Abstract
Study Objectives:
Narcolepsy is characterized by instability of sleep-wake, tonus, and rapid eye movement (REM) sleep regulation. It is associated with severe hypothalamic hypocretin deficiency, especially in patients with cataplexy (loss of tonus). As the hypocretin neurons coordinate and stabilize the brain's sleep-wake pattern, tonus, and REM flip-flop neuronal centers in animal models, we set out to determine whether hypocretin deficiency and/or cataplexy predicts the unstable sleep-wake and REM sleep pattern of the human phenotype.
Design:
We measured the frequency of transitions in patients with narcolepsy between sleep-wake states and to/from REM and NREM sleep stages. Patients were subdivided by the presence of +/- cataplexy and +/- hypocretin-1 deficiency.
Setting:
Sleep laboratory studies conducted from 2001-2011.
Patients:
In total 63 narcolepsy patients were included in the study. Cataplexy was present in 43 of 63 patients and hypocretin-1 deficiency was present in 37 of 57 patients.
Measurements and Results:
Hypocretin-deficient patients with narcolepsy had a significantly higher frequency of sleep-wake transitions (P = 0.014) and of transitions to/from REM sleep (P = 0.044) than patients with normal levels of hypocretin-1. Patients with cataplexy had a significantly higher frequency of sleep-wake transitions (P = 0.002) than those without cataplexy. A multivariate analysis showed that transitions to/from REM sleep were predicted mainly by hypocretin-1 deficiency (P = 0.011), whereas sleep-wake transitions were predicted mainly by cataplexy (P = 0.001).
Conclusions:
In human narcolepsy, hypocretin deficiency and cataplexy are both associated with signs of destabilized sleep-wake and REM sleep control, indicating that the disorder may serve as a human model for the sleep-wake and REM sleep flip-flop switches.
Citation:
Sorensen GL; Knudsen S; Jennum P. Sleep transitions in hypocretin-deficient narcolepsy. SLEEP 2013;36(8):1173-1177.
Keywords: Flip-flop, hypocretin, narcolepsy, sleep transition
INTRODUCTION
Narcolepsy with cataplexy (NC) or narcolepsy without cataplexy (NwC) is a neurological sleep disorder found in one in 2,000 individuals. It is characterized by instability of sleep-wake regulation (daytime sleepiness, short sleep latency, disrupted nocturnal sleep with awakenings) and instability of rapid eye movement (REM) sleep regulation and motor tonus regulation, causing hypnagogic hallucinations, sleep paralysis, cataplexy, and REM sleep behavior disorder.1,2 The major pathophysiology is loss of the sleep-wake pattern and motor tonus regulating hypocretinergic neurons in the hypothalamus,3,4 resulting in low or undetectable levels of cerebrospinal fluid hypocretin-1 (hcrt-1) in most patients with NC, but in only a small proportion of patients with NwC.5,6 The cause of hypocretin neuron loss is most probably autoimmunological, mainly based on a very tight association with specific HLA-types,7,8 whereas the cause of NC/NwC in patients with normal hcrt-1 levels remains unclear.
Wakefulness and arousals are believed to be regulated mainly by two ascending neuron networks in the brain, referred to as the ascending reticular activation system (ARAS).9 The first activating neuron network consists of the pedunculopontine and laterodorsal tegmental nuclei in the brainstem, which are active during wakefulness and REM sleep.10 The second activating neuron network consists of the locus coeruleus, dorsal end median raphe nuclei, ventral periaqueductal gray matter, and tuberomammillary nucleus, which are active during wakefulness, slightly active during nonrapid eye movement (NREM) sleep, and inactive during REM sleep.10 Sleep is promoted by activation of the ventrolateral preoptic nucleus in the basal forebrain, which acts by inhibiting hypocretin neurons and the neurons in ARAS.10 The currently prevailing theory is that transition from sleep to wakefulness and vice versa is regulated by a sleep-wake flip-flop switch, involving the mutual inhibition of the sleep promoting nuclei and the wakefulness-promoting nuclei.11
The REM-NREM flip-flop switch works through another mutually inhibitory system, in which gamma-aminobutyric acidergic (GABAergic) neurons in the sublaterodorsal region fire during REM sleep and inhibit GABAergic neurons in the ventrolateral periaqueductal gray matter and the adjacent lateral pontine tegmentum that in turn fire during NREM states to inhibit REM sleep.10 The hypocretin neurons play a central role in the coordination and stabilization of the sleep-wake and REM-NREM flip-flop switches.11 Consequently, hypocretin deficiency may be the cause of the observed instability of the sleep-wake pattern of the human narcoleptic phenotype. We therefore propose that instability in these systems due to hypocretin loss may lead to sleep-wake and NREM-REM transition instabilities. For this reason, narcolepsy may serve as a human model for the flip-flop switches.
METHODS
Patients
Over a 10-y period (2001-2011), patients with narcolepsy seen at the Danish Center for Sleep Medicine, Glostrup University Hospital, were identified and consecutively included after ethical approval (KA03119) and written informed consent had been obtained. All patients fulfilled the International Classification of Sleep Disorders (ICSD-2) criteria for narcolepsy,12 and were evaluated by neurological examination, determination of routine blood characteristics, polysomnography (PSG), the multiple sleep latency test (MSLT), and determination of hcrt-1 in the cerebrospinal fluid (CSF). The hypocretin measurement protocol and definition of hcrt-1 deficiency used were as previously published.6 Exclusion criteria were: additional neurological and psychiatric disorders, an apnea index > 5/h, and a periodic leg movements during sleep index > 4/h. All patients (except three with severe cataplexy) were free of antidepressants and stimu-lants 7 to 14 days before inclusion; 44 of 63 were completely drug-naïve (i.e., they had never taken medication).
A total of 63 patients fulfilled the inclusion criteria. Cataplexy was diagnosed as previously described2 and was present in 43 of 63 patients, and hcrt-1 deficiency was present in 37 patients. Six patients (five of six with cataplexy) refused lumbar puncture. Normal hcrt-1 levels were present in eight of 43 patients with cataplexy, and hcrt-1 deficiency was present in seven of 20 patients without cataplexy. All patients have been included in a previous publication by Sorensen et al.13 and 34 of 63 patients have been previously featured in publications by Knudsen et al.2,6 Demographic data of the patients are summarized in Table 1. Table 2 gives an overview of the cataplexy and hcrt-1 status for the patients in the different groups.
Table 1.
Demographic data of patients
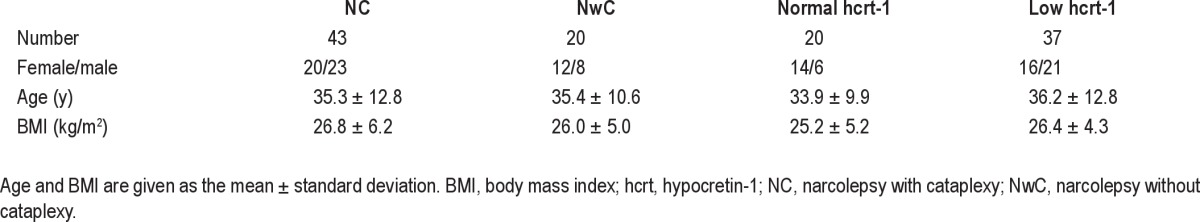
Table 2.
Overview of cataplexy and hcrt-1 status for the included patients

PSG Recordings
All patients underwent 1 night of PSG recording consisting of electroencephalography (C3-A2, C4-A1), vertical and horizontal electrooculography (EOG), surface electromyography (EMG) of the submentalis and anterior muscles, electrocardiography (ECG), nasal airflow, thoracic respiratory effort, and oxygen saturation. Patients were instructed not to consume any caffeinated or alcoholic drinks for at least 6 h before the recordings were made. Sleep stages and events were manually scored for epochs of 30 sec according to standard criteria by experienced PSG technicians supervised by PJ14,15 and without knowledge of the final diagnosis. The hypnograms and sleep events were extracted from Nervus® (V5.5, Cephalon DK, Nørresundby, Denmark) or Somnologica Studio® (V5.1, Embla, Broomfield, CO, USA), using the built-in export data tool, and imported into the Matlab¯ analysis program (R2009b, The MathWorks, Natick, MA, USA) for further processing.
Analysis of Transitions
The frequency of transitions in the hypnograms for each patient was measured as the number of transitions per h of sleep of the investigated sleep stage. Analyzed transitions included were transitions between wake and sleep, transitions to/from REM sleep (hereafter noted as REM-NREM transitions), transitions to/ from NREM stage 1, 2, and 3, and transitions between all sleep stages. Wilcoxon rank-sum tests were performed to compare the frequencies of transitions between different narcolepsy groups.
A backward-stepwise multivariate linear regression was performed to determine the relationship between transitions and age, sex, body mass index, disease duration, disease onset, +/-previous anticataplexy medication, +/- cataplexy, and +/- hcrt-1 status. The +/- anticataplexy medication indicates whether patients were pausing with previous medication or had never taken medication before inclusion (drug-naïve).
PSG variables, including sleep latency (SL), total sleep time (TST), sleep efficiency (SE), time from sleep onset to first epoch of REM sleep (REM SL), and percentage of stages 1, 2, and 3 NREM and REM sleep, were also measured. Wilcoxon rank-sum tests were performed to evaluate between-group differences (results shown in Table 3). A significance level of P < 0.05 was chosen for all statistical tests.
Table 3.
Comparison of PSG variables
RESULTS
The frequencies of sleep-wake transitions are illustrated in the box plots in Figure 1 and means and standard deviations (SDs) of each narcolepsy group are given in Table 4. Patients with narcolepsy and low hcrt-1 level had a signifi cantly higher frequency of sleep-wake transitions than patients with narco-lepsy and normal hcrt-1 level (P = 0.014). Patients with NC also had a signifi cantly higher frequency of sleep-wake transitions than those with NwC (P = 0.002).
Figure 1.
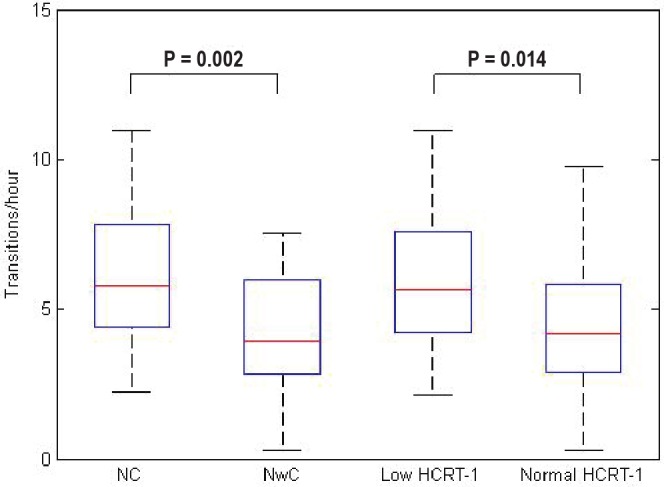
Frequency of sleep-wake transitions illustrated in box plots for each patient group. Each box plot shows the fi fth, 25th, 50th, 75th, and 95th percentile. Signifi cant P values are shown. Hcrt, hypocretin-1; NC, narcolepsy with cataplexy; NwC, narcolepsy without cataplexy.
Table 4.
Frequency of sleep transitions (mean ± standard deviation)
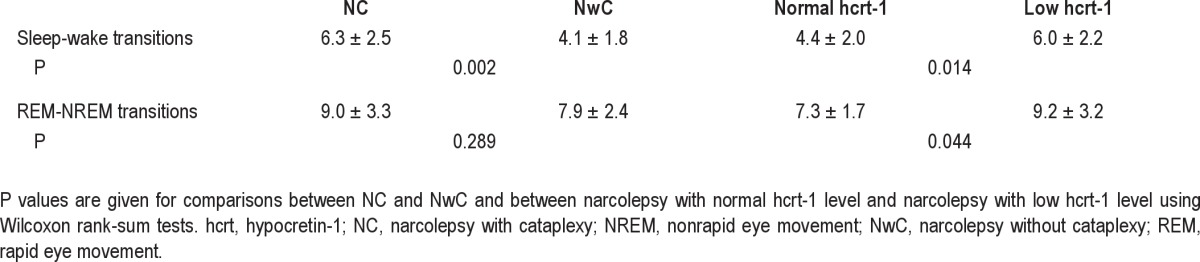
The frequencies of REM-NREM transitions are illustrated in the box plots in Figure 2 and the means and SDs are given in Table 4. Patients with narcolepsy and low hcrt-1 level had a signifi cantly higher frequency of REM-NREM transitions than patients with narcolepsy and normal hcrt-1 level (P = 0.044). Patients with NC also had a trend for more REM-NREM transitions than those with NwC, although this difference was not statistically signifi cant.
Figure 2.
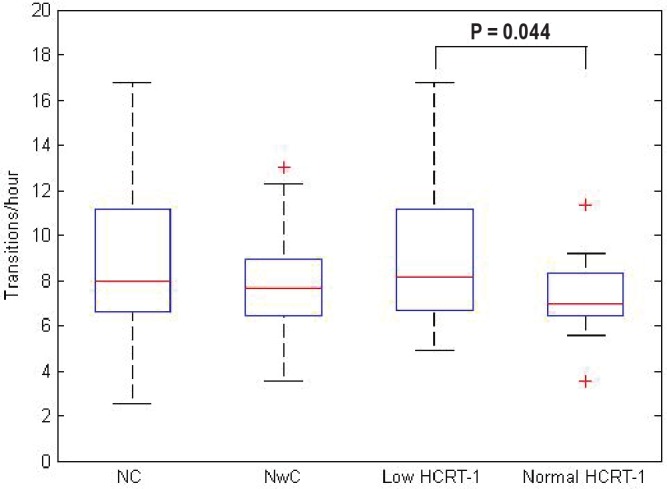
Frequency of rapid eye movement-nonrapid eye movement transitions illustrated in box plots for each patient group. Each box plot shows the fi fth, 25th, 50th, 75th, and 95th percentile. Signifi cant P values are shown. Hcrt, hypocretin-1; NC, narcolepsy with cataplexy; NwC, narcolepsy without cataplexy.
For the other analyzed transitions (to/from NREM stage 1, 2, and 3, and transitions between all sleep stages), there was no significance between any of the groups (results not shown).
In the multivariate analysis, the number of REM-NREM transitions were predicted only by hcrt-1 deficiency (P = 0.011), whereas the number of sleep-wake transitions were predicted only by cataplexy (P = 0.001).
The values for the PSG variables are displayed in Table 3. For patients with narcolepsy and hcrt-1 deficiency, the REM SL was significantly lower (P < 0.04), and the percentage of NREM 1 sleep was significantly higher (P < 0.01) than in patients with narcolepsy without hcrt-1 deficiency. The percentage of NREM 1 sleep was significantly higher in patients with NC than in those with NwC (P < 0.01).
DISCUSSION
This is the first study to show that the frequency of sleepwake transitions and REM-NREM transitions are associated with cataplexy and hypocretin deficiency in patients with narcolepsy. The study documents that sleep-wake and REM-NREM transitions are more frequent in patients with NC and hypocretin-deficient narcolepsy. These results are consistent with the flip-flop model of transitions proposed by Saper et al.,11 and are consistent with a human model in which hypocretin enforces general state stability.
Hypocretin neurons in the lateral hypothalamus reinforce the activity in the wake-promoting projections arising from neurons in the upper brainstem, whereas sleep is promoted by activation of the ventrolateral preoptic nucleus in the basal forebrain, which acts by inhibiting hypocretin neurons and the neurons in ARAS.10 The hypocretin neurons are therefore believed to play an important role in sustaining wakefulness and in stabilizing the flip-flop switch. However, other important arousal-producing neurons are present in the posterior lateral hypothalamus, which helps maintain wakefulness when hypocretin neurons are deficient. The hypocretin neurons may help to sustain activity in aminergic and cholinergic arousal regions, and in the absence of hypocretin, these arousal regions may have reduced activity, resulting in inappropriately low thresholds to transition into NREM sleep.16 In patients with narcolepsy and hypocretin deficiency, one of the most important components in the flip-flop switch is absent, making the switch unstable and entailing frequent sleep/wake switches, as seen in this study.
Hypocretin neurons are also known to suppress REM sleep by reinforcing the activity of the monoaminergic neurons in the locus coeruleus and dorsal raphe nucleus,17,18 which in turn activate REM sleep-suppressing neurons and inhibit REM on neurons. It can be hypothesized that loss of hypocretin neurons therefore also enables more frequent transitions into REM sleep and makes the REM-NREM switch unstable. In our study we detected that patients with narcolepsy and hcrt-1 deficiency had more REM-NREM transitions than those without hcrt-1 deficiency, and this higher frequency of REM-NREM transitions was primarily predicted by hcrt-1 deficiency, independent of cataplexy and other factors, supporting the theory that hypocretin destabilizes the sleep stage switch.
Quantitative electroencephalographic studies suggest changes in frequencies in NREM 2 sleep19 as well as sleep onset20 in patients with NC. Another study has shown that the occurrence of multiple spontaneous daytime sleep onset REM periods clearly identifiied patients with narcolepsy.21 Animal studies support that sleep-wake transition is under control by the hypo-cretinergic system, which facilitates wakefulness and REM-NREM cyclicity. Studies with hypocretin-deficient knockout mice and animals with narcolepsy have shown sleep stage dysregulations22 and considerably more transitions between all stages.16,23–25 As hypocretin is believed to stabilize wake and sleep, the hypocretin deficiency in patients with narcolepsy may cause a low threshold to transition between stages, causing the fragmented sleep pattern seen in patients with narcolepsy. This supports our study, showing that hypocretin-deficient patients with narcolepsy may present sleep stage instability as compared with non-hypocretin-deficient patients with narcolepsy.
One limitation of this study is that sleep-wake regulation as determined by simple macro-sleep scoring may be too simplistic a model to allow the evaluation of the sleep-wake/REM-NREM transition, because this may occur much faster and not in the 30-sec epochs used in traditional scoring. This may in part explain why we did not find a significant association with NREM transitions. However, to date, no reliable or validated sleep scoring model has been developed for scoring epochs shorter than 30 sec. Alternative methods may include a quantitative measure using a sliding window with a shorter time interval including not only the conventional sleep stages, but also transition zones between stages. One attempt using a state space analysis technique has been performed in orexin knockout mice,25 where the variations in the depth of sleep and the intensity of wakefulness were measured by electroencephalographic power in the delta and theta bands. The orexin knockout mice had an overlap between wake and REM sleep and spent more time in transition regions causing the altered quality of sleep and wake. Variations within states and changes in transition zones may be a reason for the more frequent changes in orexin knockout mice and hypocretin-deficient patients with narcolepsy. We suggest that future evaluation of sleep transition and its relation to the hypocretinergic system should include analysis of transition states and shorter windows used in the sleep scoring.
Another limitation is that interscorer variability was not considered and sleep scoring may have been subject to individual scoring. Furthermore, only nocturnal PSGs are considered, whereas daytime monitoring could be at least as interesting, because patients with narcolepsy have sleep episodes during daytime.
In conclusion, the current study shows that patients with narcolepsy and hypocretin deficiency or cataplexy have more frequent sleep-wake and REM-NREM transitions, which is consistent with the destabilization of the flip-flop switches that regulate sleep-wake and REM-NREM transitions due to the loss of hypocretin neurons. The elevated frequency of REM-NREM transitions is primarily predicted by hcrt-1 deficiency, independent of cataplexy and other factors.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENT
Gertrud Laura Sorensen and Stine Knudsen contributed equally to this work.
Footnotes
A commentary on this article appears in this issue on page 1123.
REFERENCES
- 1.Ferri R, Miano S, Bruni O, et al. NREM sleep alterations in narcolepsy/ cataplexy. Clin Neurophysiol. 2005;116:2675–84. doi: 10.1016/j.clinph.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Knudsen S, Gammeltoft S, Jennum PJ. Rapid eye movement sleep behaviour disorder in patients with narcolepsy is associated with hypocretin-1 deficiency. Brain. 2010;133:568–79. doi: 10.1093/brain/awp320. [DOI] [PubMed] [Google Scholar]
- 3.Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–7. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 4.Thannickal TC, Moore RY, Nienhuis R, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–74. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mignot E, Lammers GJ, Ripley B, et al. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol. 2002;59:1553–62. doi: 10.1001/archneur.59.10.1553. [DOI] [PubMed] [Google Scholar]
- 6.Knudsen S, Jennum PJ, Alving J, Sheikh SP, Gammeltoft S. Validation of the ICSD-2 criteria for CSF hypocretin-1 measurements in the diagnosis of narcolepsy in the Danish population. Sleep. 2010;33:169–76. doi: 10.1093/sleep/33.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kornum BR, Kawashima M, Faraco J, et al. Common variants in P2RY11 are associated with narcolepsy. Nat Genet. 2011;43:66–71. doi: 10.1038/ng.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hor H, Kutalik Z, Dauvilliers Y, et al. Genome-wide association study identifies new HLA class II haplotypes strongly protective against narcolepsy. Nat Genet. 2010;42:786–9. doi: 10.1038/ng.647. [DOI] [PubMed] [Google Scholar]
- 9.Saper CB, Scammel TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–63. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 10.Saper CB, Fuller PM, Pedersen NP, Lu J, Scamell TE. Sleep state switching. Neuron. 2010;68:1023–1042. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saper CB, Chou TC, Scamell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–31. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- 12.2nd edition. Westchester, IL: American Academy of Sleep Medicine; 2005. The International Classification of Sleep Disorders (ICSD)-diagnostic and coding manual. [Google Scholar]
- 13.Sorensen GL, Knudsen S, Pedersen ER, et al. Attenuated heart rate response is associated with hypocretin deficiency in patients with narcolepsy. Sleep. 2013;36:91–8. doi: 10.5665/sleep.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonnet MH, Carley D, Guilleminault CG, et al. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 15.Iber C, Ancoli-Israel S, Chesson AL, Quan SF. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 16.Mochizuki T, Crocker A, McCormack S, Yanagisawa M, Sakurai T, Scammell TE. Behavioral state instability in orexin knock-out mice. J Neurosci. 2004;24:6291–6300. doi: 10.1523/JNEUROSCI.0586-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bourgin P, Huitrón-Résendiz S, Spier AD, et al. Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. J Neurosci. 2000;20:7760–5. doi: 10.1523/JNEUROSCI.20-20-07760.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohlmeier KA, Watanabe S, Tyler CJ, Burlet S, Leonard CS. Dual orexin actions on dorsal raphe and laterodorsal tegmentum neurons: noisy cation current activation and selective enhancement of Ca2+ transients mediated by L-type calcium channels. J Neurophysiol. 2008;100:2265–81. doi: 10.1152/jn.01388.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hadjiyannakis K, Ogilvie RD, Alloway CE, Shapiro C. FFT analysis of EEG during stage 2-to REM transitions in narcoleptic patients and normal sleepers. Electroencephalogr Clin Neurophysiol. 1997;103:543–53. doi: 10.1016/s0013-4694(97)00064-3. [DOI] [PubMed] [Google Scholar]
- 20.Kim JW, Shin HB, Robinson PA. Quantitative study of the sleep onset period via detrended fluctuation analysis: normal vs. narcoleptic subjects. Clin Neurophysiol. 2009;120:1245–51. doi: 10.1016/j.clinph.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 21.Pizza F, Moghadam KK, Vandi S, et al. Daytime continuous polysomnography predicts MSLT results in hypersomnias of central origin. J Sleep Res. 2013;22:32–40. doi: 10.1111/j.1365-2869.2012.01032.x. [DOI] [PubMed] [Google Scholar]
- 22.Chemelli RM, Willie JT, Sinton CM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–51. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 23.Nishino S, Riehl J, Hong J, Kwan M, Reid M, Mignot E. Is narcolepsy a REM sleep disorder? Analysis of sleep abnormalities in narcoleptic Dobermans. Neurosci Res. 2000;38:437–46. doi: 10.1016/s0168-0102(00)00195-4. [DOI] [PubMed] [Google Scholar]
- 24.Kaitin KI, Kilduff TS, Dement WC. Sleep fragmentation in canine narcolepsy. Sleep. 1986;9:116–9. doi: 10.1093/sleep/9.1.116. [DOI] [PubMed] [Google Scholar]
- 25.Diniz Behn CG, Klerman EB, Mochizuki T, Lin S, Scammell TE. Abnormal sleep/wake dynamics in orexin knockout mice. Sleep. 2010;33:297–306. doi: 10.1093/sleep/33.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]


