Abstract
Study Objectives:
Light can induce an acute alerting response in humans; however, it is unknown whether the magnitude of this response is simply a function of the absolute illuminance of the light itself, or whether it depends on illuminance history preceding the stimulus. Here, we compared the effects of illuminance history on the alerting response to a subsequent light stimulus.
Design:
A randomized, crossover design was used to compare the effect of two illuminance histories (1 lux vs. 90 lux) on the alerting response to a 6.5-h 90-lux light stimulus during the biological night.
Setting:
Intensive Physiologic Monitoring Unit, Brigham and Women's Hospital, Boston, MA.
Participants:
Fourteen healthy young adults (6 F; 23.5 ± 2.9 years).
Interventions:
Participants were administered two 6.5-h light exposures (LE) of 90 lux during the biological night. For 3 days prior to each LE, participants were exposed to either 1 lux or 90 lux during the wake episode.
Measurements and Results:
The alerting response to light was assessed using subjective sleepiness ratings, lapses of attention, and reaction times as measured with an auditory psychomotor vigilance task, as well as power density in the delta/theta range of the waking EEG. The alerting response to light was greater and lasted longer when the LE followed exposure to 1 lux compared to 90 lux light.
Conclusion:
The magnitude and duration of the alerting effect of light at night depends on the illuminance history and appears to be subject to sensitization and adaptation.
Citation:
Chang AM; Scheer FAJL; Czeisler CA; Aeschbach D. Direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans depend on prior light history. SLEEP 2013;36(8):1239-1246.
Keywords: Light history, alertness and performance, light exposure
INTRODUCTION
The role of light as a primary synchronizer of the circadian timing system has been demonstrated in numerous animal and human studies. Much is known about the effects of timing,1–3 duration,4,5 intensity,6,7 and wavelength8,9 of light stimuli on the human circadian system as measured by a variety of out-put measures, such as phase resetting and suppression of the melatonin rhythm; results of these investigations have informed light treatment protocols and strategies in clinical and laboratory settings (for review see Gooley10).
In addition to the aforementioned circadian effects of phase-shifting and suppression of melatonin, light also has acute alerting effects. Light exposure reduces subjective sleepiness, improves neurobehavioral performance, reduces attentional lapses, and activates the waking electroencephalogram (EEG).6,11–16 Light-induced activation of the waking EEG is typically characterized by a decrease of power density in the delta and theta frequencies. Moreover, the alerting effects appear to be dose dependent, such that higher illuminances have greater effects.14
The neurophysiology underlying the alerting effect of light is not fully understood. It is known that intrinsically photosensitive retinal ganglion cells (ipRGCs) containing melanopsin project to a range of targets, including the suprachiasmatic nucleus (SCN) of the hypothalamus,17 and other regions of the brain that are involved in non-image-forming responses to light. The pathway by which light activates the ascending arousal system has not been elucidated, but may be mediated by the SCN.18–20
Importantly, it is unknown whether the acute alerting effect of light is simply a function of the absolute illuminance of the light stimulus itself, or whether it also depends on the level of illuminance to which an individual was exposed prior to that stimulus. In the latter case, the extent of the alerting response is a function of a relative rather than an absolute measure of the light stimulus. Previous studies in animals21,22 and humans23–26 have reported the effects of prior light history on melatonin levels, and recently we have shown that phase shifting, in addition to melatonin suppression, also depends on the illuminance history.27 Understanding whether a similar relationship exists for light-induced alerting would be important both from a mechanistic and practical point of view, since this could have implications for the use of light as a countermeasure in situations of impaired alertness and performance. Therefore, we tested the hypothesis that prior exposure to very dim light compared to typical indoor light magnifies the alerting effect of a light stimulus during the biological night, as assessed by (a) subjective alertness, (b) neurobehavioral performance, and (c) spectral composition of the waking EEG.
METHODS
Study Participants and Screening Procedures
Healthy adults (N = 14; 6 females, 8 males) free from medical or psychological conditions or disorders, between the ages of 18 and 30 years (mean ± SD: 23.5 ± 2.9 years) contributed to the data presented here. Informed written consent was obtained from all study participants prior to enrolment and they received payment for their participation. The Partners Human Research Committee of the Brigham and Women's Hospital approved the protocol, and all study procedures conformed to the Declaration of Helsinki. Potential participants were thoroughly screened using questionnaires, blood and urine tests, physical examination, and a psychological interview to determine suitability for study participation. Any reported night work or shift work in the prior 3 years and time zone travel of > 1 time zone in the previous 3 months were exclusionary. Study participants were required to maintain a stable sleep schedule (time in bed: 8 h, bedtime and wake time fixed to each individual's habitual schedule), to complete a daily sleep/wake log, and to call-in their bedtimes and rising times every day for 3 weeks before admission to the laboratory. This sleep schedule was verified by wrist actigraphy (Actiwatch-L; Mini Mitter, Bend, OR, USA) during the week prior to admission. Participants were also instructed to refrain from any medications, drugs, alcohol, nicotine products, and caffeinated products during this 3-week period. Compliance was checked with blood and urine toxicology during the screening process and upon admission to the laboratory.
Protocol and Light Exposure Procedures
The data were collected as part of a 32-day inpatient protocol in which participants were exposed to 4 light exposures (LE) during the biological night (see Chang et al.27 for details). The randomized crossover design allowed for within-participant comparison of the effect of prior illuminance history on alertness and performance in response to the LE. The LE was targeted to occur at a time that would induce a maximal response in phase shifting and suppression of the melatonin rhythm, and to show maximal alerting effects of light. Three additional participants completed the 32-day protocol but were not included in this analysis because the midpoint of the experimental LE following the very dim light condition occurred outside the targeted interval beginning 18 h after the calculated midpoint of the plasma melatonin profile on the night before the LE and ending 24.5 h after that time. In all 3 cases, the LE occurred too early relative to the endogenous circadian cycle, likely due to drift of circadian phase in the very dim light conditions over numerous days.
Lighting conditions during the study used 4100K fluorescent lamps (Philips Lighting, The Netherlands) with digital ballasts (Lutron Electronics Co., PA, USA) mounted on the ceiling. Here we show data from the two 6.5-h experimental LE of typical indoor illuminance (approximately 90 lux; ∼0.23 W/m2 at the cornea, and 137 cm from the floor in the vertical plane and a maximum of ∼150 lux at 187 cm from the floor in the horizontal plane anywhere in the room) shown in Figure 1A. Each LE was preceded by 3 days of either very dim light (1 lux; ∼0.001 W/m2 at 137 cm from the floor in the vertical plane with a maximum of < 3 lux at 187 cm from the floor in the horizontal plane) or typical indoor room light (90 lux, see above) throughout the scheduled waking episodes, in counterbalanced order. All sleep episodes were in darkness (< 0.02 lux). The two experimental LE were separated by 15 days and participants completed a constant posture/constant routine procedure the day before and the day following each LE for circadian phase estimation of the plasma melatonin rhythm. Each 6.5-h LE was scheduled to begin during the biological night, i.e., 1 h prior to habitual bed-time. Participants were required to remain awake for 15 h prior to the LE, for the duration of the LE, and for 4.5 h following the end of the LE for a total of 26 h. Participants remained in bed in a semirecumbent position with minimal activity beginning 3 h before the LE, throughout the 6.5-h LE, and for 4.5 h after the LE for a total of 14 h. To ensure wakefulness and adherence to the protocol, participants were continuously accompanied by a staff member during the constant posture. During the 6.5-h LE, participants were asked to maintain their gaze on a fixed target on the wall in front of them (fixed gaze) for 5 min, or 10 min during specific 10-min neurobehavioral tests, and then allowed to look elsewhere (free gaze) for 5 or 10 minutes. Fixed and free gazes were alternated throughout the 6.5-h session and a technician measured the light level at the eye in the angle of gaze at every gaze transition.
Figure 1.
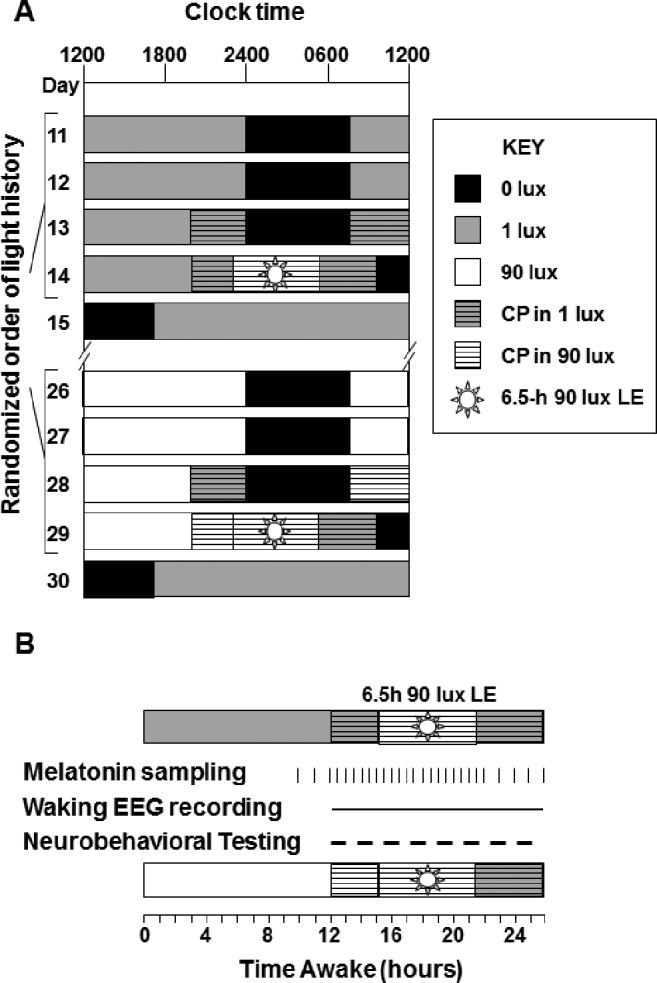
Partial raster and schema of study procedures on light exposure (LE) days. (A) The 32-day inpatient protocol included 2 experimental LE of 90 lux illuminance and 6.5 h in duration. Each LE was preceded by 3 days of either 1 lux (gray) or 90 lux (white) ambient light during the 16-h wake episodes. On LE days the wake episode lasted 26 h and included a 14-h constant posture (CP). All sleep episodes were in complete darkness (black). (B) Collection of measures during the LE day included melatonin sampling (30-60 min), waking EEG recording, and neurobehavioral performance testing (30-60 min; dashed line).
Subjective and Objective Alertness Measures
Computerized neurobehavioral test batteries were administered every 30-60 min throughout the constant posture conditions (see Figure 1B). They included: a visual analog scale (VAS) in which participants reported sleepiness by marking on a 100-mm line between “alert” and “sleepy,” a 10-min auditory psychomotor vigilance task (PVT), a reaction-time test16 in which participants were instructed to press a button using their dominant hand as soon as they heard a tone that was presented at random intervals (ranging from 1-9 sec); and the Karolinska Drowsiness Test (KDT; 3 min eyes open) in which participants were asked to maintain a fixed gaze on a black dot in front of them while avoiding movements and frequent eye blinks.28 The purpose of the KDT was to allow for a waking EEG recording under conditions in which artifacts from movement were minimized.
Neurobehavioral data were edited to exclude invalid trials. For example, PVT trials in which button presses using the non-dominant hand occurred were excluded from analysis. Moreover, errors of commission (anticipation errors), defined as button presses when no stimulus was presented, were excluded from analysis. Any trials which were interrupted or not completed (e.g., due to computer malfunction) were also excluded from analysis. Mean and median reaction time (RT) and the number of lapses (RT > 500 msec) on the auditory PVT were used to assess sustained attention.
Waking EEG
The EEG and electrooculogram (EOG) were recorded throughout the constant posture (Figure 1B) with the Vitaport-3 system (TEMEC Instruments B.V., Kerkrade, The Netherlands). The EEG included recordings from Fz, Cz, Pz, and Oz, referenced each to linked mastoids (Ax). EEG signals were both high-pass filtered (time constant: 0.33 sec) and low-pass filtered (Bessel: -6 dB at 70 Hz, 24 dB/octave), digitized (resolution: 12-bit; sampling rate 256 Hz), and stored to computer disk. Electrode impedances were checked using a GRASS F-EZM4 meter (Grass-Telefactor, Astro-Med, Inc., West Warwick, RI) to ensure that all impedances were < 10 kΩ before the start of each recording, which preceded the LE by several hours.
EEG recordings derived from Cz/Ax during the KDT were subjected to spectral analysis. To this end, signals were first visually inspected, and 2-sec epochs containing artifacts arising from body movements, eye blinks, or eye movements were removed. The remaining 2-sec epochs were subjected to fast-Fourier transformation, using a rectangular window (Vitagraph, TEMEC). EEG spectra > 20 Hz were discarded from further analysis. For 2 participants, incorrect recording montages were used during at least 1 of the 2 LE recordings and therefore were not included in the analysis.
Plasma Melatonin
Blood samples were collected via an indwelling intravenous forearm catheter at 30- to 60-min intervals throughout much of the protocol, including the day before, the day of, and the day following each LE (Figure 1B). Samples were collected into tubes containing ethylenediaminetetraacetic acid and kept on ice for < 1 h before being centrifuged (2200-2800 rpm, 2°C). Plasma melatonin samples were assayed using radioimmunoassay (Pharmasan Labs Inc., Osceola, WI) at an assay sensitivity of 0.7 pg/mL, intra-assay coefficient of variation of 5.7% to 12.1%, and inter-assay coefficient of variation of 8.4% to 13.2%.
For some analyses circadian phase angle was determined. Circadian phase angle was calculated as the difference between the time of the midpoint of the melatonin rhythm (midpoint between melatonin onset and offset) on the day following the LE and the time of the midpoint of the 6.5 h LE.
Statistical Analysis
All statistical tests were done using SAS 9.1 (SAS Institute, Cary, NC). Data collected during the 6.5-h LE and the 3-h post- LE interval were used to determine the influence of the preceding light history on subjective alertness, sustained attention, reaction time, EEG power density, and plasma melatonin concentration. Data were subjected to 2-way mixed model analysis of variance (ANOVA) and analysis of covariance (ANCOVA), with light history Condition (1 vs. 90 lux) and Time as fixed factors, Circadian Phase Angle as a covariate, and Study Participant as a random factor. In order to approximate normal distributions, some data were transformed prior to analysis, including RT (log-transform), lapses of attention [transformed using square root (lapses) + square root (1 + lapses)29], and EEG power density (log-transform). Post hoc paired Student t-tests were used for planned comparisons between conditions during and after the LE. A possible relationship between plasma melatonin levels and neurobehavioral parameters was investigated with Pearson correlations. P values less than 0.05 were considered significant for all the presented analyses.
RESULTS
Self-Reported Sleepiness
Sleepiness ratings on the VAS are shown in Figure 2A. This panel shows the time course before, during, and after the LE for the two light-history conditions. Subjective sleepiness gradually increased with longer time awake (18-26 h). A 2-way ANOVA showed significant effects for Condition (P < 0.0001) and Time (P < 0.0001) but not for Condition × Time (P = 0.81). The main effect of Condition, however, was no longer significant once Circadian Phase Angle was added to the model as a covariate, suggesting that phase contributed to this difference. The effect of Condition arose mainly from lower sleepiness ratings in the latter part of the LE as well as during the post-LE interval in the 1-lux history condition as compared to the 90-lux condition.
Figure 2.
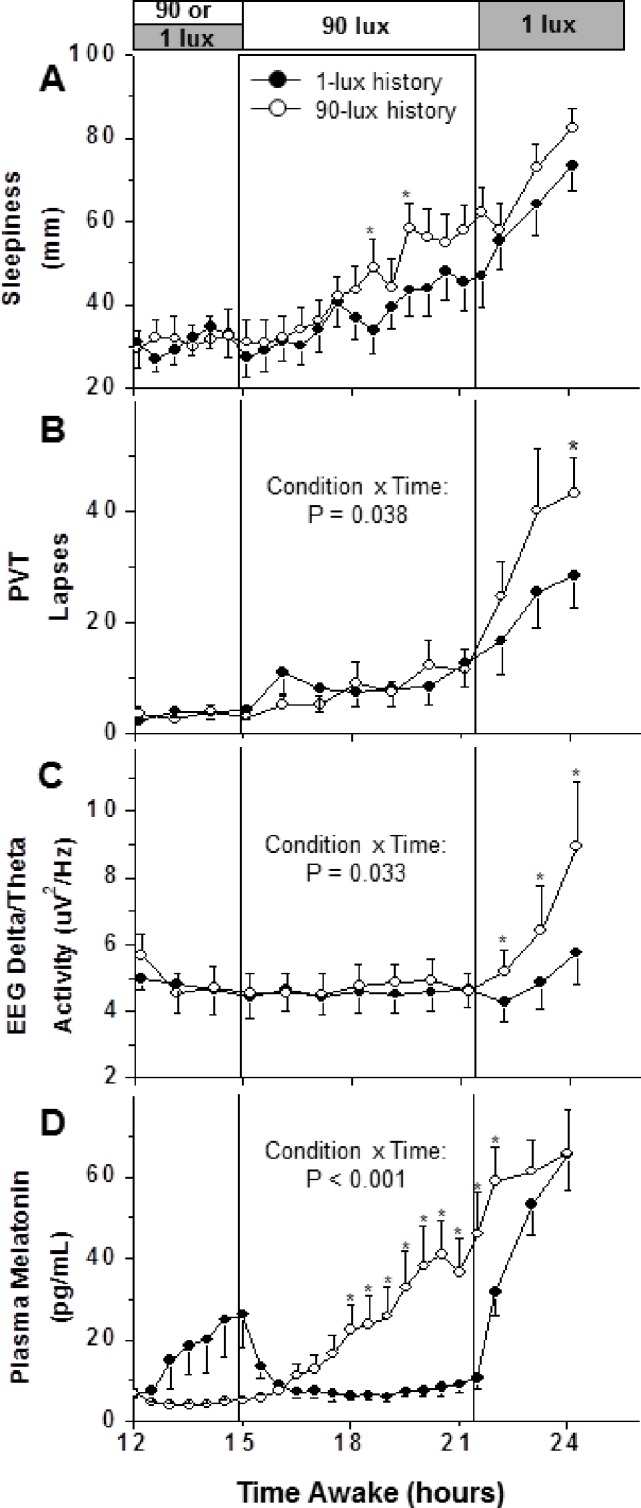
Effects of different illuminance histories on the alerting response to a 90-lux light exposure. Subjective sleepiness (derived from visual analog scale), attentional lapses (derived from the psychomotor vigilance task, PVT), EEG delta/theta activity (power density in the 2.0-5.5 Hz range), and plasma melatonin levels are shown before, during, and after a 90-lux light exposure (LE). The LE started 1 h before habitual bedtime. The open and filled symbols/bars denote 90-lux history and 1-lux history conditions, respectively. Significant differences of the interaction Condition × Time are shown in panels B, C, and D (2-way ANCOVA with Circadian Phase Angle as a covariate). Asterisks denote significant results for planned comparisons between conditions (P < 0.05, paired t-tests).
Neurobehavioral Performance
PVT lapses data are shown in Figure 2B. The time course of this performance measure showed impairment with increasing time awake in both light conditions. There were fewer PVT lapses (Figure 2B; Condition: P = 0.039; Time: P < 0.0001; Condition × Time: P = 0.053), and faster mean RTs (not shown; Condition: P = 0.043; Time: P < 0.0001; Condition × Time: P = 0.864) and median RTs (not shown; Condition: P = 0.001; Time: P < 0.0001; Condition × Time: P = 0.698) when the LE followed 1 lux compared to 90 lux. These effects mainly reflected worse performance in the post-LE interval in the 90-lux history condition. PVT data presented in Figure 2B were also subjected to a 2-way ANCOVA with Circadian Phase Angle as a covariate. The presence of a significant interaction of Condition and Time (P = 0.038) indicated that the results cannot be explained by a phase angle difference between conditions alone.
Waking EEG Activity
The time course of EEG delta/theta activity (log-transformed absolute power density in the 2.0-5.5 Hz range) is shown in Figure 2C. Delta/theta activity was lower when the LE followed 1 lux compared to 90 lux (ANOVA: Condition: P < 0.0001; Time: P < 0.0001; Condition × Time: P = 0.033). The interaction term remained significant in the 2-way ANCOVA with Circadian Phase Angle as a covariate (Figure 2C). The difference arose mainly in the post-LE interval when delta/theta activity remained relatively low in the 1-lux history condition but increased in the 90-lux history condition.
Figure 3 shows the effects of illuminance history on the entire EEG spectrum. Whereas there were no significant effects of light history on the EEG power spectrum during the LE, frequency-specific effects emerged in the post-LE interval: power density within the delta and theta frequency ranges (2.0-5.5 and 7.5 Hz) were lower following the 1-lux history than following the 90-lux history.
Figure 3.
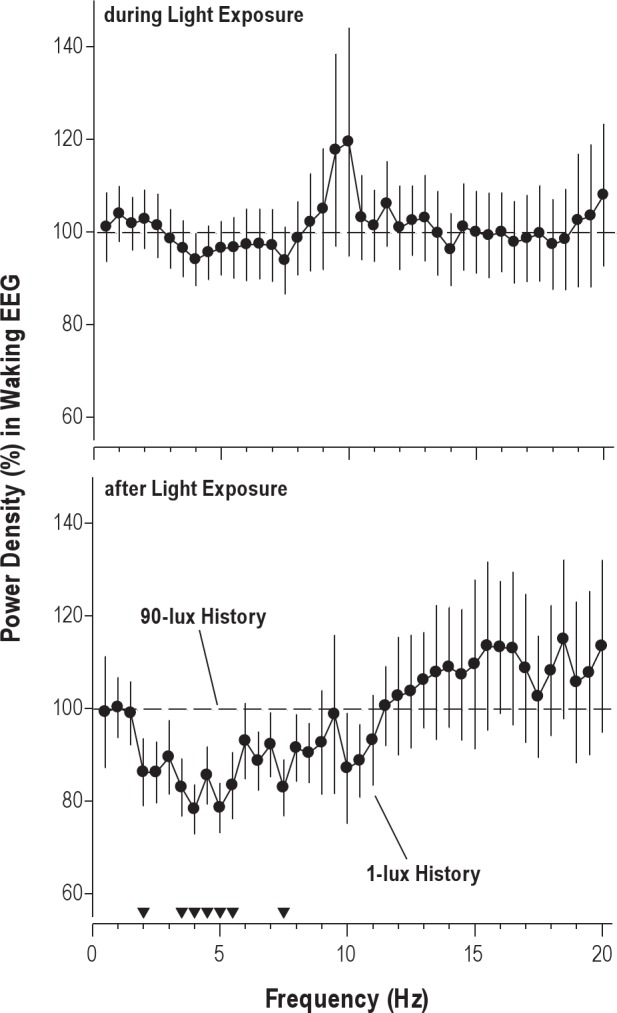
Effect of illuminance history on waking EEG response to a 90-lux light exposure (LE). Analysis of data during the 6.5-h LE is shown in the top panel; results from the 4.5 h following the LE are shown in the bottom panel. Note that participants were exposed to very dim light (1-lux history) or typical indoor light (90-lux history) prior to the LE. Filled circles represent power density in the waking EEG in the 1-lux history condition, expressed as a percentage of the 90-lux history condition (100%). The filled triangles at the bottom show EEG frequency bins for which the difference between conditions is significant (P < 0.05, paired t-tests).
Melatonin
Plasma melatonin profiles are shown in Figure 2D. Levels of plasma melatonin began increasing in the 1-lux history condition before the LE (hours 12-15) but were lower during and just after the LE compared to the 90-lux history condition (Figure 2D; Condition: P < 0.0001; Time: P < 0.0001; Condition × Time: P < 0.0001). These results remained significantly different by 2-way ANCOVA with Circadian Phase Angle as a covariate (Figure 2D).
When comparing the differences in melatonin levels between light history conditions with the differences in performance, we found no correlation for PVT lapses during the LE (r2 = 0.08, P = 0.340) or post-LE (r2 = 0.01, P = 0.788). Furthermore, there was no correlation between the differences in melatonin and median RT during and following the LE (r2 = 0.10 P = 0.305, and r2 = 0.03 P = 0.592, respectively). Although there was no correlation between differences in melatonin levels and self-reported sleepiness during the LE (r2 = 0.01, P = 0.794), there was an inverse relationship following the LE, i.e., the smaller the difference in melatonin levels, the greater the difference in sleepiness (r2 = 0.41, P = 0.018).
DISCUSSION
We have shown that the magnitude of the direct alerting response to a light stimulus depends on the illuminance to which an individual was previously exposed. Previous studies have indicated that the alerting response depends in a dose-related manner on the illuminance of the light stimulus itself.14 The current investigation now clarifies that it is not simply the absolute illuminance that is relevant for light-induced stimulation, but is modulated as a function of the immediate light history to which an individual has been exposed. In particular, the alerting response to light lasts longer if the light stimulus is preceded by very dim light. These results imply processes of sensitization and/or adaptation to light. While such processes are known to operate in the visual system and the circadian system,27 we provide evidence that they play a role in the alerting response to light.
Before we conclude, however, that processes of sensitization provide the best explanation for the present findings, we need to consider other possible explanations. For instance, a difference in alertness between the two light history conditions could be the result of a difference in circadian phase at the time of the LE. Circadian phase is known to affect alertness, neurobehavioral performance and the spectral composition of the waking EEG.30–34 Due to the acute sensitivity of melatonin secretion to light, it was not possible to use the onset of melatonin secretion during the LE as an estimate of circadian phase. Plasma melatonin samples collected in dim light 24 hours prior to, and 24 hours after the LE allowed us to compare circadian phase position between the two conditions, however. This comparison revealed that the melatonin midpoint on the day prior to the LE occurred later (P = 0.03, t-test) by (mean ± SEM) 39 ± 23 min in the 1-lux compared to the 90-lux history condition. Furthermore, the difference in phase position on the day after the LE was even greater, with the melatonin midpoint in the 1-lux condition occurring 59 ± 14 min later than in the 90-lux condition (P = 0.03, t-test). Most importantly, however, when we corrected for individual phase differences by means of an ANCOVA by using the phase position after the LE as a covariate, we found that the differences between conditions in PVT lapses, and EEG delta/theta activity remained significant, indicating that they were not simply a consequence of circadian phase disparities.
The present data show that the light history affected the secretion of melatonin during the LE, with pre-exposure to very dim light resulting in greater suppression of melatonin, as we reported before.27 We therefore tested whether the effects of illuminance history on light-induced changes in alertness are secondary to and possibly mediated by the levels of melatonin. This hypothesis derives from observations of a close temporal relationship between the circadian rhythms in circulating melatonin levels, and alertness, neurobehavioral performance, and spectral composition of the waking EEG,16,31,33–35 as well as from observations of the acute effects of melatonin administration on alertness and performance.36,37 The time course of subjective sleepiness and plasma melatonin during the LE did indeed show some similarities, which is not surprising since both pineal secretion of melatonin and the endogenous rhythm of sleep propensity are regulated by the circadian clock. On the other hand, there was no significant correlation between the difference in melatonin concentration and the difference in PVT performance between the two light-history conditions. These results are reminiscent of those previously reported showing that light can exert an alerting response during the day, in the absence of circulating melatonin levels.38–40
Photic adaptation of the human circadian timing system, measured by melatonin suppression23–25,27 and phase shifting27 is consistent with proposed models for human circadian photo-transduction,41,42 which incorporate the classic photoreceptors and the ipRGCs and accounts for the effects of light on nocturnal melatonin suppression. Animal studies have shown clear evidence for the essential role of ipRCGs in entrainment of the circadian clock and regulation of non-image-forming responses to light.43,44 The intrinsic melanopsin-mediated electrophysiological activity of ipRCGs is slow to turn on and slow to turn off in response to a light stimulus.45 In our study, the effects of illuminance history on the alerting response to light only started to emerge in the latter part of the LE, and were greatest after the LE ended. This time course may reflect the time course of the photoreceptors mediating these responses.
A limitation of the current study is the relatively small sample size (n = 14 for subjective alertness, performance, and melatonin measures; n = 12 for objective alertness measures). We may have been underpowered to detect small differences in certain measures (e.g., subjective sleepiness). Another limitation is the use of low light intensities (< 1 lux and 90 lux) given that these are not the typical conditions frequently experienced by humans in modern societies. Although most humans typically experience only a single transition from < 1 lux to 90 lux or higher per day upon awakening from sleep into room light, the present study reveals for the first time that light history can in fact affect alertness. Thus, our results warrant future studies to investigate the effects of light level histories that are more commonly experienced by humans (e.g., including bright light). One final limitation with regard to the interpretation of our results is the possible effect of behavioral modification during the prior light histories on the alerting response to a subsequent light stimulus. Although the protocol schedule was identical during the < 1 and 90 lux light history conditions and there were no differences in subjective sleepiness, PVT performance, or waking EEG between the conditions in the hours prior to the LE, there may have been some minor alteration in the participants' behavior (e.g., less time spent reading during the < 1 lux condition) that could have influenced the response.
The absence of a difference in the alerting response in the early part of the LE may reflect a type of ceiling effect, i.e., alertness was still high, such that there was no room for an alerting response to become manifest. Several factors may have contributed to this. Considering the sizable circadian wake drive at the beginning of LE and only moderate levels of homeostatic sleep pressure in the current protocol, it is not surprising that differences in alertness are not seen during the LE. Under these circumstances, the 90-lux stimulus may have been sufficient to counteract behavioral impairment, irrespective of illuminance history.
A possible explanation for this may be related to homeostatic sleep pressure. The 6.5-h light stimulus was administered beginning 1 hour prior to participants' habitual bedtimes. At the end of the LE and at the end of the extended wake episode individuals had been awake for 21.5 and 26 hours, respectively, and presumably had accumulated high sleep need. Perhaps the sleep pressure was not sufficiently high earlier in the LE (15-20 h awake) for the alerting effects of the light to become manifest. A previously published study by Cajochen et al. showed significant differences in alertness measures in response to a 6.5-h LE of different illuminance levels (3-9100 lux).14 The maximum difference in subjective alertness was seen in the latter half, with the peak difference in the last 30-60 minutes of the LE. Similarly, a more recent study by Lockley and colleagues16 showed a difference in alertness measures between different wavelengths of light during a 6.5-h LE, using a similar 9-day protocol as in the Cajochen study. In both of these protocols, participants had been kept awake for ∼50 hours prior to an 8-h sleep episode the day before LE and were thus still under increased sleep pressure. In contrast, in the current study, participants were not sleep deprived on the day prior to the LE and therefore experienced only moderate levels of sleep pressure in the early part of the LE.
Another possible explanation is that the LE procedures themselves, apart from illuminance, were alerting (e.g., interactions with technician). Indeed, in both light-history conditions, lapses of attention and EEG delta/theta activity remained at low levels throughout the LE. This suggests that the light stimulus itself—or associated LE procedures—were sufficiently strong to induce alertness, and only once they were removed did the differences between the light-history conditions fully emerge. These extended alerting effects of light are consistent with evidence from a simulated shift work protocol showing that exposure to bright light early in the night, prior to the circadian nadir of body temperature, was more effective in improving vigilance in the second part of the night than bright light administered during the second part of the night.46 Furthermore, a previous study examining the extended effects of light on subsequent sleep has shown that evening light exposure increased sleep latency.47
The current study demonstrates that exposure to a very dim light environment prior to a light stimulus increased the efficacy of this stimulus on alertness, cognitive performance, and waking EEG. From a therapeutic point of view, these results may have important implications for designing practical light treatments with perhaps shorter light exposures or stimuli that are lower in illuminance, thereby improving compliance while obtaining similar effects of improved alertness.
DISCLOSURE STATEMENT
Dr. Chang and Dr. Scheer have no conflicts of interest to disclose. Dr. Czeisler has the following disclosures for the past 2 years. Dr. Czeisler has received consulting fees from or served as a paid member of scientific advisory boards for Astra Zeneca, Bombardier, Inc., Boston Celtics, Celadon Trucking Services, Cephalon, Inc. (acquired by Teva Pharmaceutical Industries Ltd. October 2011), Eli Lilly and Co., Garda Síochána Inspectorate, Gerson Lehrman Group for Novartis, Global Ground Support, Johnson & Johnson, Koninklijke Philips electronics, N.V. (acquired Respironics, Inc. March 2008), Minnesota Timberwolves, Portland Trail Blazers, Sleep Multimedia, Inc., Somnus Therapeutics, Inc., Vanda Pharmaceuticals, Inc., and Zeo Inc. Dr. Czeisler owns an equity interest in Lifetrac, Inc., Somnus Therapeutics, Inc., Vanda Pharmaceuticals, Inc., and Zeo Inc., and received royalties from the Massachusetts Medical Society/New England Journal of Medicine, McGraw Hill, Penguin Press/Houghton Mifflin Harcourt, and Philips Respironics, Inc. Dr. Czeisler has received lecture fees from Harvard School of Public Health, Hokkaido University Graduate School of Medicine, Japan Aerospace Exploration Agency (JAXA), LOTTE Health Products, Mount Sinai School of Medicine, National Sleep Foundation, New England College of Occupational and Environmental Medicine (NECOEM), North East Sleep Society, Rockpointe (for Cephalon, Inc.), Sleep Research Society, Society of Thoracic Surgeons, Stress Research Institute, University of Stockholm, University of Chicago, University of Colorado, the World Federation of Sleep Research and Sleep Medicine Societies, and WME Entertainment LLC. Dr. Czeisler has also received research prizes with monetary awards from the American Academy of Sleep Medicine, clinical trial research contracts from Cephalon, Inc., and his research laboratory at the Brigham and Women's Hospital has received unrestricted research and education funds and/or support for research expenses from Committee for Interns and Residents, the CIR Policy and Education Initiative. The Harvard Medical School Division of Sleep Medicine (HMS/DSM), which Dr. Czeisler directs, has received unrestricted research and educational gifts and endowment funds from Boehringer Ingelheim Pharmaceuticals, Inc., Cephalon, Inc., George H. Kidder, Esq., Gerald McGinnis, GlaxoSmithKline, Herbert Lee, Hypnion, Jazz Pharmaceuticals, Jordan's Furniture, Merck & Co., Inc., Peter C. Farrell, PhD, Pfizer, ResMed, Respironics, Inc., Sanofi-Aventis, Inc., Sealy, Inc., Sepracor, Inc., Simmons, Sleep Health Centers LLC, Spring Aire, Takeda Pharmaceuticals and Tempur-Pedic. The HMS/DSM has received gifts from many outside organizations and individuals including Catalyst Group, Cephalon, Inc., Committee for Interns and Residents, Eisai, Inc., Farrell Family Foundation, Fisher & Paykel Health-care Corporation, Jordan's Furniture, Lilly USA, LLC, neurocare Center for Sleep, Philips-Respironics, Inc., Praxair US Homecare, Sanofi-Aventis, Inc., Select Comfort Corporation, Sleep HealthCenters LLC, Somaxon Pharmaceuticals, Vanda Pharmaceuticals, Inc., Wake Up Nacrcolepsy, Inc., Watermark Medical, and Zeo, Inc. The HMS/DSM Sleep and Health Education Program has received Educational Grant funding from Cephalon, Inc., Takeda Pharmaceuticals, Sanofi-Aventis, Inc. and Sepracor, Inc. Dr. Czeisler is the incumbent of an endowed professorship provided to Harvard University by Cephalon, Inc. and holds a number of process patents in the field of sleep/ circadian rhythms (e.g., photic resetting of the human circadian pacemaker). Since 1985, Dr. Czeisler has also served as an expert witness on various legal cases related to sleep and/or circadian rhythms. Dr. Aeschbach has served as a paid member of the scientific advisory board of Zeo Inc.
ACKNOWLEDGMENTS
The authors thank the individuals who participated in this study. The authors also thank the staff of the General Clinical Research Center of the Brigham and Women's Hospital and the Chronobiology Core of the Division of Sleep Medicine for their assistance in the conduct of the study, and the Sleep & EEG Core for polysomnography and EEG analysis support. The authors specifically acknowledge and thank the subject recruiters, A. Crugnale, N. McCarthy, D. McCarthy, and E. Reid for their work on this study. The authors also acknowledge Dr. Wei Wang for her assistance with statistical analysis supported by the Harvard Catalyst, The Harvard Clinical and Translational Science Center [National Center for Research Resources (NCRR) and the National Center for Advancing Translational Sciences, National Institutes of Health Award (NIH) #UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic health care centers. This work was supported by NIH grant R01HL77453. Inpatient studies were conducted in the General Clinical Research Center supported by NCRR grant M01-RR-02635. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Harvard Catalyst, NIH or NCRR. Dr. Chang was supported by NIH grant F32HL078360, Dr. Scheer by NIH grant P30-HL101299, Dr. Czeisler by National Space Biomedical Research Institute grant HFP01601, and Dr. Aeschbach in part by the German Aerospace Center.
REFERENCES
- 1.Czeisler CA, Allan JS, Strogatz SH, et al. Bright light resets the human circadian pacemaker independent of the timing of the sleep-wake cycle. Science. 1986;233:667–71. doi: 10.1126/science.3726555. [DOI] [PubMed] [Google Scholar]
- 2.Van Cauter E, Sturis J, Byrne MM, et al. Demonstration of rapid light-in-duced advances and delays of the human circadian clock using hormonal phase markers. Am J Physiol. 1994;266:E953–63. doi: 10.1152/ajpendo.1994.266.6.E953. [DOI] [PubMed] [Google Scholar]
- 3.Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol (Lond) 2003;549:945–52. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dewan K, Benloucif S, Reid K, Wolfe LF, Zee PC. Light-induced changes of the circadian clock of humans: increasing duration is more effective than increasing light intensity. Sleep. 2011;34:593–9. doi: 10.1093/sleep/34.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang AM, Santhi N, St Hilaire M, et al. Human responses to bright light of different durations. J Physiol. 2012;590:3103–12. doi: 10.1113/jphysiol.2011.226555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: Melatonin phase resetting and suppression. J Physiol (Lond) 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boivin DB, Duffy JF, Kronauer RE, Czeisler CA. Dose-response relationships for resetting of human circadian clock by light. Nature. 1996;379:540–2. doi: 10.1038/379540a0. [DOI] [PubMed] [Google Scholar]
- 8.Lockley SW, Brainard GC, Czeisler CA. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab. 2003;88:4502–5. doi: 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- 9.Revell VL, Arendt J, Terman M, Skene DJ. Short-wavelength sensitivity of the human circadian system to phase-advancing light. J Biol Rhythms. 2005;20:270–2. doi: 10.1177/0748730405275655. [DOI] [PubMed] [Google Scholar]
- 10.Gooley JJ. Treatment of circadian rhythm sleep disorders with light. Ann Acad Med Singapore. 2008;37:669–76. [PubMed] [Google Scholar]
- 11.Campbell SS, Dawson D. Enhancement of nighttime alertness and performance with bright ambient light. Physiol Behav. 1990;48:317–20. doi: 10.1016/0031-9384(90)90320-4. [DOI] [PubMed] [Google Scholar]
- 12.Badia P, Myers B, Boecker M, Culpepper J, Harsch JR. Bright light effects on body temperature, alertness, EEG and behavior. Physiol Behav. 1991;50:583–8. doi: 10.1016/0031-9384(91)90549-4. [DOI] [PubMed] [Google Scholar]
- 13.Daurat A, Aguirre A, Foret J, Gonnet P, Keromes A, Benoit O. Bright light affects alertness and performance rhythms during a 24-h constant routine. Physiol Behav. 1993;53:929–36. doi: 10.1016/0031-9384(93)90271-g. [DOI] [PubMed] [Google Scholar]
- 14.Cajochen C, Zeitzer JM, Czeisler CA, Dijk DJ. Dose-response relationship for light intensity and ocular and electroencephalographic correlates of human alertness. Behav Brain Res. 2000;115:75–83. doi: 10.1016/s0166-4328(00)00236-9. [DOI] [PubMed] [Google Scholar]
- 15.Lavoie S, Paquet J, Selmaoui B, Rufiange M, Dumont M. Vigilance levels during and after bright light exposure in the first half of the night. Chronobiol Int. 2003;20:1019–38. doi: 10.1081/cbi-120025534. [DOI] [PubMed] [Google Scholar]
- 16.Lockley SW, Evans EE, Scheer FA, Brainard GC, Czeisler CA, Aesch-bach D. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep. 2006;29:161–8. [PubMed] [Google Scholar]
- 17.Baver SB, Pickard GE, Sollars PJ. Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus. Eur J Neurosci. 2008;27:1763–70. doi: 10.1111/j.1460-9568.2008.06149.x. [DOI] [PubMed] [Google Scholar]
- 18.Gooley JJ, Lu J, Fischer D, Saper CB. A broad role for melanopsin in nonvisual photoreception. J Neurosci. 2003;23:7093–106. doi: 10.1523/JNEUROSCI.23-18-07093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chou TC, Bjorkum AA, Gaus SE, Lu J, Scammell TE, Saper CB. Afferents to the ventrolateral preoptic nucleus. J Neurosci. 2002;22:977–90. doi: 10.1523/JNEUROSCI.22-03-00977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–63. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 21.Meyer WE, Millam JR, Bradley FA. Photostimulation of Japanese quail by dim light depends upon photophase contrast, not light intensity. Biol Reprod. 1988;38:536–43. doi: 10.1095/biolreprod38.3.536. [DOI] [PubMed] [Google Scholar]
- 22.Meyer WE, Millam JR. Plasma melatonin levels in Japanese quail exposed to dim light are determined by subjective interpretation of day and night, not light intensity. Gen Comp Endocrinol. 1991;82:377–85. doi: 10.1016/0016-6480(91)90313-u. [DOI] [PubMed] [Google Scholar]
- 23.Hébert M, Martin SK, Lee C, Eastman CI. The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res. 2002;33:198–203. doi: 10.1034/j.1600-079x.2002.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith KA, Schoen MW, Czeisler CA. Adaptation of human pineal melatonin suppression by recent photic history. J Clin Endocrinol Metab. 2004;89:3610–4. doi: 10.1210/jc.2003-032100. [DOI] [PubMed] [Google Scholar]
- 25.Jasser SA, Hanifin JP, Rollag MD, Brainard GC. Dim light adaptation attenuates acute melatonin suppression in humans. J Biol Rhythms. 2006;21:394–404. doi: 10.1177/0748730406292391. [DOI] [PubMed] [Google Scholar]
- 26.Owen J, Arendt J. Melatonin suppression in human subjects by bright and dim light in Antarctica: Time and season-dependent effects. Neurosci Lett. 1992;137:181–4. doi: 10.1016/0304-3940(92)90399-r. [DOI] [PubMed] [Google Scholar]
- 27.Chang AM, Scheer FA, Czeisler CA. The human circadian system adapts to prior photic history. J Physiol. 2011;589:1095–102. doi: 10.1113/jphysiol.2010.201194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Åkerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 29.Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20:267–77. [PubMed] [Google Scholar]
- 30.Dijk DJ, Duffy JF, Czeisler CA. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J Sleep Res. 1992;1:112–7. doi: 10.1111/j.1365-2869.1992.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 31.Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am J Physiol Regul Integr Comp Physiol. 1999;277:R1152–63. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]
- 32.Aeschbach D, Matthews JR, Postolache TT, Jackson MA, Giesen HA, Wehr TA. Dynamics of the human EEG during prolonged wakefulness: Evidence for frequency-specific circadian and homeostatic influences. Neurosci Lett. 1997;239:121–4. doi: 10.1016/s0304-3940(97)00904-x. [DOI] [PubMed] [Google Scholar]
- 33.Aeschbach D, Matthews JR, Postolache TT, Jackson MA, Giesen HA, Wehr TA. Two circadian rhythms in the human electroencephalogram during wakefulness. Am J Physiol. 1999;277:R1771–9. doi: 10.1152/ajpregu.1999.277.6.R1771. [DOI] [PubMed] [Google Scholar]
- 34.Cajochen C, Wyatt JK, Czeisler CA, Dijk DJ. Separation of circadian and wake duration-dependent modulation of EEG activation during wakefulness. Neurosci. 2002;114:1047–60. doi: 10.1016/s0306-4522(02)00209-9. [DOI] [PubMed] [Google Scholar]
- 35.Wehr TA, Aeschbach D, Duncan WCJ. Evidence for a biological dawn and dusk in the human circadian timing system. J Physiol. 2001;535:937–51. doi: 10.1111/j.1469-7793.2001.t01-1-00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cajochen C, Kräuchi K, von Arx MA, Möri D, Graw P, Wirz-Justice A. Daytime melatonin administration enhances sleepiness and theta/alpha activity in the waking EEG. Neurosci Lett. 1996;207:209–13. doi: 10.1016/0304-3940(96)12517-9. [DOI] [PubMed] [Google Scholar]
- 37.Dollins AB, Zhdanova IV, Wurtman RJ, Lynch HJ, Deng MH. Effect of inducing nocturnal serum melatonin concentrations in daytime on sleep, mood, body temperature, and performance. Proc Natl Acad Sci U S A. 1994;91:1824–8. doi: 10.1073/pnas.91.5.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phipps-Nelson J, Redman JR, Dijk DJ, Rajaratnam SM. Daytime exposure to bright light, as compared to dim light, decreases sleepiness and improves psychomotor vigilance performance. Sleep. 2003;26:695–700. doi: 10.1093/sleep/26.6.695. [DOI] [PubMed] [Google Scholar]
- 39.Rüger M, Gordijn MC, Beersma DG, de Vries B, Daan S. Time-of-day-dependent effects of bright light exposure on human psychophysiology: comparison of daytime and nighttime exposure. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1413–20. doi: 10.1152/ajpregu.00121.2005. [DOI] [PubMed] [Google Scholar]
- 40.Vandewalle G, Balteau E, Phillips C, et al. Daytime light exposure dynamically enhances brain responses. Curr Biol. 2006;16:1616–21. doi: 10.1016/j.cub.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 41.Kronauer RE, Forger DB, Jewett ME. Quantifying human circadian pacemaker response to brief, extended, and repeated light stimuli over the photopic range. J Biol Rhythms. 1999;14:500–15. doi: 10.1177/074873099129001073. [DOI] [PubMed] [Google Scholar]
- 42.Rea MS, Figueiro MG, Bullough JD, Bierman A. A model of phototransduction by the human circadian system. Brain Res Rev. 2005;50:213–38. doi: 10.1016/j.brainresrev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Ruby NF, Brennan TJ, Xie X, et al. Role of melanopsin in circadian responses to light. Science. 2002;298:2211–3. doi: 10.1126/science.1076701. [DOI] [PubMed] [Google Scholar]
- 44.Panda S, Sato TK, Castrucci AM, et al. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–5. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- 45.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–3. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 46.Santhi N, Horowitz TS, Czeisler CA. Reducing nighttime attentional failures with bright light exposure: timing it right. J Sleep Res. 2008;17:23. doi: 10.1177/0748730408319863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cajochen C, Dijk DJ, Borbély AA. Dynamics of EEG slow-wave activity and core body temperature in human sleep after exposure to bright light. Sleep. 1992;15:337–43. [PubMed] [Google Scholar]


