Abstract
Study Objectives:
To examine the prevalence and clinical characteristics of REM sleep behavior disorder (RBD) and subclinical RBD in the Korean elderly population.
Design:
A community-based Korean Longitudinal Study on Cognitive Aging and Dementia and time-synchronized video-polysomnography (vPSG) in a laboratory.
Setting:
Sleep laboratory in a university hospital.
Participants:
348 individuals aged 60 years or older.
Intervention:
N/A.
Measurements and Results:
Among 696 subjects who were invited to participate in a vPSG study, 348 completed the vPSG. RBD was diagnosed when subjects showed REM sleep without atonia (RSWA) in the vPSG, and had history of complex and vigorous behaviors during sleep or abnormal REM sleep behaviors in the vPSG. Subjects with RSWA but no abnormal REM sleep behaviors were diagnosed with subclinical RBD. Seven subjects (5 male, 2 female) had RBD, three of whom (1 male, 2 female) had Parkinson disease. Two subjects reported history of sleep-related injury. The crude prevalence of RBD and idiopathic RBD was 2.01% (95% confidence interval [CI] = 0.54% to 3.49%) and 1.15% (95% CI = 0.03% to 2.27%). An age and sex-adjusted prevalence estimate of RBD and idiopathic RBD in the Korean elderly was 2.01% and 1.34%. Eighteen subjects were diagnosed with subclinical RBD, and the prevalence of subclinical RBD was estimated to be 4.95%.
Conclusions:
RBD and subclinical RBD are not rare in the elderly in the community with abnormal REM sleep behaviors of RBD being mild to injurious and violent. The clinical significance and long-term progression of subclinical RBD needs to be further explored, given the prevalence and its possible relation to RBD.
Citation:
Kang SH; Yoon IY; Lee SD; Han JW; Kim TH; Kim KW. REM sleep behavior disorder in the Korean elderly population: prevalence and clinical characteristics. SLEEP 2013;36(8):1147-1152.
Keywords: REM sleep behavior disorder, subclinical REM sleep behavior disorder, prevalence
INTRODUCTION
REM sleep behavior disorder (RBD) is a parasomnia characterized by loss of normal atonia during REM sleep and dream enacting behavior (DEB), including vocalizations, striking behaviors, or violent movements.1,2 At the time of initial diagnosis of RBD, 18.6∼18.7% of RBD patients had comorbid neurodegenerative diseases, and many such patients developed emergent neurodegenerative diseases as time passed.3–6 In a recent population-based study, probable RBD patients were found to be at 2.2-fold increased risk of developing mild cognitive impairment or Parkinson disease over 4 years.7 Therefore, investigating the prevalence of RBD is of importance for prevention of sleep-related injury and to explore the association with neurodegenerative diseases at early stage.
The prevalence of RBD has not been well researched, and only two studies were population-based. In a United Kingdom sample of 2,078 men and 2,894 women between the ages of 15 to 100 years, Ohayon found that 0.5% of the sample reported violent behaviors during sleep in the telephone interview.8 In another study with clinical evaluation and nocturnal polysomnography (NPSG), Chiu examined the prevalence of RBD and sleep-related injury in a sample of Hong Kong community-living elderly. A history of sleep-related injury was reported in 0.8% of the study population, and the prevalence of RBD was estimated at 0.38%.9 However, both studies had some limitations. In the study by Ohayon, subjects did not undergo PSG, which is essential in the diagnosis of RBD, and violent behaviors may occur in NREM as well as REM sleep.8,10,11 In the Hong Kong study, RBD prevalence might be underestimated since subjects with sleep-related injury were selected in the first stage; thus, the RBD group without violent behaviors during sleep might not be included in RBD prevalence.
Considering the association between RBD and neurodegenerative diseases, the concept of subclinical RBD was introduced for early detection of RBD. Schenck and Mahowald suggested that the criteria of subclinical RBD should include PSG abnormalities alone or with nonclinical behaviors in REM sleep such as limb twitching and jerking, and simple behavior.12 Subclinical RBD might remain asymptomatic, but it could develop into clinical RBD with progression to neurodegenerative disorders or there might be direct progression from subclinical RBD to neurodegenerative disorders.12 Therefore, our aim of this study was to investigate the prevalence of RBD and subclinical RBD using time-synchronized video-polysomnography (vPSG), and to evaluate the clinical characteristics of patients with RBD and subclinical RBD.
METHODS
Study Subjects
This study was a part of the Korean Longitudinal Study on Cognitive Aging and Dementia (KLOSCAD), a population-based prospective cohort study of cognitive function with aging and dementia in the elderly Korean population aged 60 years or older. We conducted this study from January 2010 to February 2011 on older adults living in Jukjeon-dong, Youngin-si, Korea. We randomly drew 10% (N = 696) of the 6,959 residents of Jukjeon-dong aged 60 years or older through systemic random sampling based on the residential roster. Then we invited the sampled subjects to visit Seoul National University Bundang Hospital, where subjects were given a clinical examination, and detailed medical and neurological history was taken. Parkinson disease (PD) was evaluated with the use of the clinical diagnostic criteria for PD.13 In diagnosing both RBD and subclinical RBD, subjects taking antidepressants or other psychiatric medications were excluded. Psychiatric assessments were performed as follows: The Pittsburgh sleep quality index (PSQI) was used to evaluate subjective sleep complaints, the Epworth Sleepiness Scale (ESS) to assess daytime sleepiness, geriatric depression scale (GDS) to evaluate mood symptoms, and minimental state examination (MMSE) to find patients with cognitive impairments. They were then recommended to undergo vPSG for one night in the sleep laboratory of the hospital. This study was approved by our institutional review board, and all subjects provided written informed consent themselves or via their legal guardians.
Video-polysomnography (vPSG)
We used an Embla N7000 (Embla, Reykjavik, Iceland) for PSG, and video was also recorded simultaneously with the PSG. A sleep technologist was in attendance for vPSG study. Electroencephalography electrodes were applied at C3/A2, O1/ A2, and O2/A1, and 2 electrooculography electrodes were applied at the sides of both eyes to record horizontal and vertical eye movements. Submental electromyography (EMG) electrodes were applied at the submentalis muscle and EMG at both anterior tibialis muscles recorded limb movements during sleep. Strain gauges recorded chest and abdominal respiratory movements, and nasal pressure cannulas were used to record airflow. Arterial oxygen saturation was measured using a pulse oximeter applied to an index finger. Using Rechtschaffen and Kales' criteria,14 we scored every 30-sec epoch of NPSG.
Diagnosis of RBD and Subclinical RBD
Diagnosis of RBD
RBD was diagnosed based on the criteria of International Classification of Sleep Disorders, 2nd ed. (ICSD-2)10 and American Academy of Sleep Medicine (AASM) Manual for the Scoring of Sleep and Associated Events.15 ICSD-2 defines RBD as follows: (1) presence of REM sleep without atonia (RSWA) on polysomnography—the EMG finding of excessive amounts of sustained or intermittent elevation of submental EMG tone or excessive phasic submental or limb EMG twitching; (2) sleep-related, injurious, potentially injurious or disruptive behaviors found by history and/or the presence of abnormal REM sleep behavior documented during polysomnographic monitoring; and (3) absence of EEG epileptiform activity during REM sleep. However, as the description of RSWA in the ICSD-2 was considered to be arbitrary and researcher-dependent, we used the AASM manual to define RSWA more strictly. The criteria of RSWA in the AASM manual are: (1) Sustained muscle activity in REM sleep in the chin EMG—an epoch of REM sleep with ≥ 50% of the duration of the epoch having a chin EMG amplitude greater than the minimum amplitude than in NREM sleep; or (2) Excessive transient muscle activity during REM sleep in the chin or limb EMG—in a 30-sec epoch of REM sleep divided into 10 sequential 3-sec mini-epochs, at least 5 (50%) of the mini-epochs contain bursts of transient muscle activity of 0.1∼ 5.0 seconds in duration and ≥ 4 times as high in amplitude as the background EMG activity. A minimum amount of RSWA in total REM sleep time was not defined in the AASM criteria. When subjects showed RSWA on the vPSG, we contacted the subjects and their bed partners by telephone and asked whether and how often the subjects had DEBs including talking, shouting, arm flailing, punching, or kicking. RBD was finally diagnosed when DEBs were reported by the subjects or their bed partners.
Diagnosis of Subclinical RBD
Subclinical RBD was diagnosed when subjects showed RSWA without a history of prominent abnormal behaviors during sleep or during REM sleep in the vPSG. RSWA in subclinical RBD was defined in the same way with that in RBD.
Statistical Analysis
Standardized prevalence rates for older Koreans were estimated using a direct standardization method, in which the prevalence rates were adjusted by age and sex to the total Korean population based on the 2011 national census. To examine differences in clinical characteristics between subclinical RBD subjects and non-subclinical RBD (the rest of subjects other than RBD and subclinical RBD), we used independent Student t-test or Pearson χ2 test for dichotomous variables. Analysis of covariance (ANCOVA) adjusted by gender was used for apneahypopnea index (AHI), ESS, and sleep stages. The significance criterion was defined to be P < 0.05 for 2-tailed tests. SPSS version 18.0 for Windows (SPSS Inc., Chicago, IL, USA) was used for statistical analysis.
RESULTS
The Study Process
Of 696 total sample subjects, 354 subjects participated in the vPSG study. We included 348 subjects with total sleep time ≥ 3 h and ≥ 2 episodes of REM sleep, excluding 6 subjects who did not meet the criteria of sleep time or REM sleep episodes. Thus, 348 subjects (135 men and 213 women) were analyzed; the final response rate was 50.0%. Mean age of the 348 participants was 68.4 ± 6.2 years (range 60-88), and mean body mass index was 23.7 ± 2.7 kg/m2 (16.5-32.3). There was no difference in gender ratio between the responders and elderly population living in the sampled area (χ2 = 2.857, P = 0.096). In the comparison between 348 responders and 348 non-responders, no difference was observed in gender ratio (P = 0.943), but nonresponders were older than responders (71.6 ± 8.7 vs. 68.4 ± 6.2 years, P < 0.001).
In vPSG recordings, 25 subjects showed definite RSWA. Clinically significant DEBs were reported in 7 subjects, with 2 of them showing prominent DEBs during vPSG; we diagnosed 7 subjects with RBD. The other 18 subjects had negligible or insignificant abnormal sleep behaviors and were diagnosed with subclinical RBD (14 men, 4 women). Among 7 RBD subjects (5 male, 2 female), 3 subjects (1 male, 2 female) were found to have PD. All the study processes are presented in Figure 1.
Figure 1.
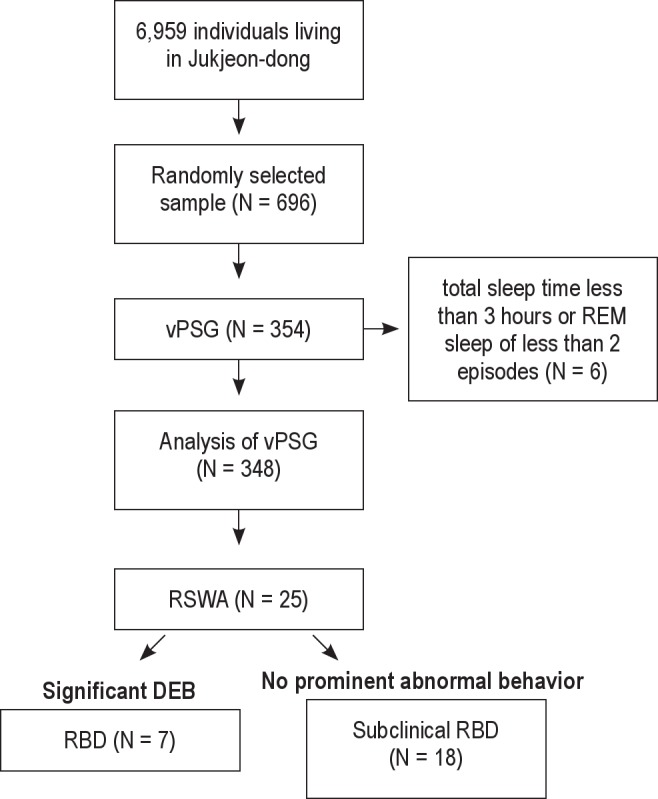
Flow chart summary of the study process. vPSG, video polysomnography; RSWA, REM sleep without atonia; DEB, dream enacting behavior; RBD, REM sleep behavior disorder
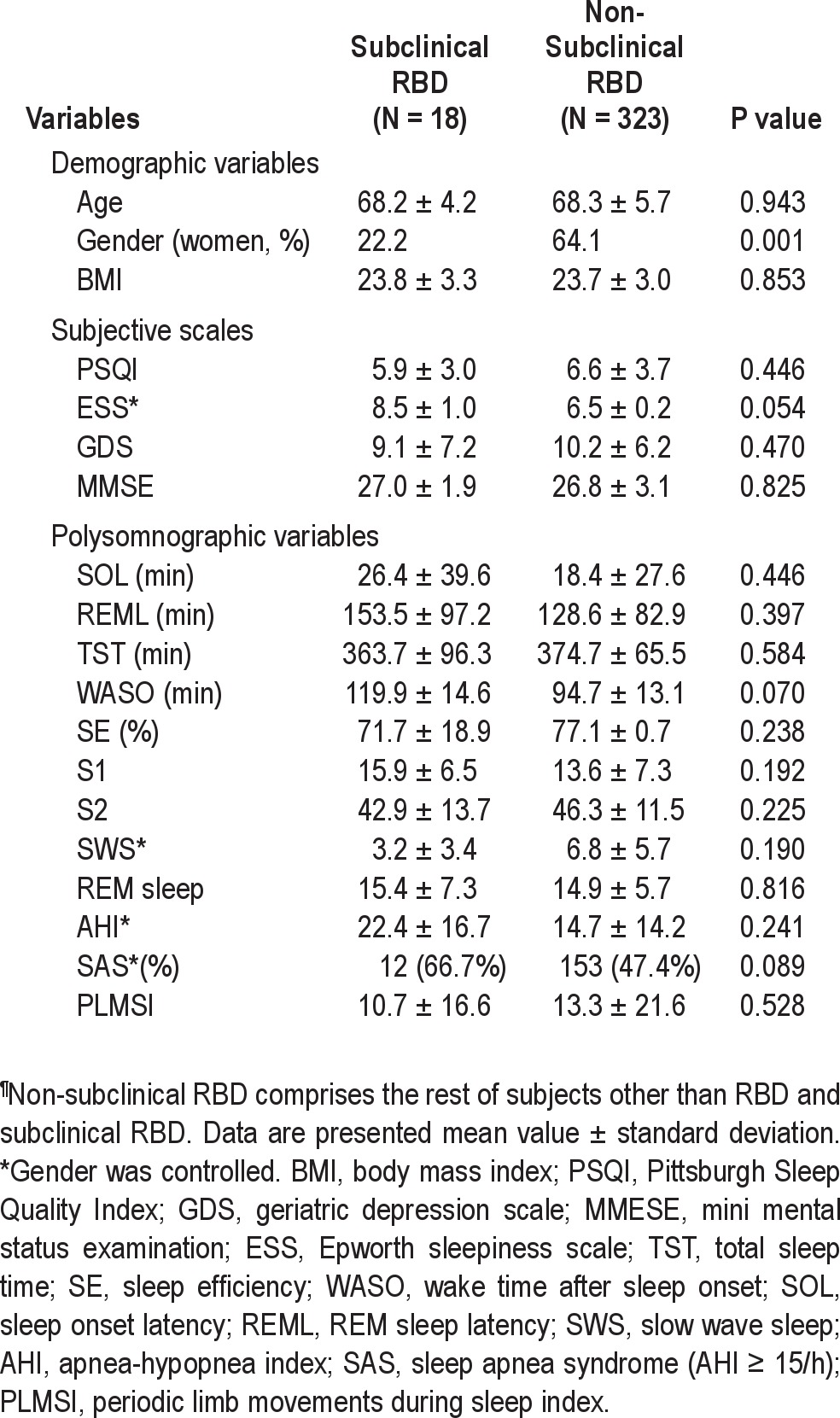
Prevalence Rates and Characteristics of RBD and Subclinical RBD The crude prevalence of RBD was 2.01% (95% confidence interval [CI] = 0.5-3.5) and crude prevalence of idiopathic RBD was 1.15% (95% CI = 0.0-2.3). An age- and genderstandardized prevalence estimate of RBD in the elderly Korean population was 2.01% (95% CI = 0.5-3.5), and prevalence of idiopathic RBD was estimated to be 1.34% (95% CI = 0.1-2.6). The crude prevalence of subclinical RBD was 5.17% (95% CI = 2.9-7.5), and prevalence of subclinical RBD was estimated to be 4.95% (95% CI = 2.7-7.2) by standardization with age and sex (Table 1). Mean duration of symptoms in all subjects with confirmed RBD was about 7.2 years, and mean DEB frequency was 2.9 ± 1.1/month (range 2-5). Two of 7 RBD subjects reported sleep-related injuries (Table 2). The comparison of clinical characteristics and PSG findings between subclinical RBD and non-subclinical RBD are presented in Table 3. No difference was found between subjects with subclinical RBD and non-subclinical RBD.
Table 1.
The prevalence of RBD, idiopathic RBD, and subclinical RBD
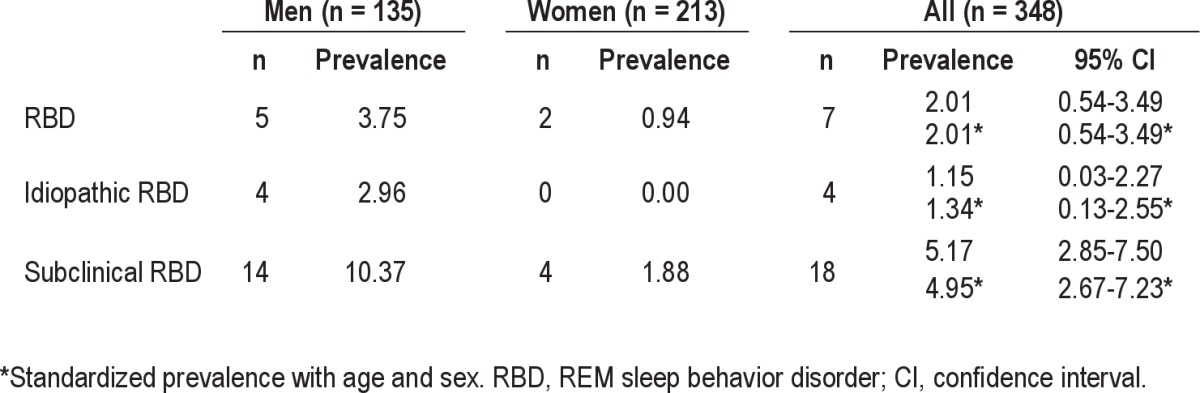
Table 2.
Clinical characteristics of patients with confirmed RBD
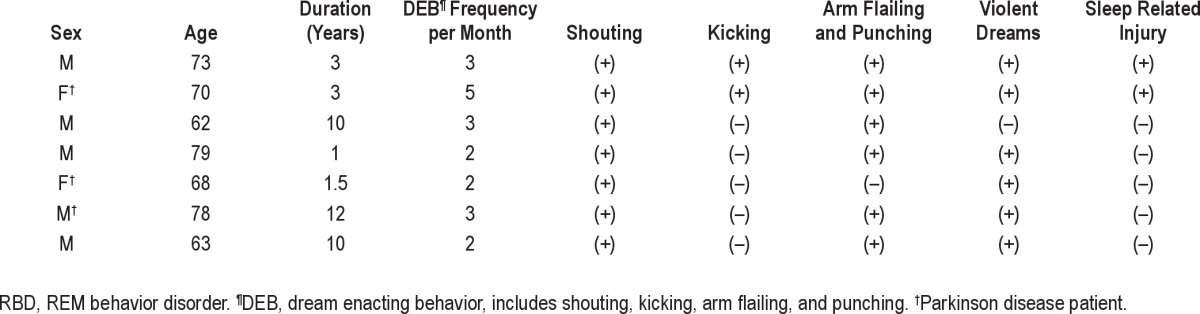
Table 3.
Comparison of demographic and clinical characteristics between subclinical RBD and non-subclinical RBD¶
DISCUSSION
To our knowledge, this is the first prevalence study of RBD and subclinical RBD in Korean elderly population using a single- stage design and vPSG. In our Korean sample of elderly population, prevalence of RBD was 2.01%, which was considerably higher than that found in of two previous studies on the general population; 0.38% in the Hong Kong study by Chiu and 0.5% in the United Kingdom study by Ohayon. This difference can partly be explained in terms of RBD severity. In both previous studies, the reported prevalence of RBD was related with relatively severe cases, as the United Kingdom study investigated violent behavior during sleep and the Hong Kong study examined sleep-related injury. Among the seven RBD subjects diagnosed in this study, two were found to have sleep-related injuries, but the other five did not report violent or injurious behaviors during sleep. Thus, if we limited RBD cases to severe ones, prevalence of RBD in this study can be estimated to be 0.57%, which is comparable to those of previous studies. However, as sleep behaviors shown in mild RBD subjects could be potentially injurious, we defined them as having RBD. Chiu of the Hong Kong study also acknowledged that the actual prevalence rate of RBD in their community might be higher than reported in their article, as patients with mild RBD who did not report sleep-related injury were excluded in the study.9 In one recent study on polysomnographically confirmed RBD patients at an academic sleep center, only 40% of them were initially referred for violent behaviors or dream enactment during sleep.16 Mild RBD cases were included in the current study, but RBD prevalence may be still underestimated in our report because we used DEBs as an entry criterion for the RBD diagnosis. One important review on RBD found that up to 35% of vPSG-documented RBD patients in the literature did not have DEBs.17 Other findings in this study of three of seven RBD subjects having PD (43%) and male predominance (male: female = 5:2) are compatible with previous reports.1,5 It was suggested that the male predominance of RBD may reflect underlying neurodegeneration, and women have later onset but comparable severity and clinical presentation to men.18,19
Some studies have suggested various characteristics of sub-clinical RBD, but there are no formal diagnostic criteria for subclinical RBD. In the early 1990s, subclinical RBD was defined when clinically unnoticed subtle movements during RSWA were present before complete diagnosis of RBD was made.20,21 In one case report, sleep bruxism and increased REM density were regarded as symptoms of subclinical RBD.22 We defined subclinical RBD based on the Schenck's criteria12; the prevalence of subclinical RBD was 4.95%, which was relatively high. In subjects with subclinical RBD, male predominance, as in RBD, was observed, with 14 of 18 subjects being male (78.8%). None of the subjects took antidepressants or other psychiatric medication known to be related to RBD.23 Subjects with subclinical RBD showed increased apneahypopnea index (AHI), prevalence of sleep apnea syndrome (SAS), and daytime sleepiness in this study, but these differences disappeared with correction for gender. That is, subclinical RBD was more common in males who showed increase of AHI, SAS prevalence, and daytime sleepiness associated with SAS compared to females; and controlling for the effect of male gender resulted in no difference in AHI, SAS prevalence, and daytime sleepiness between subjects with and without subclinical RBD. Although there was no report about the longitudinal course of subclinical RBD, Schenck suggested that subclinical RBD might not be changed, or could develop clinical RBD or clinical RBD with progression to PD/other Parkinsonism, or PD/other Parkinsonism without any clinical RBD.12 In a single-photon emission computed tomography (SPECT) study of striatal dopamine transporters (DAT), subclinical RBD was intermediate between clinically manifest RBD and normal controls in striatal DAT reduction.24 The continuum of reduced striatal DAT suggested that subclinical RBD, clinical RBD, and PD could be associated with same mechanism, and subclinical RBD might develop clinically manifested RBD and neurological diseases, particularly PD in which RBD is strongly present.25 However, the possibility remains that subclinical RBD, clinical RBD, and PD have different pathogenesis and clinical courses. There is a report that RBD has a different mechanism from PD in terms of partial involvement of nigrostiatal dopaminergic system in RBD. 26 In this study, since subjects with subclinical RBD were as old as RBD subjects, and the prevalence of RBD was already high, the possibility seems low that these older subjects with subclinical RBD could develop new RBD. Subclinical RBD might remain as it is with minimal clinical significance. Nonetheless, subjects with subclinical RBD need to be longitudinally evaluated for neurologic symptoms and closely followed up for development of clinically manifested RBD.
One major strength of this study is that we adopted a single-phase design, which avoided the use of RBD screening questionnaires that have limited sensitivity and specificity for RBD. In addition, all participants were assessed by expert physicians using standardized and structured instruments for the diagnosis of dementia and major psychiatric disorders. These two methodological strengths enhanced the accuracy of RBD diagnosis in the present study. There are two validated RBD screening questionnaires that can be used in an RBD epidemiology study with a two-stage method, but each has its own limitations. Although the RBD screening questionnaire (RBDSQ) has high sensitivity useful for screening, the benefit of using this questionnaire might be lessened because of low specificity when it is applied to population with sleep problems including elderly people.27 Also, dream-enacting behaviors are reported to be prevalent even in healthy subjects.28 RBD questionnaire-Hong Kong (RBDQ-HK), despite its robust psychometric properties, has just moderate sensitivity of 82.2% as a suitable screening tool.29 As findings from PSG are mandatory in diagnosing RBD in the International Classification of Sleep Disorders, 2nd ed. (ICSD-2),10 we invited all the subjects to undergo vPSG.
This study has several limitations to consider. First of all, this study may lack sufficient statistical power to estimate accurate population prevalence because of the limited sample size. The minimum sample size for a prevalence survey on a low prevalence condition such as RBD needs to be 1,000 or more to get a sampling error of 1% or less.30 Second, the response rate of 50.0% was not high, and non- responders were older than responders. Inviting subjects to a sleep clinic for overnight PSG might decrease participation rate in the elderly, especially in those over 80 years old. However, considering difficulties found in the elderly such as gait disturbances, reluctance to sleep in an unfamiliar environment, and avoidance of sleeping with PSG equipment attached, obtaining response rate of 50% was not easy work. Third, although the high prevalence of RBD was observed in the study, missed cases are conceivable since EMG was measured only on the legs. In addition, one-night vPSG might result in missing cases because of the night-to-night variability of RBD features in the vPSG study, 31,32 but combining PSG EMG and video features could detect nearly 95% of RBD cases with one-night vPSG. 31 Fourth, since all subjects in this study were recruited from a relatively small area, a larger sample from an area with a large population will be needed in the further study. Nevertheless, this is the first study to explore the prevalence of RBD in the elderly in a single-stage design with the use of vPSG. The high RBD prevalence of 2.01% in this study, compared to those of previous studies of 0.5% in general population and 0.38% in the elderly, might result from the single-stage design and inclusion of mild cases. Considering relatively high prevalence of subclinical RBD of 4.95%, further studies are needed to establish clinical significance and any long-term progression of subclinical RBD.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This study was supported by the Basic Science Research Program through the National Research Foundation of Ko rea (NRF) funded by the Ministry of Education, Science and Technology (MEST) (Grant No. 2010-0008886), NRF grant funded by the MEST (Grant No. 2011-0018262), and a grant from the Korean Health Technology R&D Project, Ministry for Health, Welfare, & Family Affairs, Republic of Korea (Grant No. A092077).
Footnotes
A commentary on this article appears in this issue on page 1117.
REFERENCES
- 1.Schenck CH, Hurwitz TD, Mahowald MW. Symposium: Normal and abnormal REM sleep regulation: REM sleep behaviour disorder: an update on a series of 96 patients and a review of the world literature. J Sleep Res. 1993;2:224–31. doi: 10.1111/j.1365-2869.1993.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 2.Schenck CH, Mahowald MW. REM sleep behavior disorder: clinical, developmental, and neuroscience perspectives 16 years after its formal identification in SLEEP. Sleep. 2002;25:120–38. doi: 10.1093/sleep/25.2.120. [DOI] [PubMed] [Google Scholar]
- 3.Bonakis A, Howard RS, Ebrahim IO, Merritt S, Williams A. REM sleep behaviour disorder (RBD) and its associations in young patients. Sleep Med. 2009;10:641–5. doi: 10.1016/j.sleep.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Lin FC, Lai CL, Huang P, Liu CK, Hsu CY. The rapid-eye-movement sleep behavior disorder in Chinese-Taiwanese patients. Psychiatry Clin Neurosci. 2009;63:557–62. doi: 10.1111/j.1440-1819.2009.01998.x. [DOI] [PubMed] [Google Scholar]
- 5.Olson EJ, Boeve BF, Silber MH. Rapid eye movement sleep behaviour disorder: demographic, clinical and laboratory findings in 93 cases. Brain. 2000;123:331–9. doi: 10.1093/brain/123.2.331. [DOI] [PubMed] [Google Scholar]
- 6.Postuma RB, Gagnon JF, Vendette M, Fantini ML, Massicotte-Marquez J, Montplaisir J. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology. 2009;72:1296–300. doi: 10.1212/01.wnl.0000340980.19702.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boot BP, Boeve BF, Roberts RO, et al. Probable rapid eye movement sleep behavior disorder increases risk for mild cognitive impairment and Parkinson disease: a population-based study. Ann Neurol. 2012;71:49–56. doi: 10.1002/ana.22655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohayon MM, Caulet M, Priest RG. Violent behavior during sleep. J Clin Psychiatry. 1997;58:369–76. quiz 77. [PubMed] [Google Scholar]
- 9.Chiu HF, Wing YK, Lam LC, et al. Sleep-related injury in the elderly—an epidemiological study in Hong Kong. Sleep. 2000;23:513–7. [PubMed] [Google Scholar]
- 10.American Academy of Sleep Medicine. Diagnostic and coding manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. International classification of sleep disorders. [Google Scholar]
- 11.Schenck CH, Milner DM, Hurwitz TD, Bundlie SR, Mahowald MW. A polysomnographic and clinical report on sleep-related injury in 100 adult patients. Am J Psychiatry. 1989;146:1166–73. doi: 10.1176/ajp.146.9.1166. [DOI] [PubMed] [Google Scholar]
- 12.Schenck CH, Mahowald MW. Subclinical REM sleep behavior disorder and its clinical and research implications. Sleep. 2008;31:1627. doi: 10.1093/sleep/31.12.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–9. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 14.Rechtschaffen A, Kales AA. Bethesda, MD: National Institute of Neurological Disease and Blindness; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subject. [Google Scholar]
- 15.American Academy of Sleep Medicine. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for scoring sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 16.Ju YE, Larson-Prior L, Duntley S. Changing demographics in REM sleep behavior disorder: possible effect of autoimmunity and antidepressants. Sleep Med. 2011;12:278–83. doi: 10.1016/j.sleep.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 17.Iranzo A, Santamaria J, Tolosa E. The clinical and pathophysiological relevance of REM sleep behavior disorder in neurodegenerative diseases. Sleep Med Rev. 2009;13:385–401. doi: 10.1016/j.smrv.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Wing YK, Lam SP, Li SX, et al. REM sleep behaviour disorder in Hong Kong Chinese: clinical outcome and gender comparison. J Neurol Neurosurg Psychiatry. 2008;79:1415–6. doi: 10.1136/jnnp.2008.155374. [DOI] [PubMed] [Google Scholar]
- 19.Bodkin CL, Schenck CH. Rapid eye movement sleep behavior disorder in women: relevance to general and specialty medical practice. J Womens Health (Larchmt) 2009;18:1955–63. doi: 10.1089/jwh.2008.1348. [DOI] [PubMed] [Google Scholar]
- 20.Schenck CH, Mahowald MW. Motor dyscontrol in narcolepsy: rapid-eye-movement (REM) sleep without atonia and REM sleep behavior disorder. Ann Neurol. 1992;32:3–10. doi: 10.1002/ana.410320103. [DOI] [PubMed] [Google Scholar]
- 21.Schenck CH, Mahowald MW, Kim SW, O'Connor KA, Hurwitz TD. Prominent eye movements during NREM sleep and REM sleep behavior disorder associated with fluoxetine treatment of depression and obses-sive-compulsive disorder. Sleep. 1992;15:226–35. doi: 10.1093/sleep/15.3.226. [DOI] [PubMed] [Google Scholar]
- 22.Tachibana N, Yamanaka K, Kaji R, et al. Sleep bruxism as a manifestation of subclinical rapid eye movement sleep behavior disorder. Sleep. 1994;17:555–8. [PubMed] [Google Scholar]
- 23.Teman PT, Tippmann-Peikert M, Silber MH, Slocumb NL, Auger RR. Idiopathic rapid-eye-movement sleep disorder: associations with antidepressants, psychiatric diagnoses, and other factors, in relation to age of onset. Sleep Med. 2009;10:60–5. doi: 10.1016/j.sleep.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 24.Eisensehr I, Linke R, Tatsch K, et al. Increased muscle activity during rapid eye movement sleep correlates with decrease of striatal presynaptic dopamine transporters. IPT and IBZM SPECT imaging in subclinical and clinically manifest idiopathic REM sleep behavior disorder, Parkinson's disease, and controls. Sleep. 2003;26:507–12. doi: 10.1093/sleep/26.5.507. [DOI] [PubMed] [Google Scholar]
- 25.Schenck CH, Boeve BF. The strong presence of REM sleep behavior disorder in PD: clinical and research implications. Neurology. 2011;77:1030–2. doi: 10.1212/WNL.0b013e31822e14d7. [DOI] [PubMed] [Google Scholar]
- 26.Kim YK, Yoon IY, Kim JM, et al. The implication of nigrostriatal dopaminergic degeneration in the pathogenesis of REM sleep behavior disorder. Eur J Neurol. 2010;17:487–92. doi: 10.1111/j.1468-1331.2009.02854.x. [DOI] [PubMed] [Google Scholar]
- 27.Stiasny-Kolster K, Mayer G, Schafer S, Moller JC, Heinzel-Gutenbrun-ner M, Oertel WH. The REM sleep behavior disorder screening question-naire—a new diagnostic instrument. Mov Disord. 2007;22:2386–93. doi: 10.1002/mds.21740. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen T, Svob C, Kuiken D. Dreamenacting behaviors in a normal population. Sleep. 2009;32:1629–36. doi: 10.1093/sleep/32.12.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li SX, Wing YK, Lam SP, et al. Validation of a new REM sleep behavior disorder questionnaire (RBDQ-HK) Sleep Med. 2010;11:43–8. doi: 10.1016/j.sleep.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Vaughan P, Morrow RH. Manual of epidemiology for district health management. Geneva: World Health Organization; 1989. [Google Scholar]
- 31.Zhang J, Lam SP, Ho CK, et al. Diagnosis of REM sleep behavior disorder by video-polysomnographic study: is one night enough? Sleep. 2008;31:1179–85. [PMC free article] [PubMed] [Google Scholar]
- 32.Cygan F, Oudiette D, Leclair-Visonneau L, Leu-Semenescu S, Arnulf I. Night-to-night variability of muscle tone, movements, and vocalizations in patients with REM sleep behavior disorder. J Clin Sleep Med. 2010;6:551–5. [PMC free article] [PubMed] [Google Scholar]


