previously described (22). In brief, participants received detailed instructions on accurate urine collection and specimen refrigeration. Participants were asked to void at the end of their study appointment and to begin the collection from that point forward. To avoid overcollection, research personnel arrived at the patient’s home 24 hours after the timed collection was initiated. Collections were repeated if participants reported missing the collection of any urine or if total collections were <1 or >3 L. When participants were unable to collect all urine for any reason, no data were recorded. Urine volume was recorded (in ml) and mixed thoroughly, and 5-ml aliquots were stored at −80°C. Specimens were thawed and treated with 1 mol of hydrochloric acid per L, and urine phosphorus was measured using a Cobas 6000 analyzer (Roche Diagnostics). The lower limit of detection was 3.4 mg/dl, and the coefficient of variation was 1.4%–1.7%. We calculated UPE by multiplying urine phosphorus concentration (mg/dl) by total 24-hour urine volume (dl/d).
Cardiovascular Events and Mortality.
From the baseline examination to May 2, 2012, participants (or their proxies) were contacted annually for telephone interviews that inquired about hospitalizations, cardiac procedures, or death. Two independent, blinded adjudicators reviewed all events, medical records, electrocardiography results, death certificates, and coroner’s reports and adjudicated events against prespecified criteria, as described previously (22). In the event of disagreement, the adjudicators conferred, reconsidered their classification, and requested consultation from a third blinded adjudicator, as necessary.
We considered the composite of MI, stroke, or CVD death as a CVD event. MI was defined by cardiac biomarkers, electrocardiography results, and cardiac symptoms or signs according to the American Heart Association criteria (23). We defined stroke as a new neurologic deficit not secondary to brain trauma, tumor, infection, or other cause. CVD mortality was determined by death certificates and coroner’s reports. All-cause mortality was determined by review of death certificates.
Other Measurements.
Patient demographic characteristics and comorbid diseases were determined by questionnaires. Weight and height were measured in participants wearing light clothing and no shoes. Body mass index (BMI) was calculated (kg/m2). We measured serum total cholesterol, HDL cholesterol, calcium, and phosphorus concentrations using standard clinical chemistry analyzers. Plasma FGF-23 concentrations were measured using a C-terminal human ELISA. Plasma PTH concentrations were measured using the Roche PTH immunoassay on an Elecsys E170 automated analyzer. The lower limit of detection was 6 pg/ml; coefficients of variation were 1.8% at a concentration of 167 pg/ml and 3.0% at 30 pg/ml. Serum cystatin C concentrations were measured with a particle-enhanced immunonephelometric assay, described previously (N Latex Cystatin-C, Dade Behring, Inc., Deerfield, IL), and were used to calculate estimated glomerular filtration rate (eGFR) with the following validated formula (24):
High-sensitivity C-reactive protein was measured with the Roche (Indianapolis, IN) or the Beckman Extended Range (Galway, Ireland) assays (25). In the 24-hour urine samples, we measured urine creatinine concentrations using the rate Jaffe method and urine albumin using nephelometry. Urine albumin-to-creatinine ratio was calculated (mg/g).
Statistical Analyses
We grouped participants into tertiles based on 24-hour UPE measurement. We compared baseline characteristics across tertiles by ANOVA or Kruskal-Wallis tests for continuous variables and the chi-squared test for categorical variables, as appropriate. Next, we used linear regression to evaluate the cross-sectional association of 24-hour UPE with serum phosphorus, calcium, PTH, and FGF-23, each adjusted for age, sex, race, and eGFR (PTH and FGF-23 were log-transformed because of skewed distributions). Subsequently, geometric mean concentrations and associated 95% confidence intervals (CIs) of each marker were calculated. Next, we used Cox proportional hazards models to evaluate the associations of 24-hour UPE with CVD events and death. Models were initially evaluated by UPE tertiles, and when these were observed to be fairly linear across tertiles, we evaluated UPE as a continuous predictor variable. We developed a sequence of models. The initial model was unadjusted. A subsequent model adjusted for age, sex, estrogen use in women, race, and eGFR. A final model additionally adjusted for CVD risk factors (diabetes, hypertension, smoking, BMI, total cholesterol, HDL, and C-reactive protein), and urine calcium excretion.
Because the KDIGO guidelines recommend dietary phosphorus restriction in individuals with CKD specifically, we evaluated multiplicative interactions in the associations of UPE with CVD events and mortality by CKD status (eGFR < 60 ml/min per 1.73 m2 versus higher). We developed nested models with and without a multiplicative interaction term (UPE [continuous] × CKD [binary]). Statistical significance was determined by the likelihood ratio test.
To determine whether any association between UPE and CVD events or mortality may be biased because of incomplete urine collections in patients with low UPE, we performed a sensitivity analysis by excluding participants whose 24-hour urine measured creatinine clearance was >30% different from their estimated creatinine clearance by the Cockcroft-Gault equation (26). This approach takes advantage of the fact that Cockcroft-Gault–estimated creatinine clearance relies only on serum creatinine concentration and demographic variables and is therefore not influenced by the accuracy of the 24-hour urine collection, whereas measured creatinine clearance depends on the accuracy of this collection. Thus, when discordant, the 24-hour urine collection is more likely to have been under- or overcollected.
All analyses were conducted using Stata software, version 11 (Stata Corp., College Station, TX). P values <0.05 were considered to represent statistically significant differences for all analyses, including interaction terms.
Results
Among the 880 study participants, the mean ± SD for age was 67±11 years, 18% were female, 61% were white, and the mean ± SD eGFR was 71±22 ml/min per 1.73 m2. The mean ± SD value for 24-hour UPE was 667±309 mg/d, and the mean serum phosphorus was 3.7±0.6 mg/dl. Mean 24-hour urine creatinine excretion rate was 1200±413 mg/d. During the median follow-up of 7.4 years, 340 patients died and 221 CVD events occurred.
Baseline characteristics by tertiles of 24-hour UPE are shown in Table 1. Compared with participants with lower UPE, those in the highest tertile were younger; were more frequently male and white; and had higher BMI, higher eGFR, and lower urine albumin-to-creatinine ratio. Serum phosphorus, calcium, and FGF-23 concentrations were similar across UPE tertiles, whereas participants in the highest tertile had lower serum PTH concentrations and higher 24-hour urine calcium excretion in unadjusted analysis.
Table 1.
Baseline characteristics by 24-hour urine phosphorus excretion tertiles
| Variable | UPE Tertile 1 (<508 mg/d) | UPE Tertile 2 (508–748 mg/d) | UPE Tertile 3 (>748 mg/d) | P Value |
|---|---|---|---|---|
| Participants (n) | 294 | 293 | 293 | |
| Age (yr) | 69±11 | 68±10 | 63±11 | <0.001 |
| Women | 73 (25) | 51 (17) | 34 (12) | <0.001 |
| Estrogen use in women | 19 (26) | 13 (26) | 9 (27) | 0.99 |
| Race | <0.001 | |||
| White | 156 (53) | 181 (62) | 200 (68) | |
| Black | 65 (22) | 43 (15) | 26 (9) | |
| Other | 73 (25) | 69 (24) | 67 (23) | |
| Diabetes mellitus | 78 (27) | 70 (24) | 80 (27) | 0.61 |
| Hypertension | 215 (73) | 209 (71) | 203 (69) | 0.59 |
| Smoking | 61 (21) | 51 (17) | 54 (18) | 0.57 |
| Body mass index (kg/m2) | 27.4±5.3 | 28.1±4.7 | 29.8±5.9 | <0.001 |
| eGFR (ml/min per 1.73 m2) | 66±26 | 72±21 | 76±19 | <0.001 |
| Urine albumin-to-creatinine ratio (mg/g)a | 11 (7, 23) | 8 (5, 17) | 7 (4, 14) | <0.001 |
| Diuretic use | 108 (37) | 80 (27) | 76 (26) | <0.01 |
| Vitamin use | 55 (19) | 62 (21) | 57 (19) | 0.75 |
| Serum phosphorus (mg/dl) | 3.66±0.58 | 3.66±0.56 | 3.66±0.56 | 0.75 |
| Serum calcium (mg/dl) | 9.50±0.52 | 9.55±0.54 | 9.52±0.48 | 0.10 |
| PTH (pg/ml)a | 57 (41, 77) | 52 (41, 69) | 50 (40, 66) | <0.01 |
| FGF-23 (RU/ml)a | 46 (30, 86) | 40 (26, 64) | 43 (28, 68) | 0.09 |
| Urine calcium (mg/d)a | 38 (19, 68) | 74 (46, 125) | 137 (71, 205) | <0.001 |
Data are presented as mean ± SD or number (percentage) of participants unless otherwise indicated. P values represent chi-squared tests or ANOVA. UPE, urinary phosphorus excretion; eGFR, estimated GFR; PTH, parathyroid hormone; FGF-23, fibroblast growth factor-23.
Median (interquartile range).
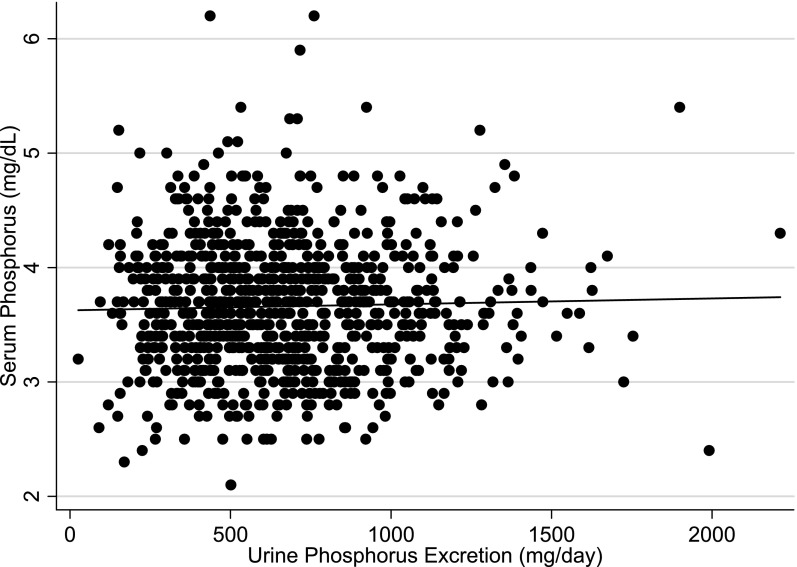
We observed no significant correlation between 24-hour UPE and fasting morning serum phosphorus concentrations when UPE was evaluated as a continuous variable (Pearson r=0.03; P=0.40) (Figure 1). With adjustment for age, sex, and eGFR, a modest direct association was revealed; however, the association did not reach statistical significance. Each 300-mg greater UPE (approximately 1 SD higher) was associated with a 0.034 mg/dl (95% CI, −0.002 to 0.072 mg/dl; P=0.07) higher serum phosphorus concentration (Table 2). This association was similar among the 269 participants with CKD, where each 300-mg greater UPE was associated with a 0.07-mg/dl (95% CI, −0.01 to 0.14 mg/dl; P=0.08) higher serum phosphorus concentration. The inverse association between 24-hour UPE and PTH observed in unadjusted analysis in Table 1 was attenuated with adjustment for eGFR. The association of 24-hour UPE with urine calcium excretion remained strong in adjusted analysis. No relationship was observed between 24-hour UPE and serum calcium or FGF-23 concentrations.
Figure 1.
Relationship of 24-hour urine phosphorus excretion with serum phosphorus concentrations in participants with kidney function ranging from normal function to moderate CKD. Pearson correlation (r) = 0.03, P=0.40.
Table 2.
Adjusted mean concentrations of mineral metabolism measures by urine phosphorus excretion tertiles and β coefficients from linear regression
| Variable | UPE Tertiles | Continuous (per 300 mg/d Higher) UPE | |||
|---|---|---|---|---|---|
| Tertile 1 <508 mg/d | Tertile 2 508–748 mg/d | Tertile 3 >748 mg/d | Difference in Variable (95% CI) | P Value | |
| Serum phosphorus (mg/dl) | 3.6 (3.6 to 3.7) | 3.7 (3.6 to 3.7) | 3.7 (3.6 to 3.7) | 0.034 (−0.002 to 0.072) | 0.07 |
| Serum calcium (mg/dl) | 9.5 (9.4 to 9.6) | 9.6 (9.5 to 9.6) | 9.5 (9.5 to 9.6) | −0.007 (−0.042 to 0.029) | 0.72 |
| Ln-PTH (pg/ml) | 4.00 (3.95 to 4.05) | 3.97 (3.92 to 4.02) | 3.98 (3.93 to 4.02) | −0.002 (−0.031 to 0.027) | 0.89 |
| Ln-FGF-23 (RU/ml) | 3.8 (3.7 to 3.9) | 3.8 (3.7 to 3.9) | 3.9 (3.8 to 4.0) | 0.022 (−0.034 to 0.079) | 0.43 |
| Urine calcium (mg/d) | 58. (51 to 66) | 90. (83 to 98) | 140. (132 to 147) | 37. (33 to 42) | <0.001 |
Concentrations given as mean (95% confidence interval). Values are adjusted for age, sex, race, and estimated GFR. UPE, urinary phosphorus excretion; CI, confidence interval; LnPTH, natural logarithm parathyroid hormone; LnFGF-23, natural logarithm fibroblast growth factor-23.
We next evaluated the association of 24-hour UPE with all-cause mortality. In unadjusted analysis, participants in the highest UPE tertile were at 44% lower risk of death compared with those in the lowest tertile, and each 300 mg higher UPE was associated with an approximately 20% lower mortality risk (Table 3). However, with adjustment for age, sex, race, oral estrogen use (in women), and eGFR, this association was attenuated and rendered no longer statistically significant. Age adjustment was responsible for most of the attenuation. Results were similar in individuals with and without CKD (P interaction = 0.95) (Supplemental Table 1).
Table 3.
Cox proportional hazards models for 24-hour urine phosphorus excretion with all-cause mortality
| Variable | UPE Tertiles | Continuous | |||
|---|---|---|---|---|---|
| Tertile 1 (<508 mg/d) | Tertile 2 (508–748 mg/d) | Tertile 3 (>748 mg/d) | Per 300 mg/d Higher UPE (95% CI) | P Value | |
| Events/at risk (n/n) | 138/294 | 112/293 | 90/293 | 340/880 | |
| Unadjusted: Hazard ratio (95% CI) | 1.00 (reference) | 0.74 (0.58 to 0.95) | 0.56 (0.43 to 0.74) | 0.81 (0.72 to 0.91) | <0.001 |
| Model 1: Hazard ratio (95% CI) | 1.00 (reference) | 0.84 (0.65 to 1.08) | 0.72 (0.54 to 0.95) | 0.91 (0.80 to 1.03) | 0.14 |
| Model 2: Hazard ratio (95% CI) | 1.00 (reference) | 0.92 (0.71 to 1.20) | 0.78 (0.56 to 1.07) | 0.96 (0.84 to 1.11) | 0.59 |
Model 1; adjusted for age, sex, oral estrogen use (women), race, and estimated GFR. Model 2; adjusted for age, sex, oral estrogen use (women), race, estimated GFR, diabetes, hypertension, smoking, body mass index, cholesterol, HDL cholesterol, C-reactive protein, and urine calcium excretion. UPE, urinary phosphorus excretion; CI, confidence interval.
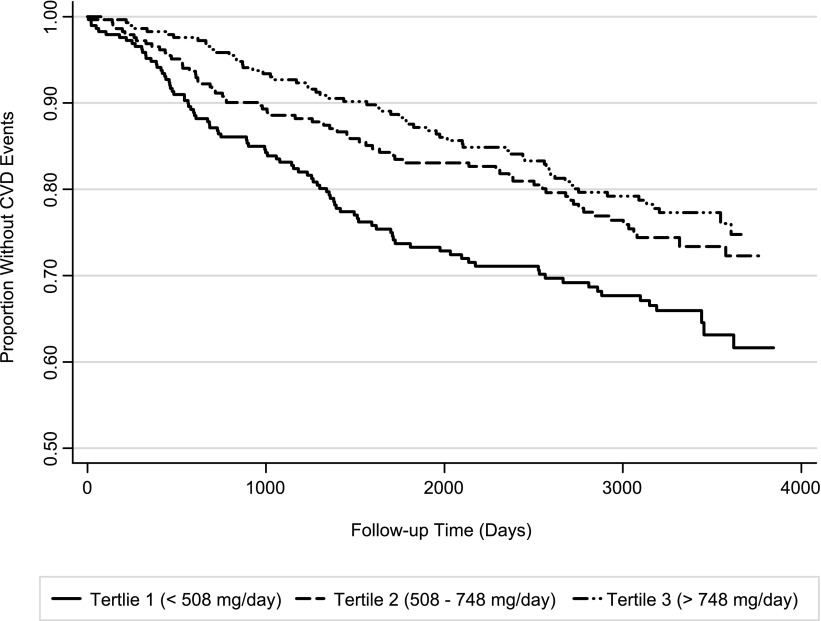
Next, we explored the association of 24-hour UPE with CVD events (Table 4) and observed that individuals with 24-hour UPE in the highest tertile were at lower risk for CVD events than those in lower UPE tertiles (Figure 2). The inverse association remained statistically significant throughout the sequence of models, such that in the fully adjusted model, participants in the highest UPE tertile were at 30% lower CVD risk compared with those in the lowest tertile, and each 300 mg/d higher UPE was associated with a 17% lower risk for CVD events. This relationship was again similar irrespective of CKD status (P interaction = 0.87 (Supplemental Table 1).
Table 4.
Cox proportional hazards models for 24-hour urine phosphorus excretion with cardiovascular disease events
| Variable | UPE Tertiles | Continuous | |||
|---|---|---|---|---|---|
| Tertile 1 (<508 mg/d) | Tertile 2 (508–748 mg/d) | Tertile 3 (>748 mg/d) | Per 300 mg/d Higher UPE (95% CI) | P Value | |
| Events/at risk (n/n) | 91/294 | 68/293 | 62/293 | 221/880 | |
| Unadjusted: Hazard ratio (95% CI) | 1.00 (reference) | 0.67 (0.49 to 0.92) | 0.57 (0.42 to 0.79) | 0.76 (0.66 to 0.88) | <0.001 |
| Model 1: Hazard ratio (95% CI) | 1.00 (reference) | 0.77 (0.56 to 1.06) | 0.73 (0.52 to 1.03) | 0.84 (0.71 to 0.98) | 0.02 |
| Model 2; Hazard ratio (95% CI) | 1.00 (reference) | 0.79 (0.57 to 1.09) | 0.70 (0.47 to 1.03) | 0.81 (0.68 to 0.97) | 0.02 |
Model 1; adjusted for age, sex, oral estrogen use (women), race, and estimated GFR. Model 2; adjusted for age, sex, oral estrogen use (women), race, estimated GFR, diabetes, hypertension, smoking, body mass index, cholesterol, HDL cholesterol, C-reactive protein, and urine calcium excretion. UPE, urinary phosphorus excretion; CI, confidence interval.
Figure 2.
Kaplan-Meier survival curve showing cardiovascular disease (CVD) events by urine phosphorus excretion tertiles. Log-rank P value=0.002.
Individuals who may have undercollected 24-hour urine would have lower 24-hour UPE, which could introduce systematic bias. To explore this possibility, we conducted a sensitivity analysis limited to individuals whose Cockcroft-Gault–estimated creatinine clearance (derived from serum creatinine and demographic characteristics and body weight only) and measured creatinine clearance (derived from 24-hour urine collection) were concordant. Specifically, we excluded the subset of individuals whose 24-hour creatinine clearance was 30% greater (n=77) or less (n=150) than their Cockcroft-Gault–estimated creatinine clearance. The remaining 653 individuals (74%) were included in the sensitivity analysis. Results were similar within this subgroup. In the fully adjusted model, each 300 mg/d greater UPE was associated with a 9% lower death risk and 18% lower CVD event risk (Supplemental Table 2).
Last, we explored the association of serum phosphorus with mortality and CVD events. For the association with mortality, the relationship appeared nonlinear. In a model adjusted for demographic characteristics, increased risk was observed among persons with serum phosphorus concentrations >4.5 mg/dl. These individuals were at approximately two-fold greater risk for death; however, power was limited, with only 28 deaths in this group (Table 5). With additional adjustment for eGFR, the nature of the relationship across phosphorus categories persisted but was attenuated and rendered no longer statistically significant. The nature of the relationship was also similar for CVD events, yet only 20 events occurred in the >4.5 mg/dl category, and the association was not statistically significant in a demographic characteristic–adjusted model or one in which eGFR was additionally included.
Table 5.
Association of serum phosphorus concentrations with all-cause mortality and cardiovascular events
| Variable | Phosphorus Categories | ||||
|---|---|---|---|---|---|
| <3.0 mg/dl | 3.0–3.4 mg/dl | 3.5–3.9 mg/dl | 4.0–4.4 mg/dl | ≥4.5 mg/dl | |
| All-cause mortality | |||||
| Events/at risk (n/n) | 31/81 | 97/244 | 117/299 | 67/184 | 28/72 |
| Model 1:a HR (95% CI) | 1.00 (reference) | 1.14 (0.76 to 1.72) | 1.35 (0.91 to 2.02) | 1.49 (0.97 to 2.29) | 2.08 (1.22 to 3.52) |
| Model 1 + eGFR: HR (95% CI) | 1.00 (reference) | 0.98 (0.65 to 1.47) | 1.22 (0.82 to 1.82) | 1.22 (0.79 to 1.89) | 1.43 (0.83 to 2.46) |
| Cardiovascular events | |||||
| Events/at risk (n/n) | 22/81 | 70/244 | 74/299 | 35/184 | 20/72 |
| Model 1:a HR (95% CI) | 1.00 (reference) | 1.12 (0.69 to 1.81) | 1.05 (0.65 to 1.70) | 0.92 (0.53 to 1.58) | 1.54 (0.82 to 2.89) |
| Model 1 + eGFR: HR (95% CI) | 1.00 (reference) | 0.97 (0.60 to 1.57) | 0.95 (0.59 to 1.53) | 0.74 (0.43 to 1.28) | 1.01 (0.53 to 1.93) |
HR, hazard ratio; CI, confidence interval; eGFR, estimated GFR.
Adjusted for age, sex, estrogen use (women), and race.
Discussion
The KDIGO international clinical practice guidelines recommend targeting serum phosphorus concentrations within the normal laboratory range in CKD stage 3–5. If serum phosphorus is higher, dietary phosphorus restriction with or without oral phosphorus binders is recommended (10). Yet, little evidence links dietary phosphorus intake with serum phosphorus concentrations in non-ESRD settings, and to our knowledge no data link greater dietary phosphorus intake with adverse clinical outcomes. Using 24-hour UPE as a marker of intestinal phosphorus absorption, we found a very modest trend between UPE and fasting morning serum phosphorus concentrations that did not reach statistical significance. Similarly, UPE was not associated with other mineral markers (FGF-23, PTH, and calcium) previously linked with CVD events. Last, greater 24-hour UPE was associated with lower, rather than higher, risk for CVD events. If confirmed, these findings may have important implications for clinical management of mineral metabolism in the general population and in persons with CKD.
Published in 2009, the KDIGO guidelines cited no references linking dietary phosphorus intake with serum phosphorus concentrations in CKD stage 3–5 (10). Since then, several intervention studies have found little or no effect of dietary phosphorus restriction on fasting morning serum phosphorus concentrations. Isakova and colleagues randomly assigned 16 patients with CKD stage 3–4 to a diet containing 1500 mg or 750 mg of dietary phosphorus for 2 weeks. Although the expected marked decline in 24-hour UPE occurred during the low-phosphorus diet, serum phosphorus concentrations did not change with either diet (15). Sigrist et al. conducted a randomized crossover study among 18 patients with CKD stage 3–4 and 10 healthy controls evaluating a high-phosphorus diet, a low-phosphorus diet, and a low-phosphorus diet plus oral phosphorus binders. Despite a >50% reduction in UPE in the group receiving a low-phosphorus diet plus binder intervention compared with the group receiving the high-phosphorus diet, no statistically significant differences in serum phosphorus concentrations was observed (17). Block et al. recently randomly assigned 148 patients with stage 3–4 CKD to high doses of oral phosphorus binders or to placebo for 9 months. The group randomly assigned to binders had marked reductions in 24-hour UPE, whereas 24-hour UPE did not change in the placebo group. Serum phosphorus concentrations were only 0.2 mg/dl lower, on average, in the binder group than in the placebo group at the end of the study (14). Similar findings have been reported by others (15,16).
Here, we extend these findings to a larger community-living population who were consuming their regular diets. Among 880 participants with kidney function ranging from normal to moderate CKD, we observed that 300 mg/d greater UPE (approximately 1 SD higher) was associated with only 0.03 mg/dl higher serum phosphorus concentration; this association approached but did not reach statistical significance despite the large sample size (P=0.07). Collectively, these data demonstrate that dietary phosphorus intake is unlikely to be a major determinant of fasting morning serum phosphorus concentrations in non-ESRD settings.
Our study, and the studies summarized above, all evaluated fasting morning serum phosphorus concentrations. Other studies have found that large dietary phosphorus loads can increase serum phosphorus concentrations shortly after a meal (27,28) and that phosphorus concentrations have diurnal variations, with the highest levels in the afternoon (20,29). In light of these findings, it is important to recognize that studies linking serum phosphorus concentrations with CVD events or death have evaluated fasting morning concentrations (7–9). Whether postprandial or afternoon phosphorus concentrations are associated with similar outcomes is unknown. Thus, if the intent of phosphorus-lowering interventions is to prevent CVD, then, on the basis of the available data, such therapies should decrease fasting morning serum phosphorus concentrations. Alternatively, if therapies influence only postprandial phosphorus concentrations, then additional studies are needed to establish whether postprandial phosphorus concentrations are associated with CVD events. To our knowledge, no such studies exist. Moreover, if 24-hour intestinal phosphorus absorption provides a more comprehensive marker of phosphorus exposure than a one-time measurement of serum phosphorus, then it too should be associated with CVD risk. To our knowledge, we are the first to evaluate the relationship of 24-hour UPE with CVD events.
Participants in our study with the highest 24-hour UPE were at lower, rather than higher, risk for CVD events. The point estimate was in the same direction for all-cause mortality, although the association did not reach statistical significance. Results of prior studies evaluating subclinical markers of CVD support these findings. In the aforementioned randomized trial of oral phosphorus binders in CKD stage 3–4, the active treatment group had substantial reductions in 24-hour UPE but greater progression of coronary artery calcification compared with the placebo group during the 9-month intervention (14). Our findings suggest that dietary phosphorus intake is not associated with a higher risk for death or CVD events. Moreover, it is possible that recommendations to decrease dietary phosphorus intake may cause harm. The primary source of dietary phosphorus is protein intake. In controlled research settings, it is possible to decrease dietary phosphorus intake while maintaining protein intake (17); however, this is difficult to achieve even in research settings, and recommendations to limit phosphorus intake in clinical practice will almost certainly lead to lower protein intake as well. The Modification of Diet in Renal Disease study was a randomized clinical trial evaluating dietary protein restriction in patients with CKD stage 3–4 (30). The low-protein intervention was associated with higher all-cause mortality in long-term follow-up, which was hypothesized to reflect consequences of skeletal muscle loss and malnutrition (31). Our data show similar findings evaluating phosphorus rather than protein because low 24-hour UPE was associated with higher CVD event rates, independent of kidney function and traditional CVD risk factors.
It is important to note that higher serum phosphorus concentrations were not independently associated with mortality or CVD events in our cohort. It is likely that the absence of statistically significant associations reported here reflects weak statistical power. We had few individuals with phosphorus levels >4.5mg/dl where the risk was increased, and few numbers of events occurred among them. Although not statistically significant, the point estimates for the hazard ratios reported here are similar to that reported in other community-living cohorts, and prior studies reporting statistically significant results between serum phosphorus and either mortality or CVD events had larger sample sizes and much longer follow-up time than those available in our study (7,8). Alternatively, it is possible that differences in study design may have led to the lack of statistically significant associations here. One important characteristic of our study sample was the requirement for prevalent CVD for inclusion. However, prior studies in populations with prevalent CVD have reported significant associations of serum phosphorus with all-cause mortality and CVD (9). Nonetheless, the lack of association of serum phosphorus with CVD events observed here raises the possibility that the relationship of 24-hour UPE with events may also differ in other settings. Thus, future studies with 24-hour UPE measurements in other settings are required to confirm our results before they are used to change clinical practice or policy.
Strengths of this study include the availability of 24-hour urine collections in a large study sample of outpatients consuming their regular diets, concurrent availability of multiple other markers of mineral metabolism, and adjudication of CVD events. The study also has important limitations. We lack data on total caloric intake, dietary sources of phosphorus, protein, or other nutrients, and urine measures of urea and sodium. We could not fully adjust for factors associated with malnutrition or inflammation. It is possible that plant versus animal sources of phosphorus, or naturally occurring phosphorus versus phosphorus additives may have different bioavailability, which may affect serum phosphorus and other markers of mineral metabolism differently (28,32). The relationships of 24-hour UPE with postprandial phosphorus and at different time points throughout the day remain unknown. The majority of study participants were men, all had stable CVD, and few had advanced CKD. Whether our results extend to other populations is unknown. Only a single 24-hour urine sample was collected.
In conclusion, in community-living individuals with a spectrum of kidney function ranging from normal to moderate CKD, 24-hour UPE was not significantly associated with fasting morning serum phosphorus concentrations or other markers of mineral metabolism. Individuals with greater 24-hour UPE had a lower risk for CVD events. In conjunction with prior studies, these findings demonstrate that dietary phosphorus intake is unlikely to be a major determinant of fasting serum phosphorus concentrations. Because higher fasting morning serum phosphorus concentrations have been associated with CVD events and all-cause mortality in other studies (7–9), future studies are needed to identify factors other than diet that might influence serum phosphorus in normal kidney function to moderate CKD. If our results are confirmed, dietary phosphorus restriction may not be an effective strategy to decrease fasting serum phosphorus concentrations and may therefore not meaningfully reduce CVD events.
Disclosures
None.
Supplementary Material
Acknowledgments
This study was supported by grants from the National Heart, Lung, and Blood Institute (1R01HL096851) (J.H.I.), an American Heart Association Fellow-to-Faculty Transition Award (J.H.I.), and an award from the Sandra Daugherty Foundation (J.H.I.). Heather Palomino was supported by a training grant from the National Institutes of Health (TL1TR00098). The Heart and Soul Study was funded by the Department of Veterans Affairs, American Federation of Aging Research, Robert Wood Johnson Foundation, Nancy Kirwan Heart Research Fund, Ischemia Research and Education Foundation, and the National Heart, Lung, and Blood Institute (R01 HL079235).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11181012/-/DCSupplemental.
See related editorial, “Preeclampsia and Subsequent Cardiovascular Disease: Villain or Innocent Bystander?,” on pages 1061–1063.
References
- 1.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Criqui MH, Kamineni A, Allison MA, Ix JH, Carr JJ, Cushman M, Detrano R, Post W, Wong ND: Risk factor differences for aortic versus coronary calcified atherosclerosis: The multiethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol 30: 2289–2296, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA: Serum phosphorus levels associate with coronary atherosclerosis in young adults. J Am Soc Nephrol 20: 397–404, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ix JH, De Boer IH, Peralta CA, Adeney KL, Duprez DA, Jenny NS, Siscovick DS, Kestenbaum BR: Serum phosphorus concentrations and arterial stiffness among individuals with normal kidney function to moderate kidney disease in MESA. Clin J Am Soc Nephrol 4: 609–615, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kendrick J, Ix JH, Targher G, Smits G, Chonchol M: Relation of serum phosphorus levels to ankle brachial pressure index (from the Third National Health and Nutrition Examination Survey). Am J Cardiol 106: 564–568, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meng J, Wassel CL, Kestenbaum BR, Collins TC, Criqui MH, Lewis CE, Cummings SR, Ix JH, Osteoporotic Fractures in Men (MrOS) Study Group : Serum phosphorus levels and the spectrum of ankle-brachial index in older men: The Osteoporotic Fractures in Men (MrOS) study. Am J Epidemiol 171: 909–916, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhingra R, Sullivan LM, Fox CS, Wang TJ, D’Agostino RB, Sr, Gaziano JM, Vasan RS: Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 167: 879–885, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Foley RN, Collins AJ, Ishani A, Kalra PA: Calcium-phosphate levels and cardiovascular disease in community-dwelling adults: The Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J 156: 556–563, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G, Cholesterol And Recurrent Events Trial Investigators : Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 112: 2627–2633, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group : KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl S1–S130, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Tentori F, Blayney MJ, Albert JM, Gillespie BW, Kerr PG, Bommer J, Young EW, Akizawa T, Akiba T, Pisoni RL, Robinson BM, Port FK: Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 52: 519–530, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Kopple JD, Coburn JW: Metabolic studies of low protein diets in uremia. II. Calcium, phosphorus and magnesium. Medicine (Baltimore) 52: 597–607, 1973 [DOI] [PubMed] [Google Scholar]
- 13.Ramirez JA, Emmett M, White MG, Fathi N, Santa Ana CA, Morawski SG, Fordtran JS: The absorption of dietary phosphorus and calcium in hemodialysis patients. Kidney Int 30: 753–759, 1986 [DOI] [PubMed] [Google Scholar]
- 14.Block GA, Wheeler DC, Persky MS, Kestenbaum B, Ketteler M, Spiegel DM, Allison MA, Asplin J, Smits G, Hoofnagle AN, Kooienga L, Thadhani R, Mannstadt M, Wolf M, Chertow GM: Effects of phosphate binders in moderate CKD. J Am Soc Nephrol 23: 1407–1415, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isakova T, Gutiérrez OM, Smith K, Epstein M, Keating LK, Jüppner H, Wolf M: Pilot study of dietary phosphorus restriction and phosphorus binders to target fibroblast growth factor 23 in patients with chronic kidney disease. Nephrol Dial Transplant 26: 584–591, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliveira RB, Cancela AL, Graciolli FG, Dos Reis LM, Draibe SA, Cuppari L, Carvalho AB, Jorgetti V, Canziani ME, Moysés RM: Early control of PTH and FGF23 in normophosphatemic CKD patients: A new target in CKD-MBD therapy? Clin J Am Soc Nephrol 5: 286–291, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sigrist M, Tang M, Beaulieu M, Espino-Hernandez G, Er L, Djurdjev O, Levin A: Responsiveness of FGF-23 and mineral metabolism to altered dietary phosphate intake in chronic kidney disease (CKD): Results of a randomized trial. Nephrol Dial Transplant 28: 161–169, 2013 [DOI] [PubMed] [Google Scholar]
- 18.de Boer IH, Rue TC, Kestenbaum B: Serum phosphorus concentrations in the third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis 53: 399–407, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gutiérrez OM, Katz R, Peralta CA, de Boer IH, Siscovick D, Wolf M, Diez Roux A, Kestenbaum B, Nettleton JA, Ix JH: Associations of socioeconomic status and processed food intake with serum phosphorus concentration in community-living adults: The Multi-Ethnic Study of Atherosclerosis (MESA). J Ren Nutr 22: 480–489, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isakova T, Xie H, Barchi-Chung A, Smith K, Sowden N, Epstein M, Collerone G, Keating L, Jüppner H, Wolf M: Daily variability in mineral metabolites in CKD and effects of dietary calcium and calcitriol. Clin J Am Soc Nephrol 7: 820–828, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whooley MA, de Jonge P, Vittinghoff E, Otte C, Moos R, Carney RM, Ali S, Dowray S, Na B, Feldman MD, Schiller NB, Browner WS: Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA 300: 2379–2388, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ix JH, de Boer IH, Wassel CL, Criqui MH, Shlipak MG, Whooley MA: Urinary creatinine excretion rate and mortality in persons with coronary artery disease: the Heart and Soul Study. Circulation 121: 1295–1303, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, Goldberg RJ, Hand MM, Jaffe AS, Julian DG, Levy D, Manolio T, Mendis S, Mensah G, Pajak A, Prineas RJ, Reddy KS, Roger VL, Rosamond WD, Shahar E, Sharrett AR, Sorlie P, Tunstall-Pedoe H, AHA Council on Epidemiology and Prevention. AHA Statistics Committee. World Heart Federation Council on Epidemiology and Prevention. European Society of Cardiology Working Group on Epidemiology and Prevention. Centers for Disease Control and Prevention. National Heart, Lung, and Blood Institute : Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation 108: 2543–2549, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS: Estimating GFR using serum cystatin C alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 51: 395–406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whooley MA, Caska CM, Hendrickson BE, Rourke MA, Ho J, Ali S: Depression and inflammation in patients with coronary heart disease: Findings from the Heart and Soul Study. Biol Psychiatry 62: 314–320, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cockcroft DW, Gault MH: Prediction of creatinine clearance from serum creatinine. Nephron 16: 31–41, 1976 [DOI] [PubMed] [Google Scholar]
- 27.Shuto E, Taketani Y, Tanaka R, Harada N, Isshiki M, Sato M, Nashiki K, Amo K, Yamamoto H, Higashi Y, Nakaya Y, Takeda E: Dietary phosphorus acutely impairs endothelial function. J Am Soc Nephrol 20: 1504–1512, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bell RR, Draper HH, Tzeng DYM, Shin HK, Schmidt GR: Physiological responses of human adults to foods containing phosphate additives. J Nutr 107: 42–50, 1977 [DOI] [PubMed] [Google Scholar]
- 29.Portale AA, Halloran BP, Morris RC, Jr: Dietary intake of phosphorus modulates the circadian rhythm in serum concentration of phosphorus. Implications for the renal production of 1,25-dihydroxyvitamin D. J Clin Invest 80: 1147–1154, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, Striker G, Modification of Diet in Renal Disease Study Group : The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med 330: 877–884, 1994 [DOI] [PubMed] [Google Scholar]
- 31.Menon V, Kopple JD, Wang X, Beck GJ, Collins AJ, Kusek JW, Greene T, Levey AS, Sarnak MJ: Effect of a very low-protein diet on outcomes: Long-term follow-up of the Modification of Diet in Renal Disease (MDRD) Study. Am J Kidney Dis 53: 208–217, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Moe SM, Zidehsarai MP, Chambers MA, Jackman LA, Radcliffe JS, Trevino LL, Donahue SE, Asplin JR: Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol 6: 257–264, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.