Abstract
Introduction:
The obstructive sleep apnea syndrome (OSAS) is associated with increased visceral adipose tissue (VAT) in adults; however, few studies have evaluated VAT in relation to upper airway function in adolescents. We hypothesized that increased neck circumference (NC) and VAT would be associated with increased upper airway collapsibility.
Methods:
Adolescents (24 obese patients with OSAS, 22 obese control patients, and 29 lean control patients) underwent abdominal magnetic resonance imaging, and measurement of upper airway pressure-flow relationships in the activated and hypotonic upper airway states.
Results:
Patients with OSAS had a greater activated slope of the pressure-flow relationship (SPF) than control groups (P < 0.001), whereas hypotonic SPF was greater in both obese groups compared with lean control patients (P = 0.01). NC and VAT were greater in obese control patients and those with OSAS than in lean control patients (P < 0.001), but did not differ between obese patients with OSAS and obese control patients. In lean control patients and those with OSAS, increased NC was associated with increased activated SPF, whereas in obese control patients it was associated with decreased activated SPF (P = 0.03). In contrast, increased NC was associated with increased hypotonic SPF in all groups (P < 0.001). There was no significant effect of VAT on either activated or hypotonic SPF for any of the three groups.
Conclusions:
Increased neck circumference was associated with increased upper airway collapsibility in adolescents in the hypotonic but not activated state. These data suggest that obese adolescents without OSAS, despite a narrowed upper airway from adipose tissue, are protected from developing OSAS by upper airway neuromotor activation. Neither neck circumference nor visceral adipose tissue is useful in predicting upper airway collapsibility in obese adolescents.
Citation:
Yuan H; Schwab RJ; Kim C; He J; Shults J; Bradford R; Huang J; Marcus CL. Relationship between body fat distribution and upper airway dynamic function during sleep in adolescents. SLEEP 2013;36(8):1199-1207.
Keywords: Adipose tissue, pressure-flow relationship
INTRODUCTION
In adults, most cases of obstructive sleep apnea syndrome (OSAS) are associated with obesity. Obesity can contribute to OSAS in a number of ways, including mechanical compression of the upper airway by extrinsic fat deposited in the neck or intrinsic fat infiltrating into the upper airway structures,1 fat deposition in the abdomen leading to thoracic restriction and changes in upper airway length,2 and central hypoventilation. In particular, visceral fat has been shown to contribute to OSAS more than subcutaneous fat.3
In previous decades, pediatric OSAS was associated primarily with adenotonsillar hypertrophy rather than obesity, and failure to thrive was a common presentation.4 However, obesity is becoming more of an issue in the pathogenesis of pediatric OSAS. The prevalence of childhood obesity has increased dramatically, such that 17% of adolescents in the United States are currently obese.5 Obesity increases the risk of OSAS throughout life, from infancy through adulthood.6–10 However, the causal association between obesity and OSAS in the pediatric population is not well understood.11 In particular, little is known about the relationship between obesity and OSAS during the important developmental transition phase of adolescence. Several recent studies have shown an association between visceral fat and OSAS in pediatric patients.12–14 However, pediatric studies to date have focused on polysomnographically evident obstruction, and no studies have evaluated the relationship between fat distribution and functional upper airway measurements. Furthermore, no pediatric studies have included the entire spectrum of body size, including studies of lean patients. We therefore evaluated the relationship between upper airway dynamic function during sleep and body fat distribution in a sample of adolescents with varying degrees of obesity and OSAS. We hypothesized that adolescents with increased abdominal visceral fat would have a greater tendency for upper airway collapse. An advantage of measuring upper airway dynamics rather than simply the apnea-hypopnea index (AHI) is that this technique allows evaluation of the entire spectrum of upper airway collapsibility, whereas the AHI characterizes the upper airway only above the threshold for airway collapse.
METHODS
Adolescents with OSAS, age 12-16 y, were recruited from the Sleep Center at Children's Hospital of Philadelphia, and nonsnoring adolescents were recruited from the general population by means of advertisements, as part of a larger study evaluating the pathophysiology of OSAS.15 The study was approved by the Institutional Review Board of Children's Hospital of Philadelphia. Written informed consent was obtained from parents/ guardians, and assent from adolescents. Adolescents with a history of adenotonsillectomy were ineligible. Obesity was defined as a body mass index (BMI) > 95th percentile for age and sex, or > 30 kg/m2.16 To limit overlap between groups, patients with OSAS were included only if they had an AHI ≥ 5/h, and control patients were included if they had an AHI < 1.5/h.17–20 Neck circumference (NC) was measured at the level of the cricothyroid cartilage in the Frankfort horizontal plane. Patients underwent standard baseline polysomnography,21,22 followed by a separate polysomnogram with pressure-flow measurements, and abdominal magnetic resonance imaging (MRI). Tanner pubertal staging was performed using a validated self-assessment form.23
Upper Airway Pressure-Flow Measurements during Sleep
In addition to routine polysomnographic measurements, the patient wore a mask (Philips Respironics, Murrysville, PA) attached to a heated pneumotachometer (Hans Rudolph, Inc., Kansas City, MO) and transducer (Validyne Engineering Corp., Northridge, CA). Nasal pressure (PN) was measured at the mask, using a differential pressure transducer referenced to atmosphere. PN was altered in either a positive or subatmo-spheric direction, using a device provided by Philips Respironics. A toggle switch allowed the patient to be switched rapidly between positive and negative pressure, ranging from -25 to +25 cm H2O. Measurements were performed during nonrapid eye movement (NREM) sleep.24
Activated Technique
Patients slept while receiving a level of PN sufficient to abolish inspiratory airflow limitation (the holding pressure). PN was then lowered in 2 cm H2O decrements every 5 breaths until flow approached zero or an arousal occurred. This slow, stepwise protocol allowed for recruitment of upper airway reflexes in response to subatmospheric pressure, and resulted in a neuromuscularly activated airway.25,26
Hypotonic Technique
PN was decreased abruptly from the holding pressure by 2 cm H2 O for five breaths, following which it was rapidly returned to the holding pressure. PN was dropped repeatedly to incrementally lower levels (by 2 cm H2O each drop), with a return each time to the holding pressure, until either flow approached zero or arousal occurred. Previous studies have shown that it takes several breaths at subatmospheric pressure before the upper airway reflexes are activated,26–28 thus analyzing only the first three breaths provides data on a relatively hypotonic upper airway.
Analysis
For the activated runs, the average midinspiratory flow was taken from the lowest two consecutive breaths at each level of pressure. For hypotonic runs, data were taken from the first three breaths after the pressure drop. Pressure-flow curves were constructed by plotting maximal inspiratory airflow (VImax) against PN. PN versus VImax curves were fitted by least squares linear regression, and the slope of the pressure-flow curve (SPF) and the X-axis intercept, i.e., the critical closing pressure (Pcrit) where VImax = 0, were determined. As many pediatric patients are able to maintain airflow even at markedly subatmospheric pressures, the X-intercept cannot always be determined without extreme extrapolation. Therefore, a threshold value of -25 cm H2O (the lowest PN deliverable by our equipment) was assigned to Pcrit data that were extrapolated to < -25 cm H2O,26,29 and SPF was used as the primary parameter to characterize the upper airway response.26,30
Abdominal MRI
Patients underwent abdominal MRI using a 1.5 T scanner (Siemens Avanto, Germany) with a body coil. Axial images were acquired with a magnetic resonance gradient echo pulse sequence. The abdominal compartment was defined as extending from the superior aspect of the xiphoid process to the most inferior slice depicting the L5-S1 interspace. Magnetic resonance images were obtained in 1-cm contiguous intervals (slice thickness of 1 cm) throughout the abdominal compartment. Analysis was performed using image analysis software (Amira 4.1.2, Visage Imaging Inc., Andover, MA). Each MRI slice was manually examined. Anatomical landmarks were identified and adipose tissue beds were labeled and segmented into either subcutaneous adipose tissue (SAT) or visceral adipose tissue (VAT) (Figure 1). The adipose areas from each slice were then summed across the entire abdominal compartment and total SAT and VAT volumes were calculated.13,31
Figure 1.
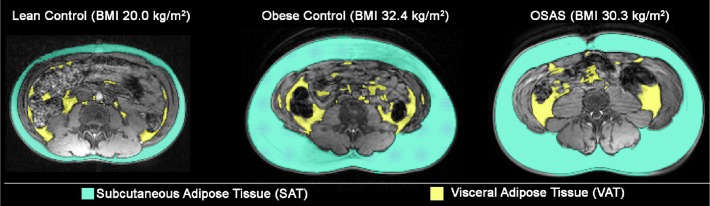
Examples of MRI images showing fat distribution. Representative examples of abdominal MRIs from a male lean control patient, male obese control patient, and male patient with obstructive sleep apnea syndrome (OSAS) are shown. Subcutaneous adipose tissue is shown in blue and visceral adipose tissue in yellow.
Statistical Analysis
Data are presented as mean ± standard deviation unless otherwise stated. A P value < 0.05 was considered significant. Statistical analyses were conducted by SPSS version 17.0 (SPSS, Inc., Chicago, IL). Group differences in demographics and polysomnography results were determined using chi-square analysis for categorical variables. One-way analysis of variance (ANOVA) was used to compare means of continuous variables between the OSAS, lean control, and obese control groups. If ANOVA identified a significant difference between the three groups, Bonferroni multiple comparison tests were then applied to identify significant pairwise differences in group means. Correction of VAT and NC for height and height2 was assessed by conducting ANOVA (with subsequent Bonferroni corrected pairwise comparisons) for VAT and NC divided by height, and VAT and NC divided by height.2 Spearman correlation coefficients were calculated to estimate association between continuous variables. Correlations between pressure-flow measurements and anthropometric measurements were estimated within each group to assess relationships and identify potential interactions. A linear regression model was constructed for the primary outcome (activated slope of the pressure-flow curve) with an indicator for subject group (OSAS and obese control groups versus the reference group of lean control patients) and parameter of interest (NC, VAT, or SAT) as predictors. Regression models for secondary outcomes such as Pcrit were also fitted. Significant interactions (OSAS × neck circumference and obese control × neck circumference) (P < 0.05) were included in the final regression model; the significant interaction terms indicated that the association between activated slope and NC differed between groups. The final model was also modified to include sex. The assumptions of linear regression were assessed using standard tests and graphical displays; these included the construction of quantile-quantile plots to assess the normality of the residuals.
RESULTS
Study Population
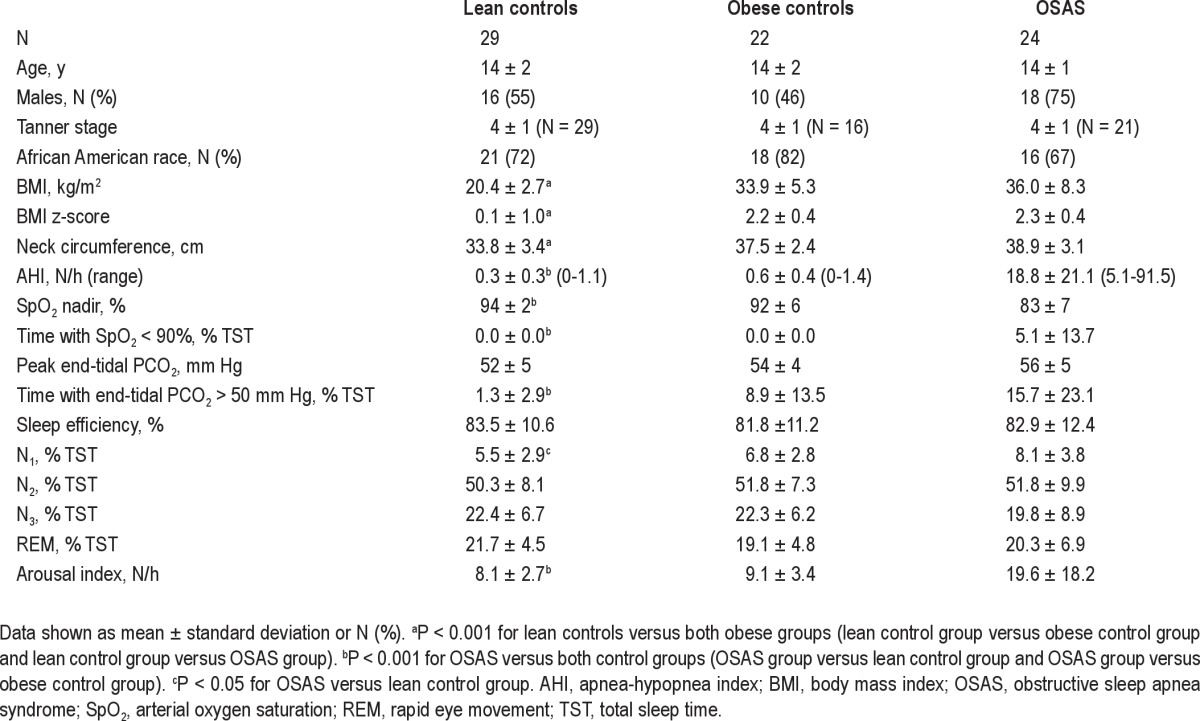
Seventy-five patients (24 with OSAS, 29 lean control patients, and 22 obese control patients) were recruited. Study group characteristics are shown in Table 1. Patients ranged from very thin to morbidly obese, with weight ranging from 36.8 to 151.8 kg, and BMI z-scores ranging from -3.45 to 3.16. By design, the obese OSAS and obese control patients had a significantly higher BMI than the lean control patients, and the OSAS group had a significantly higher AHI than either control group. No control patient had significant desaturation (the highest percentage of total sleep time with SpO2 < 90% in control patients was 0.1%). Three obese control patients had hypoventilation (> 25% of total sleep time with endtidal PCO2 > 50 mm Hg), with a range of 0-48.5% total sleep time in the obese control group.
Table 1.
Demographic and polysomnographic data
Pressure-Flow Measurements
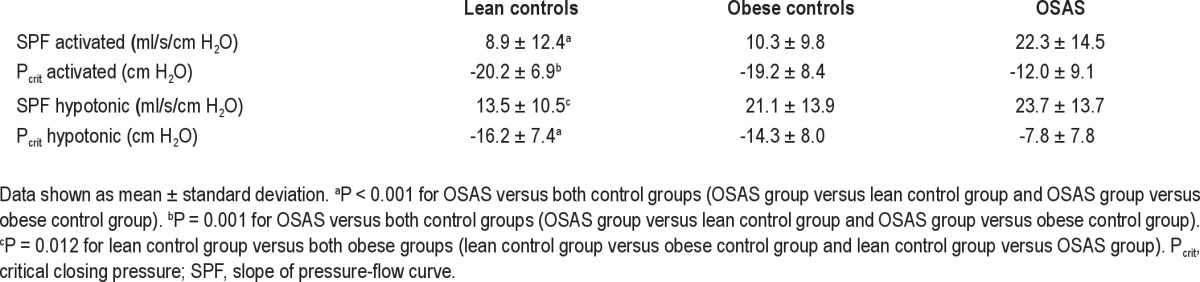
Pressure-flow measurement results are shown in Table 2. Forty-three percent of all control patients and 21% of patients with OSAS had extrapolated activated Pcrit measures that were < -25 cm H2O (P = 0.007). In the activated condition, SPF was higher in patients with OSAS compared with both obese and lean control patients, indicating a more collapsible upper airway in the OSAS group; whereas in the hypotonic condition, the SPF was higher in both obese groups (patients with OSAS and obese control patients) compared with lean control patients, indicating a more collapsible upper airway in the obese groups than the lean patients. Pcrit values were higher in the OSAS group compared with the two control groups in both the activated and hypotonic conditions.
Table 2.
Pressure-flow measurements during sleep
Adipose Tissue as Measured by MRI
The volumes of visceral, subcutaneous, and total adipose tissue were all significantly greater in the OSAS and obese control groups compared to the lean control patients (Table 3). However, there were no differences in any of these volumes between the two obese groups (OSAS and obese control). Similarly, when corrected for height or height2, VAT remained greater in the OSAS and obese control groups compared to the lean control patients (all P < 0.0005), but did not differ between the obese control and obese OSAS groups. There were similar findings for NC corrected for height or height2 (all P < 0.0005).
Table 3.
Volume of adipose tissue by magnetic resonance imaging

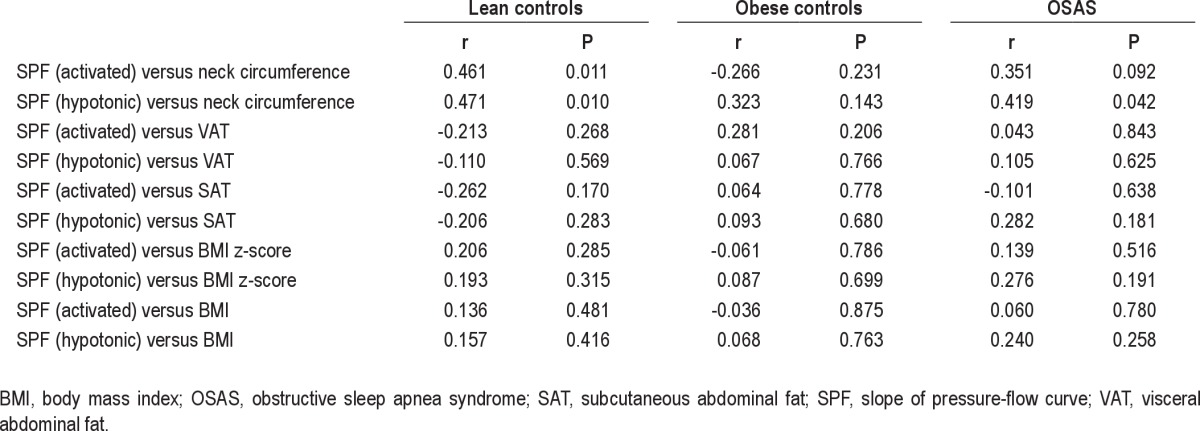
Relationship between Measurements of Adiposity and Upper Airway Dynamic Function during Sleep
Primary data for the relationships between measurements of adiposity and upper airway dynamic function during sleep are shown in Figures 2 through 4 and Table 4.
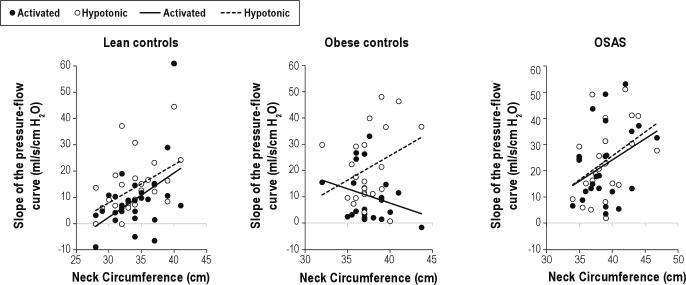
Figure 2.
The relationship between neck circumference (NC) and the slope of the pressure flow relationship (SPF) is shown for the three groups. In the lean control patients and the OSAS group, increased NC was associated with increased activated SPF, whereas in obese control patients it was associated with decreased activated SPF. In contrast, increased NC was associated with increased hypotonic SPF in all three groups.
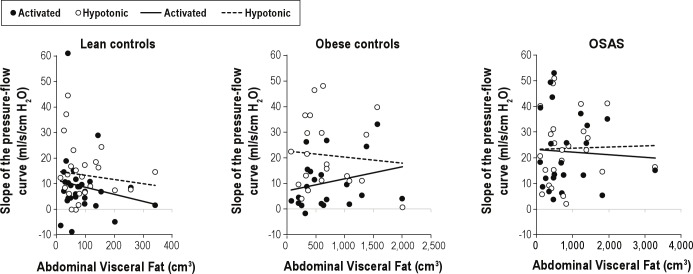
Figure 3.
The relationship between visceral adipose tissue (VAT) and the slope of the pressure flow relationship (SPF) is shown for the three groups. There was no significant association between SPF and VAT for any group in either the activated or hypotonic condition.
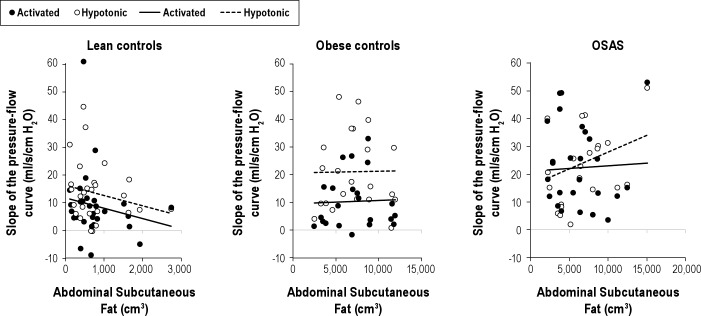
Figure 4.
The relationship between subcutaneous adipose tissue (SAT) and the slope of the pressure flow relationship (SPF) is shown for the three groups. There was no significant association between SPF and SAT for any group in either the activated or hypotonic condition.
Table 4.
Correlations between pressure-flow measurements and anthropometric measurements for the three groups
Relationship between NC and Upper Airway Function
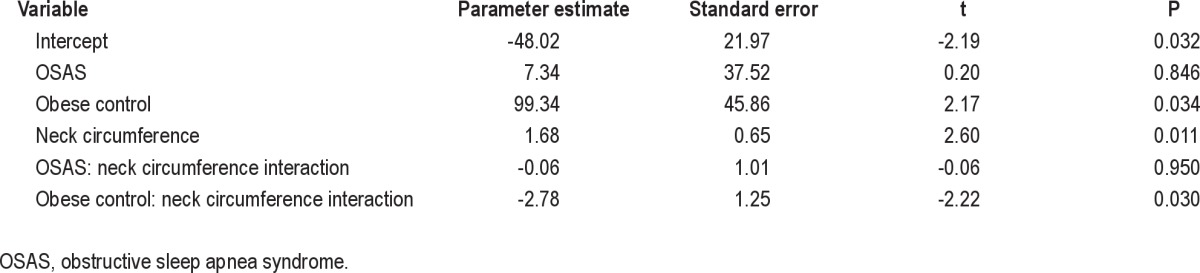
A significant interaction between NC and SPF in the activated state was found (Table 5). However, this interaction differed by group (Figure 2). Lean control patients and those with OSAS both showed a positive correlation between NC and activated SPF, such that a 1-unit increase in NC was associated with a 1.68- and 1.62-unit increase in activated slope, respectively. In contrast, obese control patients showed an inverse relationship, with a 1-unit increase in NC being associated with a 1.09-unit reduction in activated slope. A similar relationship was noted for activated Pcrit: in lean control patients and those with OSAS, a 1-unit increase in NC was associated with 0.51 and 0.85 unit increases in Pcrit, respectively. Among obese control patients, a 1 unit increase in NC was associated with a 1.57 unit reduction in Pcrit.
Table 5.
Linear regression examining the effects of group (with lean controls as the reference) and neck circumference on the activated slope of the pressure-flow curve
The relationship between NC and hypotonic pressure-flow relationships was evaluated (Figure 2). In all three groups, increasing NC was associated with increasing SPF in the hypotonic condition (P < 0.001). No significant differences were found between the three groups. A similar association between NC and hypotonic Pcrit was found, with no differences between the three groups (P = 0.033).
Relationship between Adipose Tissue on MRI and Upper Airway Function
There were no significant relationships between the volume of visceral fat and activated SPF, activated Pcrit, hypotonic SPF, or hypotonic Pcrit. Similarly, there was no relationship between visceral fat as a percentage of total fat and any of the outcomes (data not shown). The effects of subcutaneous fat and total fat were also evaluated. As with visceral fat, the effect of these predictors on activated SPF and Pcrit or hypotonic SPF and Pcrit were not significant (Figures 3 and 4).
Effect of Sex
There were more males in the OSAS group than in the control groups. Although this difference was not significant, the effect of sex on the correlations between upper airway dynamics and NC and abdominal adipose tissue was examined. When the regression model was adjusted for male sex, the overall results did not change.
Effect of OSAS Severity
The correlations between the AHI and other parameters were evaluated for the OSAS group only (as control patients all had an AHI < 1.5/h). In the activated condition, AHI correlated with SPF (r = 0.69, P < 0.001) and there was a trend for a correlation with Pcrit (r = 0.38, P = 0.07). There were no signifi cant correlations in the hypotonic condition (SPF: r = 0.23, P = 0.28; Pcrit: r = 0.28, P = 0.19). There were no signifi cant correlations between AHI and NC (r = 0.05, P = 0.83), VAT (r = 0.30, P = 0.16), or SAT (r = 0.02, P = 0.92). However, the distribution of VAT differed signifi cantly when patients with OSAS were categorized as having mild to moderate (AHI 5-15/h, n = 17) versus severe OSAS (AHI > 15/h, n = 7). Patients with severe OSAS had a signifi cantly higher VAT (1,327 + 1,055 versus 648 ± 466 cm3, P = 0.037). There were no signifi cant differences in NC (P = 0.449), SAT (P = 0.383), or total abdominal fat (P = 0.237) between the mild to moderate OSAS and severe OSAS groups.
DISCUSSION
In summary, this study has shown that neither NC nor VAT differed between obese patients with OSAS and BMI-matched obese control patients. Under activated upper airway conditions, when upper airway dilatory muscles were active, upper airway collapsibility correlated with NC in lean patients and those with OSAS, but an inverse relationship was observed in obese control patients. In contrast, under hypotonic conditions, when minimal upper airway muscle activity was present, upper airway collapsibility correlated with NC in all patient groups including obese control patients. This finding is consistent with published data showing that some obese adolescents are protected from OSAS by the presence of intact upper airway reflexes during sleep.15 However, VAT did not correlate with SPF in either the activated or hypotonic state in any of the three groups, except for at the most severe range of OSAS. Unique aspects of this study were the inclusion of patients across the entire spectrum of adiposity including lean patients, recruitment of control patients from an asymptomatic community sample rather than a clinic-based sample, and the use of functional upper airway measurements rather than the AHI alone, allowing for detection of more subtle deficits in upper airway function during sleep.
Measurement of Upper Airway Function
The technique used for measuring upper airway pressure-flow relationships is a noninvasive means of evaluating upper airway function during sleep.32 Upper airway patency is affected by mechanical and structural factors as well as neural mechanisms. The techniques used provide a useful tool for the comprehensive evaluation of upper airway function,33 with the hypotonic technique primarily evaluating structural factors (such as obesity) that promote airway collapsibility and the activated technique evaluating the combined effect of structural and neuromuscular factors. The AHI is defined as the number of apneic and hypopneic events occurring per h during sleep, and is used to diagnose and evaluate the severity of OSAS. In contrast with AHI, the technique of measuring the pressure-flow relationship allows the evaluation of the full spectrum of upper airway function, including patients without overt upper airway obstruction.
In this study, SPF was used rather than Pcrit to characterize upper airway collapsibility. Normal children and adolescents have an upper airway that is resistant to collapse; thus, previous studies in the pediatric population have found a very flat SPF, such that Pcrit cannot be determined without extreme extrapolation.26,29,30,34–36 Because the extreme extrapolation cannot be relied upon to produce a physiologically valid value, studies in the literature have used a value of -25 cm H2O to represent Pcrit when the extrapolated Pcrit is < -25 cm H2O, as -25 cm H2O is the lowest pressure the equipment provides.26,29,30,34–36 However, this results in a floor effect. Therefore, we have used SPF rather than Pcrit as the primary means to characterize upper airway function.
Adolescence
Adolescence is a transitional stage from childhood to adult-hood and is associated with many changes in sleep, including major changes in sleep state organization and circadian rhythm.37 The etiology of OSAS is multifactorial, especially in adolescents, and includes neuromuscular and anatomic factors. Thus, the association between obesity and OSAS in adolescents may be affected by many factors such as reduction in upper airway tone with age,26,33,36 differences in lymphoid tissue and in airway size,13 and developmental changes such as sex hormone secretion and differences in fat deposition. Although many of these factors will be different between children and adolescents, most pediatric studies have included both school-aged children and adolescents. The distinctive characteristics described in this paragraph warrant the study of adolescents as a separate group.
Obesity, Body Fat Distribution, and OSAS in Adolescents
In adults, there is a clear association between obesity and OSAS. 6 In the pediatric population, the association does not appear to be as robust. There appears to be a discrepancy in the strength of the association between older versus younger children. One study showed a correlation between BMI z-score and AHI in adolescents 12 y of age or older, but not in younger children.38 However, few studies have evaluated adolescents as a separate group, despite the importance of this developmental stage.
Body fat stores are typically partitioned into visceral fat and subcutaneous fat. Visceral fat has been shown to be more closely associated with metabolic complications of obesity, including insulin resistance, cardiovascular disease and diabetes.3,39 In adults, VAT has been associated with OSAS.3 However, few studies have evaluated the relationship between OSAS and VAT in the pediatric population.12–14 In the largest study, Arens et al.13 found increased VAT in children and adolescents with OSAS compared with control patients, but no correlation between AHI and VAT. Hannon et al.12 similarly found increased VAT in adolescents with OSAS compared with control patients, but also found a correlation between AHI and VAT. In a small study of children and adolescents undergoing MRI, Canapari et al. 14 did not find a significant difference in VAT between patients with OSAS and control patients, but did show a relationship between VAT and AHI. In the current study, there was no relationship between VAT and either the presence of OSAS or the AHI. The discrepancy between studies may be due to a number of issues. The other studies in the literature included only obese patients compared with the range of lean and obese patients, and most included a mixture of school-aged children and adolescents.
Interaction between Structural and Neuromotor Factors and OSAS in Adolescents
This study showed a correlation between NC and hypotonic SPF in all patients, but a divergence in the relationship between NC and activated SPF among the three groups (Figure 2). Although the lean control patients and the obese OSAS adolescents both had a correlation between NC and activated SPF, the obese control patients showed an inverse response— the greater their NC, the flatter their SPF. In other studies in the same cohort, we have shown that obese adolescents with-out OSAS have increased genioglossal activity in response to subatmospheric pressure during sleep when compared with obese adolescents with OSAS or lean control patients.15 Thus, these studies indicate that obese control patients increase upper airway neuromuscular activity during sleep to counteract collapsibility caused by anatomical narrowing from adipose tissue. This finding is consistent with previous data showing that young children have active upper airway reflexes during sleep, and that these reflexes decrease during adolescence,26,30,36 albeit with significant individual variability.36 Thus, when a wide range of adolescents are studied, including those without any symptoms of snoring or sleep disordered breathing, neuromotor factors appear to play a role in addition to structural factors, and it is therefore not surprising that a clear-cut relationship between measures of adiposity and obstructive apnea cannot be demonstrated. This may be different in adults, in whom upper airway reflexes during sleep are much diminished.26,30
Other anatomical factors, such as adenotonsillar hypertro-phy, play a role in the etiology of OSAS in adolescents. Further studies are needed to explore the interaction between adiposity, lymphoid tissue, and upper airway collapsibility.
Limitations
The sample sizes of 29, 22, and 24 in the lean control, obese control, and OSAS groups, respectively, were relatively small; smaller sample sizes result in increased likelihood of type I and type II errors. Future studies with larger sample sizes are needed to confirm the findings of this study.
CONCLUSIONS
In conclusion, this study suggests that neck size, presumably primarily reflecting neck adipose tissue, plays a role in the pathophysiology of OSAS in adolescents, but that abdominal visceral fat is not a major contributor to OSAS in this age group except, perhaps, at the most extreme end of the OSAS spectrum. Furthermore, neuromotor factors also play an important role in the etiology of OSAS in this age group. These data do not support routine measurement of either neck size or visceral fat in adolescents with suspected OSAS, as these measurements cannot predict OSAS without taking into consideration neuromotor factors during sleep.
DISCLOSURE STATEMENT
Philips Respironics, Inc. provided the airway pressure device.
ACKNOWLEDGMENTS
The authors thank all of the Children's Hospital of Philadelphia sleep laboratory technologists who helped conduct this study. We are grateful to the children and their families for their enthusiastic participation in this study. This study was supported by NIH grants U54-RR023567, R01-HL58585, R01HL089447, and P01HL094307. Dr. Yuan is a recipient of the state scholarship from the China Scholarship Council.
REFERENCES
- 1.Horner RL, Mohiaddin RH, Lowell DG, et al. Sites and sizes of fat deposits around the pharynx in obese patients with obstructive sleep apnoea and weight matched controls. Eur Respir J. 1989;2:613–22. [PubMed] [Google Scholar]
- 2.Stanchina ML, Malhotra A, Fogel RB, et al. The influence of lung volume on pharyngeal mechanics, collapsibility, and genioglossus muscle activation during sleep. Sleep. 2003;26:851–6. doi: 10.1093/sleep/26.7.851. [DOI] [PubMed] [Google Scholar]
- 3.Shinohara E, Kihara S, Yamashita S, et al. Visceral fat accumulation as an important risk factor for obstructive sleep apnoea syndrome in obese subjects. J Intern Med. 1997;241:11–8. doi: 10.1046/j.1365-2796.1997.63889000.x. [DOI] [PubMed] [Google Scholar]
- 4.Brouillette RT, Fernbach SK, Hunt CE. Obstructive sleep apnea in infants and children. J Pediatr. 1982;100:31–40. doi: 10.1016/s0022-3476(82)80231-x. [DOI] [PubMed] [Google Scholar]
- 5.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 6.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015–21. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 7.Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children. Associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159:1527–32. doi: 10.1164/ajrccm.159.5.9809079. [DOI] [PubMed] [Google Scholar]
- 8.Marcus CL, Curtis S, Koerner CB, Joffe A, Serwint JR, Loughlin GM. Evaluation of pulmonary function and polysomnography in obese children and adolescents. Pediatr Pulmonol. 1996;21:176–83. doi: 10.1002/(SICI)1099-0496(199603)21:3<176::AID-PPUL5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 9.Chay OM, Goh A, Abisheganaden J, et al. Obstructive sleep apnea syndrome in obese Singapore children. Pediatr Pulmonol. 2000;29:284–90. doi: 10.1002/(sici)1099-0496(200004)29:4<284::aid-ppul8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 10.Kahn A, Mozin MJ, Rebuffat E, et al. Sleep pattern alterations and brief airway obstructions in overweight infants. Sleep. 1989;12:430–8. doi: 10.1093/sleep/12.5.430. [DOI] [PubMed] [Google Scholar]
- 11.Verhulst SL, Schrauwen N, Haentjens D, et al. Sleep-disordered breathing in overweight and obese children and adolescents: prevalence, characteristics and the role of fat distribution. Arch Dis Child. 2007;92:205–8. doi: 10.1136/adc.2006.101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hannon TS, Lee S, Chakravorty S, Lin Y, Arslanian SA. Sleep-disordered breathing in obese adolescents is associated with visceral adiposity and markers of insulin resistance. Int J Pediatr Obes. 2011;6:157–60. doi: 10.3109/17477166.2010.482156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arens R, Sin S, Nandalike K, et al. Upper airway structure and body fat composition in obese children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2011;183:782–7. doi: 10.1164/rccm.201008-1249OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canapari CA, Hoppin AG, Kinane TB, Thomas BJ, Torriani M, Katz ES. Relationship between sleep apnea, fat distribution, and insulin resistance in obese children. J Clin Sleep Med. 2011;7:268–73. doi: 10.5664/JCSM.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang J, Pinto SJ, Yuan H, et al. Upper airway collapsibility and genioglossus activity in adolescents during sleep. Sleep. 2012;35:1345–52. doi: 10.5665/sleep.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Himes JH, Dietz WH. Guidelines for overweight in adolescent preventive services: recommendations from an expert committee. The Expert Committee on Clinical Guidelines for Overweight in Adolescent Preventive Services. Am J Clin Nutr. 1994;59:307–16. doi: 10.1093/ajcn/59.2.307. [DOI] [PubMed] [Google Scholar]
- 17.Traeger N, Schultz B, Pollock AN, Mason T, Marcus CL, Arens R. Polysomnographic values in children 2-9 years old: additional data and review of the literature. Pediatr Pulmonol. 2005;40:22–30. doi: 10.1002/ppul.20236. [DOI] [PubMed] [Google Scholar]
- 18.Marcus CL, Omlin KJ, Basinki DJ, et al. Normal polysomnographic values for children and adolescents. Am Rev Respir Dis. 1992;146:1235–9. doi: 10.1164/ajrccm/146.5_Pt_1.1235. [DOI] [PubMed] [Google Scholar]
- 19.Uliel S, Tauman R, Greenfeld M, Sivan Y. Normal polysomnographic respiratory values in children and adolescents. Chest. 2004;125:872–8. doi: 10.1378/chest.125.3.872. [DOI] [PubMed] [Google Scholar]
- 20.Witmans MB, Keens TG, Davidson Ward SL, Marcus CL. Obstructive hypopneas in children and adolescents: normal values. Am J Respir Crit Care Med. 2003;168:1540. doi: 10.1164/ajrccm.168.12.954. [DOI] [PubMed] [Google Scholar]
- 21.Pinto S, Huang J, Tapia I, et al. Effects of race on upper airway dynamic function during sleep in children. Sleep. 2011;34:495–501. doi: 10.1093/sleep/34.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iber C, Ancoli-Israel S, Chesson A, Quan SF. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications. [Google Scholar]
- 23.Duke PM, Litt IF, Gross RT. Adolescents' self-assessment of sexual maturation. Pediatrics. 1980;66:918–20. [PubMed] [Google Scholar]
- 24.Huang J, Karamessinis LR, Pepe ME, et al. Upper airway collapsibility during REM sleep in children with the obstructive sleep apnea syndrome. Sleep. 2009;32:1173–81. doi: 10.1093/sleep/32.9.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horner RL. Motor control of the pharyngeal musculature and implications for the pathogenesis of obstructive sleep apnea. Sleep. 1996;19:827–53. doi: 10.1093/sleep/19.10.827. [DOI] [PubMed] [Google Scholar]
- 26.Marcus CL, Fernandes Do Prado LB, Lutz J, et al. Developmental changes in upper airway dynamics. J Appl Physiol. 2004;97:98–108. doi: 10.1152/japplphysiol.00462.2003. [DOI] [PubMed] [Google Scholar]
- 27.Katz ES, Marcus CL, White DP. Influence of airway pressure on genioglossus activity during sleep in normal children. Am J Respir Crit Care Med. 2006;173:902–9. doi: 10.1164/rccm.200509-1450OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwartz AR, O'Donnell CP, Baron J, et al. The hypotonic upper airway in obstructive sleep apnea: role of structures and neuromuscular activity. Am J Respir Crit Care Med. 1998;157:1051–7. doi: 10.1164/ajrccm.157.4.9706067. [DOI] [PubMed] [Google Scholar]
- 29.Marcus CL, Katz ES, Lutz J, Black CA, Galster P, Carson KA. Upper airway dynamic responses in children with the obstructive sleep apnea syndrome. Pediatr Res. 2005;57:99–107. doi: 10.1203/01.PDR.0000147565.74947.14. [DOI] [PubMed] [Google Scholar]
- 30.Marcus CL, Lutz J, Hamer A, Smith PL, Schwartz A. Developmental changes in response to subatmospheric pressure loading of the upper airway. J Appl Physiol. 1999;87:626–33. doi: 10.1152/jappl.1999.87.2.626. [DOI] [PubMed] [Google Scholar]
- 31.Arnardottir ES, Maislin G, Schwab RJ, et al. The interaction of obstructive sleep apnea and obesity on the inflammatory markers C-reactive protein and interleukin-6: the icelandic sleep apnea cohort. Sleep. 2012;35:921–32. doi: 10.5665/sleep.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith PL, Wise RA, Gold AR, Schwartz AR, Permutt S. Upper airway pressure-flow relationships in obstructive sleep apnea. J Appl Physiol. 1988;64:789–95. doi: 10.1152/jappl.1988.64.2.789. [DOI] [PubMed] [Google Scholar]
- 33.Arens R, Marcus CL. Pathophysiology of upper airway obstruction: a developmental perspective. Sleep. 2004;27:997–1019. doi: 10.1093/sleep/27.5.997. [DOI] [PubMed] [Google Scholar]
- 34.Marcus CL, McColley SA, Carroll JL, Loughlin GM, Smith PL, Schwartz AR. Upper airway collapsibility in children with obstructive sleep apnea syndrome. J Appl Physiol. 1994;77:918–24. doi: 10.1152/jappl.1994.77.2.918. [DOI] [PubMed] [Google Scholar]
- 35.Huang J, Pinto SJ, Allen JL, et al. Upper airway genioglossal activity in children with sickle cell disease. Sleep. 2011;34:773–8. doi: 10.5665/SLEEP.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bandla P, Huang J, Karamessinis L, et al. Puberty and upper airway dynamics during sleep. Sleep. 2008;31:534–41. doi: 10.1093/sleep/31.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007;8:602–12. doi: 10.1016/j.sleep.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Kohler MJ, Thormaehlen S, Kennedy JD, et al. Differences in the association between obesity and obstructive sleep apnea among children and adolescents. J Clin Sleep Med. 2009;5:506–11. [PMC free article] [PubMed] [Google Scholar]
- 39.Cornier MA, Despres JP, Davis N, et al. Assessing adiposity: a scientific statement from the american heart association. Circulation. 2011;124:1996–2019. doi: 10.1161/CIR.0b013e318233bc6a. [DOI] [PubMed] [Google Scholar]