Abstract
Study Objectives:
To simultaneously explore the associations between mortality and insomnia, sleep duration, and the use of hypnotics in older adults.
Design:
A fixed cohort study.
Setting:
A community in Shih-Pai area, Taipei, Taiwan.
Participants:
A total of 4,064 participants over the age of 65 completed the study.
Intervention:
N/A.
Measurements and Results:
Insomnia was classified using an exclusionary hierarchical algorithm, which categorized insomnia as “no insomnia,” “subjective poor sleep quality,” “Pittsburgh Sleep Quality Index > 5 insomnia,” “1-month insomnia disorder,” and “6-month insomnia disorder.” The main outcome variables were 9-year all-cause mortality rates. In the all-cause mortality analyses, when hypnotic use, depressive symptoms and total sleep time were excluded from a proportional hazards regression model, subjects with “Pittsburgh Sleep Quality Index > 5 insomnia” had a higher mortality risk (HR: 1.21, 95% CI: 1.01-1.45). In the full model, frequent hypnotic use and long sleep duration predicted higher mortality rates. However, the increased mortality risk for subjects with “Pittsburgh Sleep Quality Index > 5 insomnia” was not observed in the full model. On the contrary, individuals with a 6-month DSM-IV insomnia disorder had a lower risk for premature death (HR: 0.64, 95% CI: 0.43-0.96).
Conclusions:
Long sleep duration and frequent hypnotics use predicted an increased mortality risk within a community-dwelling sample of older adults. The association between insomnia and mortality was affected by insomnia definition and other parameters related to sleep patterns.
Citation:
Chen HC; Su TP; Chou P. A nine-year follow-up study of sleep patterns and mortality in community-dwelling older adults in Taiwan. SLEEP 2013;36(8):1187-1198.
Keywords: Insomnia, sleep duration, sleep pattern, older adults, use of hypnotics
INTRODUCTION
Sleep disturbance is a prevalent health problem that has substantial consequences for older adults.1,2 Longitudinal studies have shown that sleep difficulties predict several adverse health outcomes.3–7 The quality and quantity of sleep have been associated with coping ability, timely engagement with activities, and resilience in the face of adversity8; and these outcomes are often indicative of successful aging. Therefore, it is important to examine the factors that influence sleep quality and health outcomes among older adults. However, the risk predictors identified within older adult samples seem to differ from those of the general population.
Predictors of the association between sleep patterns and mortality have been summarized within three dimensions: insomnia, sleep duration, and use of hypnotics. Evidence for the link between sleep duration and mortality is consistent and robust. Meta-analytic studies have observed that both longer and shorter sleep durations are related to increased mortality.9,10 In contrast, the association between insomnia and mortality is not as conclusive. Increased risk,11–15 no association,15–22 and even decreased risk of mortality19,23 have been reported among subjects with insomnia. Specifically, among studies using older adult samples, one study has revealed a gender-specific association between insomnia and elevated risk for mortality12; most other studies do not observe this relationship.17,19–22 Similarly, although some studies report that the use of hypnotics predicts a significant risk for mortality,13,18,23–26 this trend is unusual in older adult samples.12,15,17,20 Thus, divergent associations between sleep parameters and mortality suggest a necessity for research examining these relationships in older adults.
Inconsistent definitions of sleep parameters, difficulty in identifying confounds, and difficulty in determining causality underlie the discrepant associations between sleep patterns and mortality.27 Although the National Institute of Health has encouraged specific research efforts that apply well-defined diagnostic criteria for insomnia in sleep studies,28,29 no longitudinal epidemiological studies have examined the association between the risk of mortality and the various definitions of insomnia. Additionally, although sleep parameters are interrelated, short sleep duration is not equivalent to insomnia, long sleep duration is not equivalent to good sleep, and taking hypnotics is not equivalent to poor sleep.23,30 This suggests that insomnia, sleep duration, and use of hypnotics are not necessarily colinear but could confound each other. The inclusion of all potential predictors related to specific sleep patterns might help to illustrate each parameter's specific relationship with mortality.
Certain longitudinal studies have taken into account the independent effects of insomnia, sleep duration, and hypnotics within the same investigation.12,13,15,17,18,23,26 Among these studies, only a few have included older adult samples.12,17 Therefore, the present study investigated how insomnia, sleep duration, and hypnotics predicted mortality in a community-dwelling sample of older adults. Furthermore, insomnia classification was defined a priori in order to examine whether a differential relationship exists between insomnia and mortality.
METHODS
Study Site and Participants
This study is part of the Shih-Pai Sleep Study, which is a community-based, fixed cohort study. The study cohort was established in the Shih-Pai area of Taipei, Taiwan. Eligible participants were identified from the government household registration system. According to the 1999 official resident registration database, 9,141 residents over the age of 65 years lived in the Shih-Pai area. Excluding 523 institutionalized older adults, 175 who died before an interview could be conducted, and 1,292 vacant households, there were 7,151 eligible subjects. After door-to-door interviews, 1,255 eligible subjects were not contacted for the required 3 visits, and 1,832 refused to be interviewed. Thus, 4,064 subjects completed the interview process between 1999 and 2002. The response rate was 56.8%. The institutional review board of Taipei Veterans General Hospital approved this study.
Mortality Data
The dependent variable of interest was the occurrence of death between the initial interview and December 31, 2008. Nine-year mortality data, which provide the major cause and date of death but not time of death, were acquired from the national death registry of the Department of Health, Taiwan. Causes of death were classified by the International Classification of Disease, 9th Revision, Clinical Modification (ICD-9). The follow-up period included the time from the date of the initial interview until the date of death or the end of this study (December 31, 2008). Deaths related to neoplasm (ICD-9 codes 140-208), cardiovascular disease (ICD-9 codes 390-459), and pulmonary disease (ICD-9 codes 460-519) were specified for cause-specific analyses.
Social Demographic Data, Lifestyle, and General Medical History
In addition to basic demographic data, information regarding history of cigarette smoking, alcohol consumption, and medical illness was also collected. If a subject's poor physical condition precluded cooperation with data collection procedures, height and weight were coded as missing values. History of medical illness was obtained with a checklist that screened for hypertension, diabetes mellitus, cardiovascular diseases, stroke, and gouty arthritis. Data on cancer were not collected. Medical illness was coded only for subjects who confirmed a history of diagnosis and treatment for one of the above ailments. Pain was assessed with a single-item question that asked about pain severity over the past month. Severity was rated as “none,” “mild,” “moderate,” and “severe.” Those who responded with moderate or severe were coded as experiencing significant pain. Depressive symptoms were evaluated using the Geriatric Depression Scale-Short Form. 31,32 Subjects with scores ≥ 5 of 15 questions were considered to have significant depressive symptoms. Daytime sleepiness was assessed by asking participants their propensity for sleeping in the daytime after an adequate night's sleep. Responses were rated as “not at all,” “mild,” “moderate,” or “severe.” Responses of moderate or severe were coded as experiencing significant daytime sleepiness. Subjects reported habitual snoring as “none,” “mild,” “moderate,” or “severe.” Subjects responding with moderate or severe were defined as prominent and disturbing snorers, respectively.
Nighttime Sleep Pattern
The parameters of sleep patterns (i.e., insomnia and sleep duration) were investigated using specific questions and items obtained from the Pittsburgh Sleep Quality Index (PSQI). The cutoff for PSQI-defined poor sleep quality was a score > 5.33
Exclusionary Hierarchical Algorithm to Classify Insomnia Categories
Insomnia was classified into 5 mutually exclusive categories defined by an a priori exclusionary hierarchical algorithm. According to the frequency of symptoms, daytime repercussions, and duration of symptoms, insomnia was classified as “no insomnia,” “subjective poor sleep quality,” “PSQI > 5 insomnia,” “1-month insomnia disorder,” and “6-month insomnia disorder,” respectively. Subjective sleep quality was evaluated through an item of the PSQI asking participants how they would rate their sleep quality overall during the past month. Responses of “fairly bad” and “very bad” were coded as “subjective poor sleep quality.” Subjects with total PSQI scores > 5 were classified as PSQI > 5 insomnia. If subjects' sleep complaints met DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, IV)34 criteria for insomnia disorder, they were defined as having insomnia disorder regardless of their PSQI scores. Along with the establishment of DSM-IV insomnia disorder, if subjects' sleep disturbances had lasted ≥ 6 months and they reported no intervals of symptom-free sleep lasting > 1 month within the past 6 months, then 6-month insomnia disorder was confirmed. All other cases that met the DSM-IV insomnia disorder criteria for < 6 months were classified as 1-month insomnia disorder.
The principle behind utilizing item combinations of the PSQI to make a DSM-IV diagnosis of insomnia disorder has been reported in a previous study.35 PSQI items are divided into a cluster of nighttime symptoms and a cluster of poor sleep quality/ daytime functional impairment. To satisfy the diagnostic criteria for DSM-IV insomnia disorder, subjects must experience ≥ 1 nighttime symptom with a frequency ≥ 3 times per week. In addition to the criterion involving cluster 1, subjects must score 3 or 4 out of 4 severity classes on any item from cluster 2. The cluster definitions are as follows:
Cluster 1
Difficulty falling asleep, defined as failing to fall asleep within 30 min after going to bed or
Difficulty maintaining sleep (≥ 3 interruptions of sleep during the night) or early-morning awakening (waking up ≥ 2 h earlier than usual)
Each symptom must occur ≥ 3 times per week to qualify for cluster 1.
Cluster 2
Poor subjective sleep quality (scoring 3 or 4 among the 4 severity levels) or
At least a moderate degree of daytime dysfunction in mood, work efficiency, or daily activities (scoring 3 or 4 among the 4 severity levels)
Use of Hypnotics
The frequency of use of hypnotics was quantified on the basis of an additional question not derived from the PSQI. It specifically inquired “During the last 4 weeks, on how many nights did you use sedatives/hypnotics to help fall sleep?” Subjects who reported ≥ 21 instances of use of sedatives/hypnotics within the specified period were identified as “frequent hypnotic users.”
Sleep Duration
Sleep duration was estimated from responses to the following self-report question: “During the past month, how many hours of actual sleep did you get at night?” Sleep duration was categorized in 1-h units. All responses were rounded down to the nearest whole digit. For comparison with other studies, we chose a sleep duration of 7 hours as our reference category.23,36–39
Statistical Analysis
Mortality rates were estimated using the person-time method. Hazard ratios (HRs) were estimated using Cox proportional hazards regression. A formal test of the proportional hazards assumption was also performed using Schoenfeld residuals. The results are presented as crude and adjusted hazard ratios with 95% confidence intervals (CI). The minimum statistical significance level for all analyses was P < 0.05. In addition to the main predictors of sleep pattern (insomnia, sleep duration, and use of hypnotics), another 17 covariates were included in the full model (Cox regression) to control for potential confounding effects. Another 4 Cox regression models were conducted with the predictors of insomnia, depression, use of hypnotics, and sleep duration added stepwise to illustrate the mutual relationships between various categories of insomnia and other major predictors. In our analysis of the determinants of cause-specific mortality, subjects who died of other causes were treated as censored observations.
Despite controlling for many potential confounds, it is possible that undiagnosed diseases or underlying health conditions could cause both sleep disturbance and eventual death.40 This problem may result in reverse causality and bias the estimate of the association between sleep patterns and mortality.38,41–43 The exclusion of decedents (those who died in the first 2 years after the initial assessment) reduces the risk of reverse causality.44 After excluding decedents from the first 2-year post-baseline assessment, the present study addressed this issue by establishing 2-year lag models that repeated all multivariate Cox regression analyses for all causes of mortality.
RESULTS
The average age of the study cohort at baseline assessment was 73.8 (SD 5.7) years. During the follow-up period, a total of 1,004 deaths occurred, making an overall mortality rate of 24.7%. The average follow-up period was 7.0 (SD 2.1) years, with a total 28,474 person-years observed. There were no significant differences between the participants and the registered 1999 data on the entire population aged ≥ 65 years throughout Taipei city in terms of age (χ2 = 3.49, df = 1, P = 0.06) or gender (χ2 = 2.01, df = 1, P = 0.16). Significant depressive symptoms were observed in 9.8% of the subjects. According to our definition of insomnia, 19.9% of participants could be classified into at least one category of insomnia. A total of 5.9% of subjects had DSM-IV insomnia disorder, and two-thirds of these sub-jects (67.8%) had 6-month insomnia disorder. In total, 12.3% of the subjects had used sedatives/hypnotics to help them fall asleep in the last 4 weeks, and 7.3% were defined as “frequent hypnotic users (≥ 21 days/4 weeks).” The average nighttime sleep duration was 6.3 h (SD 1.5). In total, 52.5% of subjects reported sleeping 6-7 h per night (Table 1).
Table 1.
Demographic characteristics and clinical features of participants at the baseline interview (N = 4,064)*
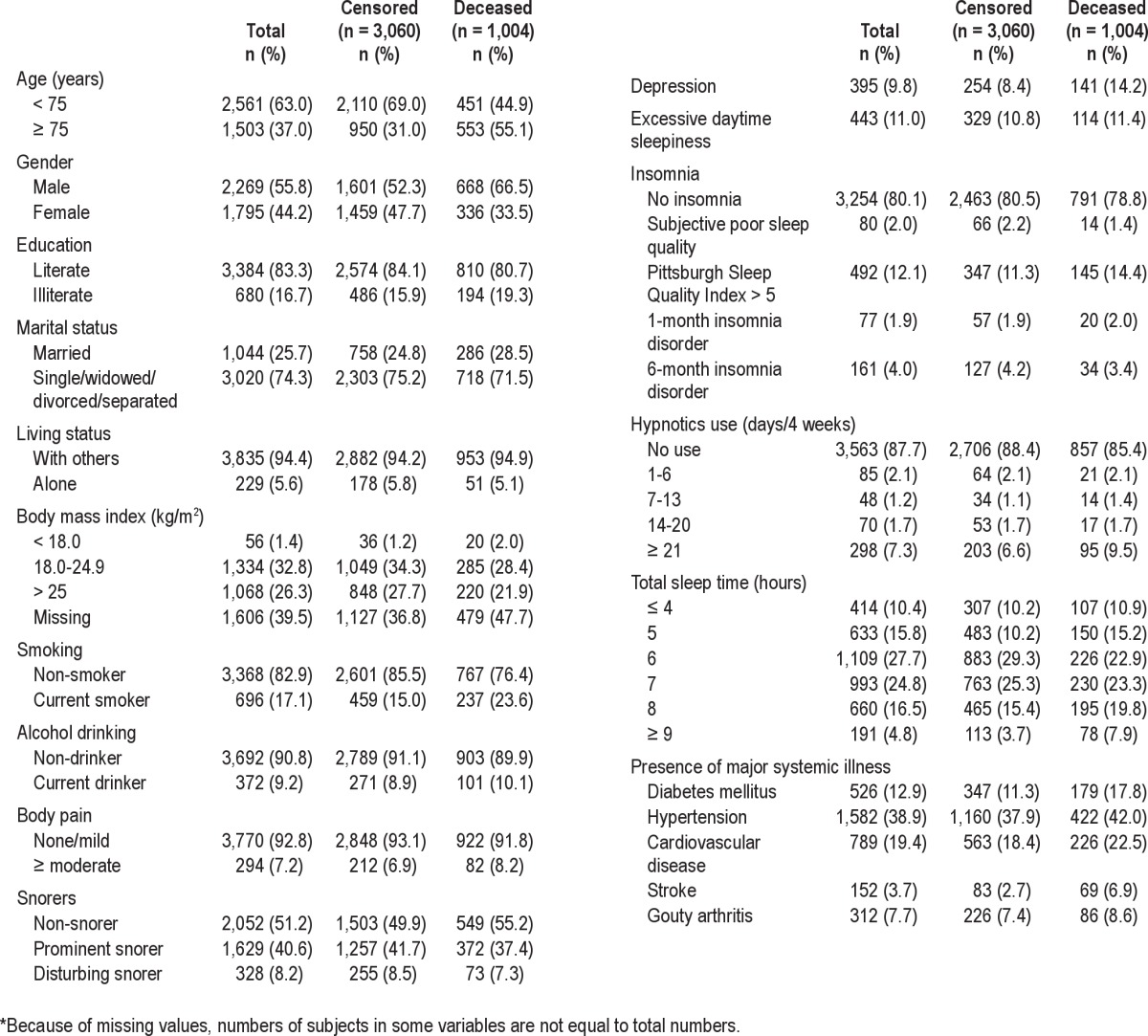
Table 2 shows comparisons of PSQI scores among various definitions of insomnia. The omnibus tests for between-group comparisons of the total scores and 7 subscores were all significant (all Ps < 0.001). In terms of post hoc comparisons, all classifications of insomnia differed from each other in total scores (all Ps < 0.001). Subjects with insomnia disorder scored higher than those with PSQI > 5 insomnia within all other subscores. However, this trend was not observed among scores derived from components of sleep duration and efficiency. Specifically, older adults with PSQI > 5 insomnia had the worst sleep efficiency. Among subjects with DSM- IV insomnia disorder, except for the subscore related to the use of sleep medication, there were no significant differences with respect to subscores between subjects with 1-month and 6-month insomnia disorder. In addition, before applying the exclusionary hierarchical algorithm, 18.0% of subjects scored ≥ 6 on the PSQI. Among subjects with 1 -month and 6- month insomnia disorder, 98.5% and 98.0% reached that score threshold, respectively. Conversely, of those who scored > 5 on the PSQI, only 9.3% and 20.5% of subjects satisfied the definitions of 1-month and 6-month insomnia disorder, respectively (Table 2).
Table 2.
Comparison and distribution of the Pittsburgh Sleep Quality Index (PSQI) scores in various insomnia categories
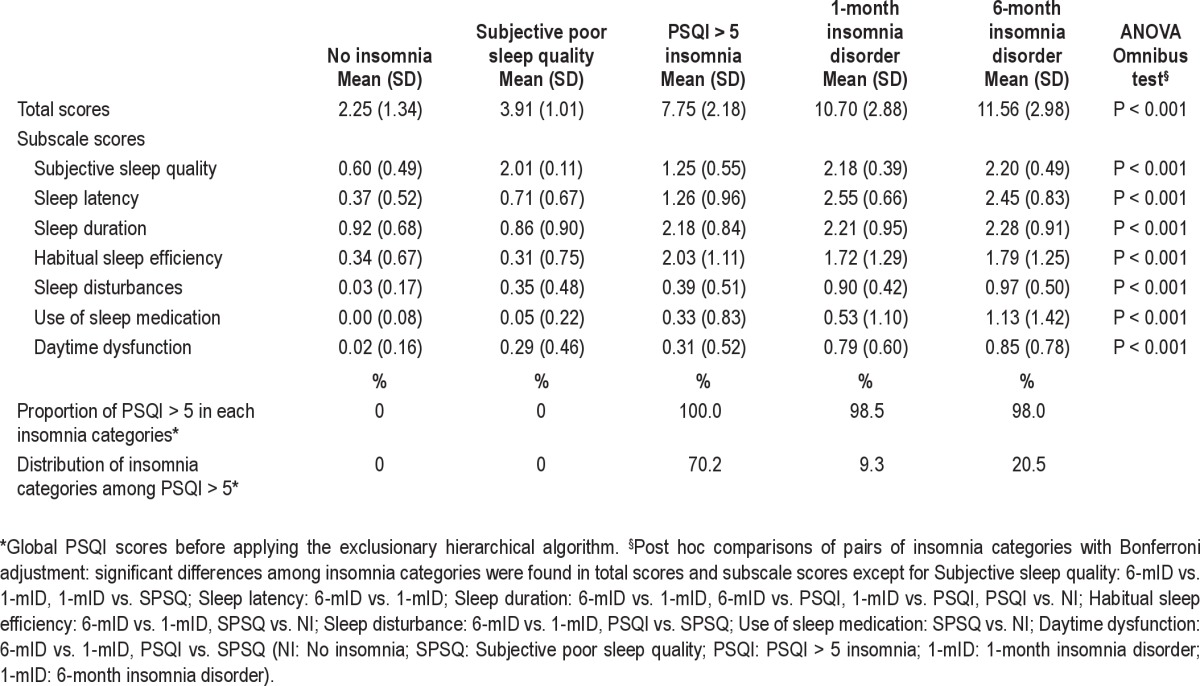
Figure 1 illustrates the interrelated distribution between insomnia, sleep duration, and the use of hypnotics in our sample. Panel A reveals that only subjects with subjective poor sleep quality tend to sleep longer (40.8% sleep ≥ 8 h per night). About half of the older adults (52.0%-54.8%) with insomnia disorder reported ≤ 4 h of sleep per night. Panel B suggests that among the older adults who were frequent hypnotic users (≥ 21 days/4 weeks), 50.7% of the older adults reported being free from insomnia. However, 49.3% met various criteria for insomnia categories; 50.5% of these subjects met the stringent criteria for DSM-IV insomnia disorder. Panel C shows that 38.1% of subjects slept < 6 h per night despite their frequent use of hypnotics. In contrast, only 5.2% of frequent hypnotic users slept ≥ 9 h per night with the assistance of hypnotics.
Figure 1.
The interrelated distribution between insomnia, sleep duration, and use of hypnotics among older adults. (A) The distribution of sleep duration by insomnia categories. (B) The distribution of sleep disturbance among hypnotic users. (C) The distribution of sleep hours among hypnotic users. PSQI, Pittsburgh Sleep Quality Index
Table 3 compares the differences in demographic data and clinical features between the 5 categories of insomnia. Older adults with insomnia, regardless of classifications, tended to be women (50.0%-61.0%). The age distribution did not differ significantly across various definitions of insomnia (P = 0.61). Subjects with insomnia—either PSQI > 5 insomnia or insomnia disorder—were prone to reporting pain, depressive symptoms, hypertension, cardiovascular diseases, and stroke. Compared with subjects without insomnia, those with insomnia disorder were more likely to sleep for shorter lengths of time and frequently use hypnotics (16.9%-37.9%).
Table 3.
Characteristics of participants by categories of sleep disturbance (N = 4,064)
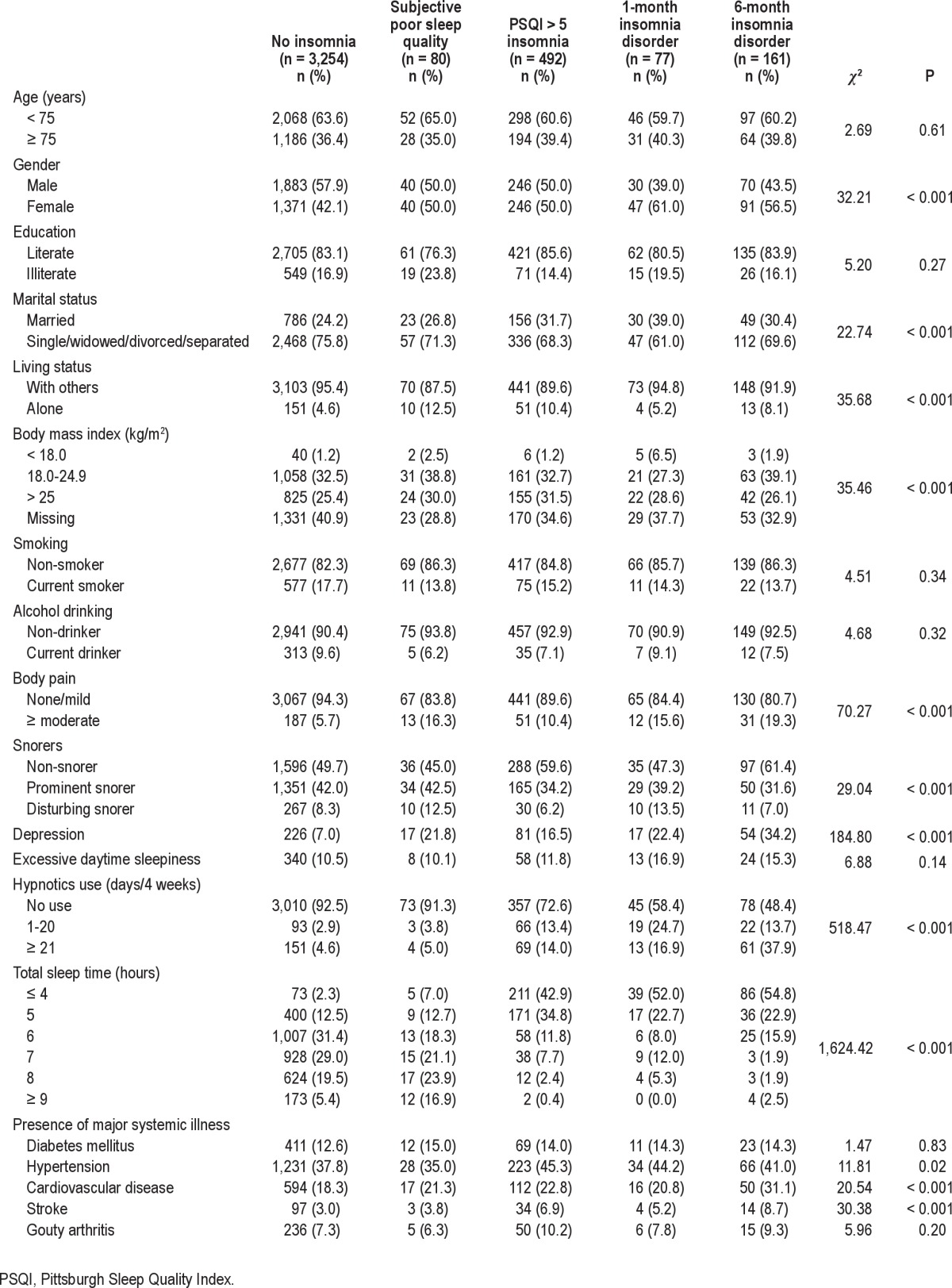
Table 4 shows the crude and adjusted hazard ratios for various predictors. Except for variables related to BMI, hypertension, and insomnia, significant predictors were similar across the unadjusted and adjusted HRs. In the full model, when compared with those without insomnia, subjects with PSQI > 5 insomnia had a marginally significant elevated mortality risk (HR: 1.24, 95% CI: 0.98-1.55, P = 0.07). In contrast, people with 6-month insomnia disorder had a lower mortality risk (HR: 0.64, 95% CI: 0.43-0.96). Frequent hypnotics users had an increased mortality risk (HR: 1.37, 95% CI: 1.09-1.73). In comparison with subjects from the reference group (sleep duration: 7 h), older adults who slept 8 h (HR: 1.26, 95% CI: 1.04-1.53) or ≥ 9 h (HR: 1.66, 95% CI: 1.28-2.17) had a higher mortality risk. No significant elevated mortality risk was found among subjects who slept for short durations.
Table 4.
Cox regression model for factors predicting all-cause of mortality at 9-year follow-up
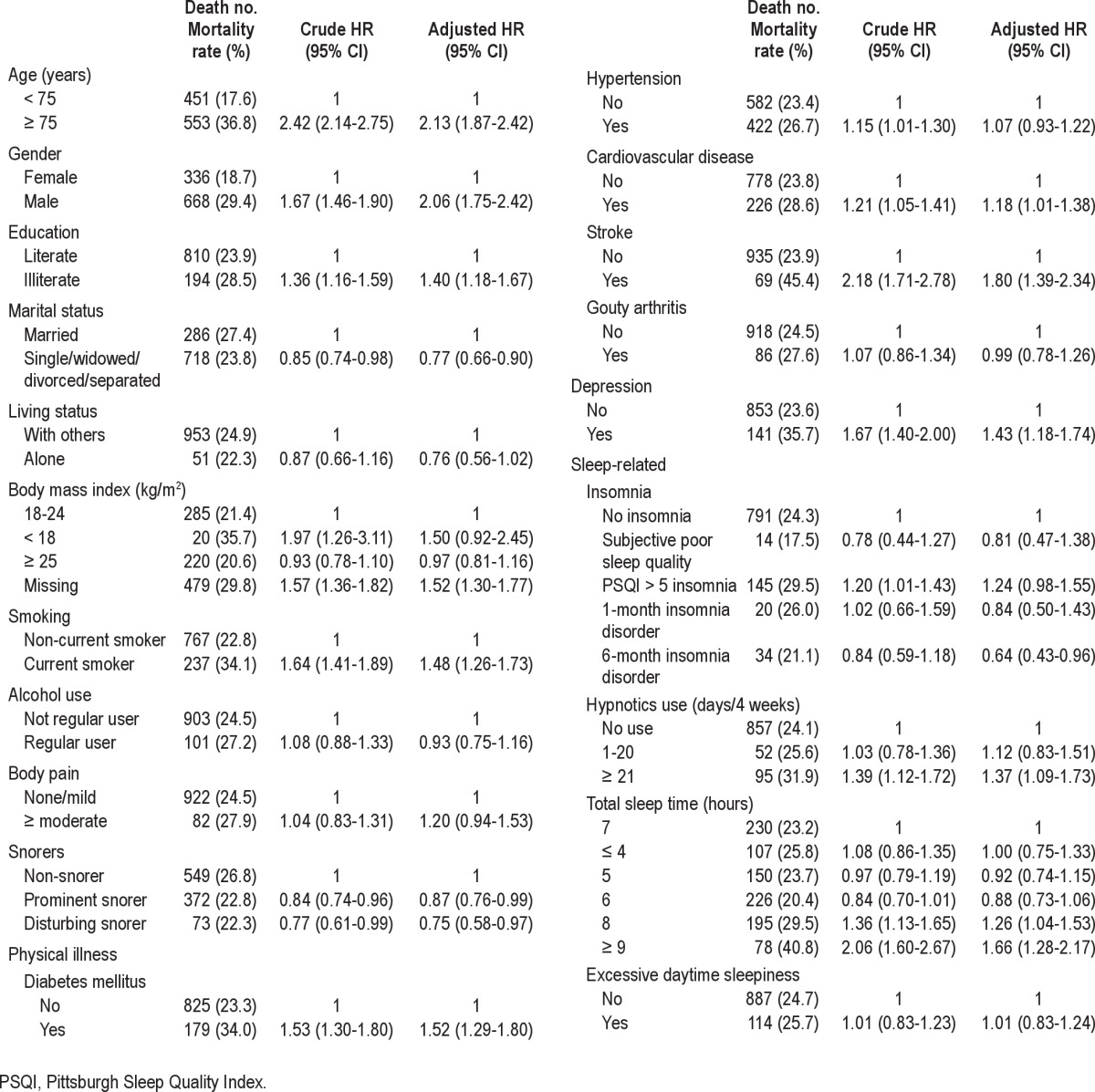
Table 5 summarizes the HR change after inclusion of different predictors for all causes of mortality. After major confounding factors other than depression, use of hypnotics, and sleep duration were controlled for, Model 1 showed that subjects with PSQI > 5 insomnia (rather than those with insomnia disorder) had an increased mortality risk (HR: 1.21, 95% CI: 1.01-1.45); this was comparable to the crude HR. Once depression (Model 2) or use of hypnotics (Model 3) were included as covariates, the increased mortality risk for subjects with PSQI > 5 insomnia was no longer significant, and people with 6-month insomnia disorder turned out to have a lower risk of mortality. Of the variables included in the full model, only sleep duration was not included in Model 4. The predictive patterns between insomnia and hypnotics use with all causes of mortality were similar with the HR of the full model. In contrast, Model 5 showed that the inclusion of sleep duration, but not depression and use of hypnotics, maintained the increased mortality risk for subjects with PSQI > 5 insomnia featured in Model 1. The comparison between Models 4 and 5 and the full model indicated that the elevated mortality risk among subjects with PSQI > 5 insomnia was significantly affected by depression and use of hypnotics rather than sleep duration. The reverse causality analysis indicated that the increased mortality risk for subjects with PSQI > 5 insomnia in Model 1 was attenuated (HR: 1.22, 95% CI: 0.99-1.50, P = 0.06), but all the other significant effects remained within the 2-year lag models.
Table 5.
Cox regression models for factors predicting all causes of mortality at 9-year follow-up*
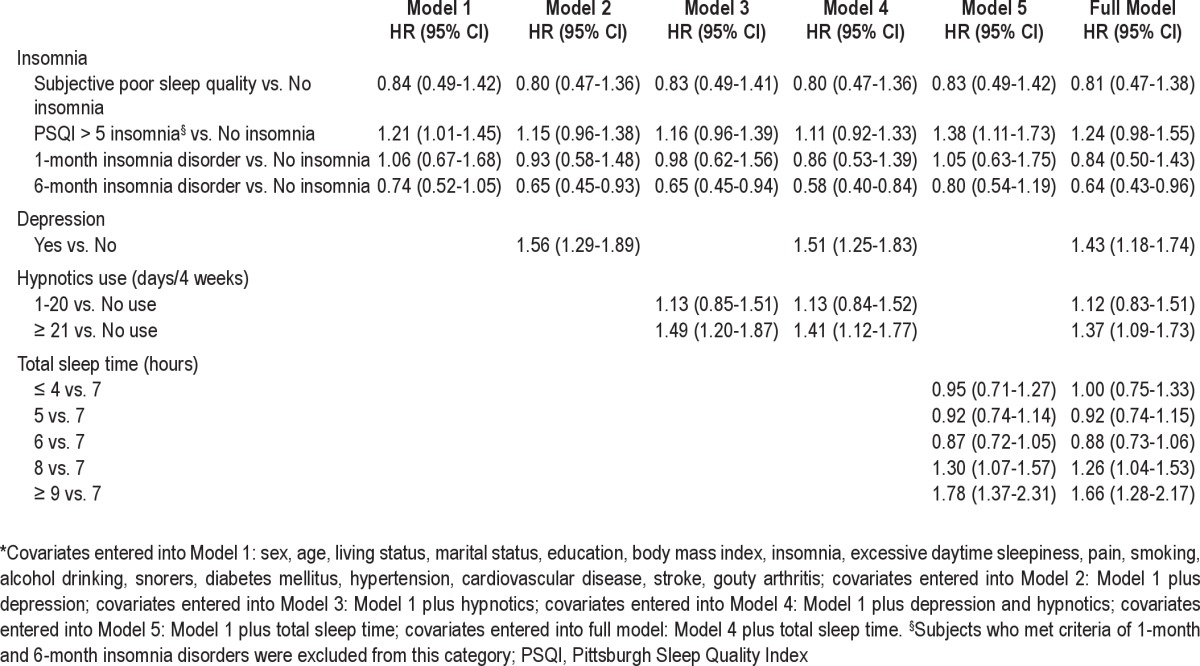
As for the cause-specific analyses, older adults with 6-month insomnia disorder had a lower risk of dying from neoplasm (HR: 0.32, 95% CI: 0.13-0.78). In contrast, a sleep duration ≥ 9 hours and frequent use of hypnotics predicted increased mortality risk from cardiovascular (HR: 2.36, 95% CI: 1.46 -3.80) and pulmonary (HR: 2.56, 95% CI: 1.38-4.72) diseases (Table 6).
Table 6.
Cox regression models for factors predicting cause-specific mortality at 9-year follow-up*
DISCUSSION
The present study provides important insights to help explain inconsistent findings in the literature regarding the relationship between sleep patterns and mortality. We were able to assess this relationship with a large sample, which allowed for the inclusion of a variety of potential confounding variables. Since few studies have simultaneously assessed the effects of insomnia, sleep duration, and use of hypnotics, the present study provides a significant contribution to the literature. Additionally, we were able to examine the differential impacts of various definition of insomnia. Our findings support the advantage of classifying insomnia through an a priori-defined, exclusionary hierarchical algorithm. This approach not only helps to clarify inconsistent findings between insomnia and mortality, but also identifies priority groups that should be targeted for interventions. To our knowledge, only one other study has used this method to examine the moderating role of age on the correlates of insomnia.45
Prevalence of Insomnia
In the present study, 18.0% of the subjects scored > 5 on the PSQI, and 5.9% met the criteria for DSM-IV insomnia disorder. In the literature, 8% to 18% of community-dwelling subjects were dissatisfied with their sleep, and 6% had DSM-IV insomnia diagnoses.1 Besides, the prevalence of insomnia symptoms generally increases with age, while the rates of sleep dissatis-faction and diagnosis of sleep disorders vary little with age.1 Therefore, the prevalence rates of poor sleep quality (scores > 5 on the PSQI) and DSM-IV insomnia disorder observed in the present study are compatible with those reported in the literature. Interestingly, only 29.8% of the older adults who scored > 5 on the PSQI met the DSM-IV criteria for insomnia diagnoses. The poor correlation between scoring > 5 on the PSQI and DSM-IV diagnosis of insomnia disorder may reflect differences in definitions between these two constructs. The stringent frequency quantifier and the requirement for request of the daytime repercussions differentiates those who only scored > 5 on the PSQI from those who concurrently met the DSM-IV criteria for insomnia disorder. Although 50.7% of the frequent hypnotic users met no classification criterion for insomnia and may have found the medication to be efficacious, 49.3% of the frequent users still suffered from insomnia and met the criteria for one of the insomnia categories. Furthermore, nearly half of these older adults still had DSM-IV insomnia disorder. This finding suggests that older adults who frequently take hypnotics to fall asleep may not be satisfied with their resulting sleep quality or may experience prominent residual symptoms. Because sleep disturbances in older adults are usually multifactorial in etiology, no treatment can be expected to be adequate without a comprehensive evaluation and treatment strategy tailored to the needs of the individual patient.46
Insomnia and Mortality
In the present study, depressive symptoms and use of hypnotics attenuated the association between PSQI > 5 insomnia and mortality. Paradoxically, insomnia disorder correlated more closely with depressive symptoms and frequent use of hypnotics than did PSQI > 5 insomnia. Controlling for the effects of depressive symptoms and hypnotics should reduce the mortality risk among those with 6-month insomnia disorder. This suggests that depressive symptoms and the use of hypnotics might act as confounds or intermediate variables that spuriously magnify the mortality risk among those with PSQI > 5 insomnia. Conversely, these variables might counterbalance the effect of mortality risk among those with 6-month insomnia disorder. This finding implies inherent differences in the associations between various categories of insomnia and mortality. Thus, the nature of this heterogeneous relationship deserves further discussion.
First, this heterogeneous relationship might originate from differential risks of specific insomnia complaints. By dissecting subscores on the PSQI, we found that the worst level of sleep efficiency differentiates PSQI > 5 insomnia from subjective poor sleep quality and insomnia disorder. Evidence from polysomnography studies also supports the increased mortality risk associated with poor sleep efficiency.11 Thus, this heterogeneous relationship might stem partly from the differences in symptom profiles among various categories of insomnia.
Second, the population of older adults with short-term insomnia might differ in some aspects from those with chronic insomnia. Among the subjects with insomnia disorder, more than 98% scored > 5 on the PSQI. In other words, in addition to PSQI scores, another major difference between PSQI > 5 insomnia and 6-month insomnia disorder should be the duration of severe insomnia. In light of our stringent definitions, the older adults with 6-month insomnia disorder might have experienced sleep issues even at younger ages. In contrast, subjects with PSQI > 5 insomnia might represent a group that developed new sleep issues at older ages. In terms of chronicity, 6-month insomnia disorder is similar to prevalent insomnia, and PSQI > 5 insomnia is closer to incident insomnia. Therefore, prevalent insomnia and incident insomnia are fundamentally different from each other. Compared with prevalent insomnia, incident insomnia is characterized by both a higher remission rate and a higher mortality rate.47 The examination of reverse causality diminished the increased mortality risk of PSQI > 5 insomnia but retained the lower mortality risk for those with 6-month insomnia disorder. This implies that PSQI > 5 insomnia (incident insomnia) might correlate with undiagnosed diseases or conditions that cause death. In contrast, 6-month insomnia disorder (prevalent insomnia) late in life might represent unobserved factors that confer a survival advantage. These factors might have existed early in life and provided protection into old age.48 In sum, PSQI > 5 insomnia may represent a recent undiagnosed/unfavorable condition, and 6-month insomnia disorder might indicate undetected protective traits for survival. In fact, a few large-scale longitudinal epidemiological studies have associated a lower risk of insomnia symptoms19,23,29 and benzodiazepine use25 with adverse health outcomes. The question of whether survival mortality contributes to these unexpected findings warrants further research.
Use of Hypnotics and Mortality
We observed a consistent, increased mortality risk among frequent hypnotics users, even after controlling for insomnia and depression. This finding addresses the argument about whether mortality associated with use of hypnotics can be attributed to insomnia or depression.49,50 Specifically, frequent use of hypnotics predicted a higher risk of death from pulmonary diseases. Numerous pulmonary diseases—such as chronic obstructive pulmonary disease, accumulated bronchial secretions, nocturnal bronchospasm, sleep-related laryngospasm, and rhinitis/sinusitis—and their treatment agents affect sleep quality in older adults.51 Because the present study was not able to control for all the confounding effects relating to pulmonary comorbidities, the mortality risk from use of hypnotics may be overestimated. However, data from the 2003 National Sleep Foundation's annual Sleep in America poll demonstrated that sleep disturbances in lung diseases mainly manifest as a shorter total sleep time, pauses in breathing, and lack of refreshment from sleep upon awakening.52 In contrast with the present study, the covariates of total sleep time, excessive day-time sleepiness, and snoring have been forced into the previous regression models. Therefore, the confounding effects of pulmonary comorbidities have been (at least partly) removed in this study.
It remains unclear what mechanisms underlie the links between use of hypnotics and increased overall mortality risk, and mortality from pulmonary diseases. The adverse respiratory effects of sedatives/hypnotics have been considered as a potential cause of complications. Some classes of hypnotics—such as benzodiazepines—depress the respiratory system, particularly in patients with chronic obstructive pulmonary disease.53–56 In contrast, although newer pyridine derivatives such as zolpidem and zaleplon also have some potential to worsen pulmonary function, they appear less likely to do so.54,57,58,59 Furthermore, though limited data are available regarding sedative antidepressants and sedative antipsychotics, they also appear to be well-tolerated by the respiratory system.56 The participants were not asked about the types of sedatives/hypnotics used in the present study, and collapsing all agents into one category could dilute the risks of other agents that may impair respiratory function, which consequently leads to mortality. Therefore, when prescribing sedatives/hypnotics to older adults at increased risk of adverse respiratory effects—such as those with advanced disease and hypercarbia—extra caution is highly required.
In the present study, only older adults who frequently used sedatives/hypnotics (≥ 21 days/4 weeks) had a higher all-cause mortality risk than non-users. Because numerous studies have consistently reported the elevated risk of hypnotics use, the absence of increased mortality risk in older adults who used sedatives/hypnotics with a frequency of less than 21 instances in 4 weeks deserves attention. In fact, a comparable number of studies that failed to illustrate the mortality risk of hypnotic use. In these studies, because of limitations related to the probing questions or a relatively small-scale sample size, the frequency of hypnotic exposure was often dichotomized into users vs. non-users or frequent users vs. non-frequent users.12,15–17,20,60–63 On the contrary, in studies that successfully demonstrated a higher mortality risk, the frequency of hypnotics use was usually categorized into various levels to reflect different loadings. Compared with no exposure to hypnotics, a higher-frequency exposure rather than a low-frequency exposure has consistently predicted an elevated mortality risk.23,25,26,64,65 Moreover, in a study with a large sample, a dose-response association has been demonstrated in the association between hypnotics use and mortality.65 Obviously, collapsing the different frequencies of hypnotic exposure altogether may attenuate the genuine risk of hypnotic use, especially in high frequency users.
Sleep Duration and Mortality
A U-shaped association between sleep duration and mortality has been previously documented among older adults36; however, this pattern was not observed in the present study. Confounds related to comorbidity have been proposed to underlie the association between shorter sleep duration and increased mortality risk. Given that our participants were relatively healthy community-dwelling older adults, the confounding effects from comorbidities might be too subtle to detect. Additionally, we found that longer sleep duration predicted higher mortality in the present study, consistent with the findings of a previous study in Taiwan.38
Some mechanisms that link long sleep duration with increased mortality have been proposed. First, poor sleep quality among those with insomnia might result in a need for prolonged time in bed to restore energy.36 Although this assumption is consistent with the present study's observation that older adults with subjective poor sleep quality were more likely to sleep longer, the significant predictability of longer sleep duration did not diminish after controlling for the effect of insomnia. In contrast, the subjects with PSQI > 5 and insomnia disorder tended to sleep for shorter (instead of longer) durations. Second, proinflammatory cytokines, such as interleukin (IL)-1 and IL-2, have been shown to promote sleep.66,67 Therefore, sleep durations at baseline might have been lengthened as a consequence of inflammatory responses to medical conditions, and these medical conditions may have ultimately played roles in the subjects' deaths. The inflammatory process has been shown to be a key factor in both the pathogenesis and pathophysiology of cancer and cardiovascular disease.68,69 This potential role of inflammation was partly supported by our finding that subjects who slept longer tended to die of cardiovascular diseases. Third, increased sleep duration has been found to occur during the last few months of life, and subtle increases in sleep might occur during the last few years before death.40 However, the analyses of reverse causality in the present study did not fully mitigate the increased mortality risk resulting from long sleep duration. Therefore, our present results seem to provide at least partial support to previous studies examining the role of long sleep duration in mortality.
Limitations
One problem with studies that examine the associations between sleep patterns and adverse outcomes is the difficulty in selecting confounding factors.27 Regression models that simultaneously include insomnia, sleep duration, and hypnotics as predictors need to account for the statistical collinearity among variables. Introducing collinear variables into a model can nullify certain predictors, mask significant associations, and allow for the emergence of spurious associations. However, the advantage of simultaneously including sleep parameters may out-weigh the concern of redundancy between variables. First, in concordance with previous studies, the present study also found that insomnia, sleep duration, and use of hypnotics are not necessarily equal to each other. Second, we can infer the particular roles of parameters that are potentially collinear with parameters of sleep according to established knowledge. The temporal relationships between variables help define the specific roles of covariates.70 The temporal precedence between certain predictors—such as insomnia and use of hypnotics—is apparent in the present study, but other predictors show no such clear patterns, exemplified by the well-known bidirectional relationship between insomnia and depression.71–73 In the analyses of Models I-III shown in Table 5, the mortality risk associated with PSQI > 5 insomnia vanished after the use of hypnotics and depression were controlled for. Causal step analysis of the mediation effects indicates that depression and the use of hypnotics may either confound (in the case of depression) or totally mediate the relationship between PSQI > 5 insomnia and elevated risk of mortality.74 This implies that the correlates of PSQI > 5 insomnia (i.e., depression and use of hypnotics), rather than PSQI > 5 insomnia per se, determine the association between PSQI > 5 insomnia and elevated mortality risk. Similarly, depression, use of hypnotics, and total sleep time may also confound or mediate each other's relationships with mortality. The analyses of Models IV and V and the full model shown in Table 5 found significant independent associations between depression, use of hypnotics, and total sleep time with mortality, although the magnitude of these associations decreased slightly. This finding suggests that if mediation effects do exist, the observed elevated mortality risk associated with depression, use of hypnotics, and total sleep time is only partially mediated by other covariates.
We have yet to determine which combination of sleep covariates can justify both the confounding effects and the risk of collinearity. Nonetheless, simultaneous consideration of the effects of several sleep parameters helps provide a comprehensive understanding of the independent and interrelated effects of sleep patterns on mortality. Accordingly, the present study illustrated how frequent use of hypnotics and long sleep time serve as strong risk factors for increased mortality, even though the variety of sleep parameters included in the models may introduce the problem of multicollinearity.
Other limitations of this study include the insufficient amount of information gathered on primary sleep disorders and other comorbidities. Details on the type of, dosage of, and duration of exposure to hypnotics during the follow-up period were also not collected. Further, the validity of comorbidity history is uncertain. First, overlooked medical comorbidities may bias our findings. For example, because the PSQI includes indicators suggesting sleep apnea, the marginally significant elevation in all-cause mortality associated with PSQI > 5 insomnia might be attributable to primary sleep disorders, such as sleep apnea or even periodic limb movement disorder. Besides, the cause-specific mortality analysis indicates that 6-month insomnia disorder predicts a lower mortality risk from neoplasms. Failure to control for history of neoplasms may bias the results toward the null hypothesis. In contrast, frequent use of hypnotics predicts a higher risk for mortality risk from pulmonary diseases. The inability to control for the effects of various pulmonary diseases may overestimate the risks associated with use of hypnotics. Second, the sedatives/hypnotics used as helping sleep aids in Taiwan may comprise not only benzodiazepine/ non-benzodiazepine hypnotics, but also antihistamines, sedative antidepressants, and sedative antipsychotics. Besides, it is unknown whether participants discontinued or commenced taking hypnotics over the follow-up period. Failure to identify the types of sedatives/hypnotics used and the total exposure duration during the study period may also underestimate the impact of hypnotics on mortality. Thirdly, we defined medical illnesses through self-reported diagnoses and associated treatments for the current study. A correlational study in Taiwan found that self-reports regarding medical illness were highly reliable, particularly among those who reported receiving treatment.75 Thus, the validity of our comorbidity assessment is likely high.
CONCLUSIONS
From a clinical perspective, older adults with recently developed insomnia, long sleep durations, and frequent use of hypnotics warrant more indepth assessment. Overall, the simultaneous inclusion of insomnia, sleep duration, and use of hypnotics in the model was advantageous. Further classification of insomnia by various definitions should help us identify high-risk groups. Finally, before solving the analytical problem of under-control vs. over-control, it is premature to draw conclusions regarding the specific effects of chronic DSM-IV insomnia disorder on mortality.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This study was conducted at the Community Medicine Research Center of National Yang-Ming University, Taipei, Taiwan.
ABBREVIATIONS
- CI
confidence interval
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- HR
hazard ratio
- ICD-9
International Classification of Disease, 9th Revision
- IL
interleukin
- NI
no insomnia
- PSQI
Pittsburgh Sleep Quality Index
- SPSQ
Subjective Poor Sleep Quality
- 1-mID
1-month Insomnia Disorder
- 6-mID
6-month Insomnia Disorder
Footnotes
A commentary on this article appears in this issue on page 1127.
REFERENCES
- 1.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 2.Ancoli-Israel S, Cooke JR. Prevalence and comorbidity of insomnia and effect on functioning in elderly populations. J Am Geriatr Soc. 2005;53:S264–71. doi: 10.1111/j.1532-5415.2005.53392.x. [DOI] [PubMed] [Google Scholar]
- 3.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2006;61:405–10. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- 4.Latimer Hill E, Cumming RG, Lewis R, Carrington S, Le Couteur DG. Sleep disturbances and falls in older people. J Gerontol A Biol Sci Med Sci. 2007;62:62–6. doi: 10.1093/gerona/62.1.62. [DOI] [PubMed] [Google Scholar]
- 5.Stone KL, Ancoli-Israel S, Blackwell T, et al. Actigraphy-measured sleep characteristics and risk of falls in older women. Arch Intern Med. 2008;168:1768–75. doi: 10.1001/archinte.168.16.1768. [DOI] [PubMed] [Google Scholar]
- 6.Dam TT, Ewing S, Ancoli-Israel S, et al. Association between sleep and physical function in older men: the osteoporotic fractures in men sleep study. J Am Geriatr Soc. 2008;56:1665–73. doi: 10.1111/j.1532-5415.2008.01846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reid KJ, Martinovich Z, Finkel S, et al. Sleep: a marker of physical and mental health in the elderly. Am J Geriatr Psychiatry. 2006;14:860–6. doi: 10.1097/01.JGP.0000206164.56404.ba. [DOI] [PubMed] [Google Scholar]
- 8.Driscoll HC, Serody L, Patrick S, et al. Sleeping well, aging well: a descriptive and cross-sectional study of sleep in “successful agers” 75 and older. Am J Geriatr Psychiatry. 2008;16:74–82. doi: 10.1097/JGP.0b013e3181557b69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33:585–92. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. J Sleep Res. 2009;18:148–58. doi: 10.1111/j.1365-2869.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- 11.Dew MA, Hoch CC, Buysse DJ, et al. Healthy older adults' sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Med. 2003;65:63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- 12.Pollak CP, Perlick D, Linsner JP, Wenston J, Hsieh F. Sleep problems in the community elderly as predictors of death and nursing home placement. J Community Health. 1990;15:123–35. doi: 10.1007/BF01321316. [DOI] [PubMed] [Google Scholar]
- 13.Mallon L, Broman J-E, Hetta J. Is usage of hypnotics associated with mortality? Sleep Med. 2009;10:279–86. doi: 10.1016/j.sleep.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Vgontzas AN, Liao D, Pejovic S, et al. Insomnia with short sleep duration and mortality: the Penn State cohort. Sleep. 2010;33:1159–64. doi: 10.1093/sleep/33.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mallon L, Broman JE, Hetta J. Sleep complaints predict coronary artery disease mortality in males: a 12-year follow-up study of a middle-aged Swedish population. J Intern Med. 2002;251:207–16. doi: 10.1046/j.1365-2796.2002.00941.x. [DOI] [PubMed] [Google Scholar]
- 16.Phillips B, Mannino DM. Does insomnia kill? Sleep. 2005;28:965–71. doi: 10.1093/sleep/28.8.965. [DOI] [PubMed] [Google Scholar]
- 17.Rumble R, Morgan K. Hypnotics, sleep, and mortality in elderly people. J Am Geriatr Soc. 1992;40:787–91. doi: 10.1111/j.1532-5415.1992.tb01850.x. [DOI] [PubMed] [Google Scholar]
- 18.Hublin C, Partinen M, Koskenvuo M, Kaprio J. Sleep and mortality: a population-based 22-year follow-up study. Sleep. 2007;30:1245–53. doi: 10.1093/sleep/30.10.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–32. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 20.Brabbins CJ, Dewey ME, Copeland JRM, et al. Insomnia in the elderly: Prevalence, gender differences and relationships with morbidity and mortality. Int J Geriatr Psychiatry. 1993;8:473–80. [Google Scholar]
- 21.Ganguli M, Reynolds CF, Gilby JE. Prevalence and persistence of sleep complaints in a rural older community sample: the MoVIES project. J Am Geriatr Soc. 1996;44:778–84. doi: 10.1111/j.1532-5415.1996.tb03733.x. [DOI] [PubMed] [Google Scholar]
- 22.Althuis MD, Fredman L, Langenberg PW, Magaziner J. The relationship between insomnia and mortality among community-dwelling older women. J Am Geriatr Soc. 1998;46:1270–3. doi: 10.1111/j.1532-5415.1998.tb04544.x. [DOI] [PubMed] [Google Scholar]
- 23.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 24.Hammond EC. Some preliminary findings on physical complaints from a prospective study of 1,064,004 men and women. Am J Public Health Nations Health. 1964;54:11–23. doi: 10.2105/ajph.54.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kripke DF, Klauber MR, Wingard DL, Fell RL, Assmus JD, Garfinkel L. Mortality hazard associated with prescription hypnotics. Biol Psychiatry. 1998;43:687–93. doi: 10.1016/s0006-3223(97)00292-8. [DOI] [PubMed] [Google Scholar]
- 26.Kripke DF, Simons RN, Garfinkel L, Hammond EC. Short and long sleep and sleeping pills. Is increased mortality associated? Arch Gen Psychiatry. 1979;36:103–16. doi: 10.1001/archpsyc.1979.01780010109014. [DOI] [PubMed] [Google Scholar]
- 27.Ohayon MM. Insomnia: a dangerous condition but not a killer? Sleep. 2005;28:1043–4. doi: 10.1093/sleep/28.9.1043. [DOI] [PubMed] [Google Scholar]
- 28.Narional Institutes of Health. National Institutes of Health State of the Science Conference statement on Manifestations and Management of Chronic Insomnia in Adults, June 13-15, 2005. Sleep. 2005;28:1049–57. doi: 10.1093/sleep/28.9.1049. [DOI] [PubMed] [Google Scholar]
- 29.Phillips B, Buzkova P, Enright P. Insomnia did not predict incident hypertension in older adults in the cardiovascular health study. Sleep. 2009;32:65–72. [PMC free article] [PubMed] [Google Scholar]
- 30.Buysse DJ, Ganguli M. Can sleep be bad for you? Can insomnia be good? Arch Gen Psychiatry. 2002;59:137–8. doi: 10.1001/archpsyc.59.2.137. [DOI] [PubMed] [Google Scholar]
- 31.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS). recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–73. [Google Scholar]
- 32.Lee HCB, Chiu HFK, Kowk WY, Leung CM, Kwong PK, Chung DWS. Chinese elderly and the GDS short form: A preliminary study. Clin Gerontol. 1993;14:37–42. [Google Scholar]
- 33.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 34.American Psychiatric Association. 4th ed. Washington, DC: American Psychiatric Association; 1994. Diagnostic and statistical manual of mental disorders, DSM-IV. [Google Scholar]
- 35.Su TP, Huang SR, Chou P. Prevalence and risk factors of insomnia in community-dwelling Chinese elderly: a Taiwanese urban area survey. Aust N Z J Psychiatry. 2004;38:706–13. doi: 10.1080/j.1440-1614.2004.01444.x. [DOI] [PubMed] [Google Scholar]
- 36.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Sleep duration associated with mortality in elderly, but not middle-aged, adults in a large US sample. Sleep. 2008;31:1087–96. [PMC free article] [PubMed] [Google Scholar]
- 37.Youngstedt SD, Kripke DF. Long sleep and mortality: rationale for sleep restriction. Sleep Med Rev. 2004;8:159–74. doi: 10.1016/j.smrv.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Lan TY, Lan TH, Wen CP, Lin YH, Chuang YL. Nighttime sleep, Chinese afternoon nap, and mortality in the elderly. Sleep. 2007;30:1105–10. doi: 10.1093/sleep/30.9.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chien KL, Chen PC, Hsu HC, et al. Habitual sleep duration and insomnia and the risk of cardiovascular events and all-cause death: report from a community-based cohort. Sleep. 2010;33:177–84. doi: 10.1093/sleep/33.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teno JM, Weitzen S, Fennell ML, Mor V. Dying trajectory in the last year of life: does cancer trajectory fit other diseases? J Palliat Med. 2001;4:457–64. doi: 10.1089/109662101753381593. [DOI] [PubMed] [Google Scholar]
- 41.Baker DW, Gazmararian JA, Williams MV, et al. Functional health literacy and the risk of hospital admission among Medicare managed care enrollees. Am J Public Health. 2002;92:1278–83. doi: 10.2105/ajph.92.8.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel SR, Ayas NT, Malhotra MR, et al. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27:440–4. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
- 43.Castro-Costa É, Dewey ME, Ferri CP, et al. Association between sleep duration and all-cause mortality in old age: 9-year follow-up of the Bambuí Cohort Study. Brazil J Sleep Res. 2011;20:303–10. doi: 10.1111/j.1365-2869.2010.00884.x. [DOI] [PubMed] [Google Scholar]
- 44.Tamakoshi A, Ohno Y. Self-reported sleep duration as a predictor of all-cause mortality: results from the JACC study, Japan. Sleep. 2004;27:51–4. [PubMed] [Google Scholar]
- 45.Stewart R, Besset A, Bebbington P, et al. Insomnia comorbidity and impact and hypnotic use by age group in a national survey population aged 16 to 74 years. Sleep. 2006;29:1391–7. doi: 10.1093/sleep/29.11.1391. [DOI] [PubMed] [Google Scholar]
- 46.Ancoli-Israel S, Alessi C. Sleep and aging. Am J Geriatr Psychiatry. 2005;13:341–3. doi: 10.1176/appi.ajgp.13.5.341. [DOI] [PubMed] [Google Scholar]
- 47.Morgan K, Clarke D. Longitudinal trends in late-life insomnia: implications for prescribing. Age Ageing. 1997;26:179–84. doi: 10.1093/ageing/26.3.179. [DOI] [PubMed] [Google Scholar]
- 48.Kaplan GA, Seeman TE, Cohen RD, Knudsen LP, Guralnik J. Mortality among the elderly in the Alameda County Study: behavioral and demographic risk factors. Am J Public Health. 1987;77:307–12. doi: 10.2105/ajph.77.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kripke DF, Langer RD, Kline LE. Do no harm: not even to some degree. J Clin Sleep Med. 2012;8:353–4. doi: 10.5664/jcsm.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bianchi MT, Thomas RJ, Ellenbogen JM. Hypnotics and mortality risk. J Clin Sleep Med. 2012;8:351–2. doi: 10.5664/jcsm.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ancoli-Israel S, Ayalon L. Diagnosis and treatment of sleep disorders in older adults. Am J Geriatr Psychiatry. 2006;14:95–103. doi: 10.1097/01.JGP.0000196627.12010.d1. [DOI] [PubMed] [Google Scholar]
- 52.Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 53.Guilleminault C. Benzodiazepines, breathing, and sleep. Am J Med. 1990;88:25S–8S. doi: 10.1016/0002-9343(90)90282-i. [DOI] [PubMed] [Google Scholar]
- 54.Murciano D, Armengaud MH, Cramer PH, et al. Acute effects of zolpidem, triazolam and flunitrazepam on arterial blood gases and control of breathing in severe COPD. Eur Respir J. 1993;6:625–9. [PubMed] [Google Scholar]
- 55.Ranlov PJ, Nielsen SP. Effect of zopiclone and diazepam on ventilatory response in normal human subjects. Sleep. 1987;10(Suppl 1):40–7. doi: 10.1093/sleep/10.suppl_1.40. [DOI] [PubMed] [Google Scholar]
- 56.George CFP, Bayliff CD. Management of insomnia in patients with chronic obstructive pulmonary disease. Drugs. 2003;63:379–87. doi: 10.2165/00003495-200363040-00004. [DOI] [PubMed] [Google Scholar]
- 57.Beaumont M, Goldenberg F, Lejeune D, Marotte H, Harf A, Lofaso F. Effect of zolpidem on sleep and ventilatory patterns at simulated altitude of 4,000 meters. Am J Respir Crit Care Med. 1996;153:1864–9. doi: 10.1164/ajrccm.153.6.8665047. [DOI] [PubMed] [Google Scholar]
- 58.Beaumont M, Batejat D, Coste O, et al. Effects of zolpidem and zaleplon on sleep, respiratory patterns and performance at a simulated altitude of 4,000 m. Neuropsychobiology. 2004;49:154–62. doi: 10.1159/000076723. [DOI] [PubMed] [Google Scholar]
- 59.Coyle MA, Mendelson WB, Derchak PA, James SP, Wilson MG. Ventilatory safety of zaleplon during sleep in patients with obstructive sleep apnea on continuous positive airway pressure. J Clin Sleep Med. 2005;1:97. [PubMed] [Google Scholar]
- 60.Ahmad R, Bath PA. Identification of risk factors for 15-year mortality among community-dwelling older people using Cox regression and a genetic algorithm. J Gerontol A Biol Sci Med Sci. 2005;60:1052–8. doi: 10.1093/gerona/60.8.1052. [DOI] [PubMed] [Google Scholar]
- 61.Hays JC, Blazer DG, Foley DJ. Risk of napping: excessive daytime sleep-iness and mortality in an older community population. J Am Geriatr Soc. 1996;44:693–8. doi: 10.1111/j.1532-5415.1996.tb01834.x. [DOI] [PubMed] [Google Scholar]
- 62.Kojima M, Wakai K, Kawamura T, et al. Sleep patterns and total mortality: a 12-year follow-up study in Japan. J Epidemiol. 2000;10:87–93. doi: 10.2188/jea.10.87. [DOI] [PubMed] [Google Scholar]
- 63.Merlo J, Ostergren PO, Mansson NO, et al. Mortality in elderly men with low psychosocial coping resources using anxiolytic-hypnotic drugs. Scand J Public Health. 2000;28:294–7. [PubMed] [Google Scholar]
- 64.Hausken AM, Skurtveit S, Tverdal A. Use of anxiolytic or hypnotic drugs and total mortality in a general middle-aged population. Pharmacoepidemiol Drug Saf. 2007;16:913–8. doi: 10.1002/pds.1417. [DOI] [PubMed] [Google Scholar]
- 65.Kripke DF, Langer RD, Kline LE. Hypnotics' association with mortality or cancer: a matched cohort study. BMJ Open. 2012;2:e000850. doi: 10.1136/bmjopen-2012-000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krueger JM, Majde JA. Humoral links between sleep and the immune system: research issues. Ann N Y Acad Sci. 2003;992:9–20. doi: 10.1111/j.1749-6632.2003.tb03133.x. [DOI] [PubMed] [Google Scholar]
- 67.Krueger JM, Obal FJ, Fang J, Kubota T, Taishi P. The role of cytokines in physiological sleep regulation. Ann N Y Acad Sci. 2001;933:211–21. doi: 10.1111/j.1749-6632.2001.tb05826.x. [DOI] [PubMed] [Google Scholar]
- 68.Haffner SM. The metabolic syndrome: inflammation, diabetes mellitus, and cardiovascular disease. Am J Cardiol. 2006;97:3A–11A. doi: 10.1016/j.amjcard.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 69.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 70.Kraemer HC, Stice E, Kazdin A, Offord D, Kupfer D. How do risk factors work together? Mediators, moderators, and independent, overlapping, and proxy risk factors. Am J Psychiatry. 2001;158:848–56. doi: 10.1176/appi.ajp.158.6.848. [DOI] [PubMed] [Google Scholar]
- 71.Perlis ML, Smith LJ, Lyness JM, et al. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med. 2006;4:104–13. doi: 10.1207/s15402010bsm0402_3. [DOI] [PubMed] [Google Scholar]
- 72.Riemann D, Voderholzer U. Primary insomnia: a risk factor to develop depression? J Affect Disord. 2003;76:255–9. doi: 10.1016/s0165-0327(02)00072-1. [DOI] [PubMed] [Google Scholar]
- 73.Thase ME. Correlates and consequences of chronic insomnia. Gen Hosp Psychiatry. 2005;27:100–12. doi: 10.1016/j.genhosppsych.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 74.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 75.Chou YC. Taipei, Taiwan: National Yang-Ming University; 2006. Agreement between survey and claims data of chronic disease in Taiwan [Master] [Google Scholar]


