Abstract
Study Objectives:
To determine rapid eye movement (REM) sleep phase preference in a crepuscular mammal (Octodon degus) by challenging the specific REM sleep homeostatic response during the diurnal and nocturnal anticrepuscular rest phases.
Design:
We have investigated REM sleep rebound, recovery, and documented REM sleep propensity measures during and after diurnal and nocturnal selective REM sleep deprivations.
Subjects:
Nine male wild-captured O. degus prepared for polysomnographic recordings
Interventions:
Animals were recorded during four consecutive baseline and two separate diurnal or nocturnal deprivation days, under a 12:12 light-dark schedule. Three-h selective REM sleep deprivations were performed, starting at midday (zeitgeber time 6) or midnight (zeitgeber time 18).
Measurements and Results:
Diurnal and nocturnal REM sleep deprivations provoked equivalent amounts of REM sleep debt, but a consistent REM sleep rebound was found only after nocturnal deprivation. The nocturnal rebound was characterized by a complete recovery of REM sleep associated with an augment in REM/total sleep time ratio and enhancement in REM sleep episode consolidation.
Conclusions:
Our results support the notion that the circadian system actively promotes REM sleep. We propose that the sleep-wake cycle of O. degus is modulated by a chorus of circadian oscillators with a bimodal crepuscular modulation of arousal and a unimodal promotion of nocturnal REM sleep.
Citation:
Ocampo-Garcés A; Hernández F; Palacios AG. REM sleep phase preference in the crepuscular Octodon degus assessed by selective REM sleep deprivation. SLEEP 2013;36(8):1247-1256.
Keywords: crepuscular chronotype, Octodon degus, REM sleep, REM sleep homeostasis, REM sleep rebound
INTRODUCTION
Crepuscular mammals exhibit a characteristic bimodal profile of activity, with activity bouts peaking just before dawn (morning peak) and dusk (evening peak). The crepuscular chronotype of rest-activity rhythm is widely distributed among mammals and has been reported in both free-ranging and captive animals.1–3 Physiological and behavioral measures such as body temperature,4 wheel-running,5,6 cortisol secretion,7 and electro-encephalographic (EEG) data8 have been also found to display bimodal temporal profiles among crepuscular species.
A crepuscular activity pattern has been described in Octodon degus (Rodentia: Hystricognatha) living in natural conditions and confinement.1,9 Polysomnographic recordings have shown a bimodal crepuscular profile of wakefulness, with phase-opposed modes at zeitgeber time (ZT)11 and ZT23 under a 12:12 light-dark cycle.10 Wake bouts delimit two sleep-predominant rest plateaus occurring outside the crepuscular periods (anticrepuscular rest phases, as defined by McElhinny et al.4). No significant diurnal-nocturnal difference has been found for the amount of rapid eye movement (REM) or non-REM (NREM) sleep,10,11 casting doubts on the idea of a diurnal or nocturnal phase sleep state preference. The crepuscular bimodal sleep-wake pattern departs from the typical unimodal profile observed among nocturnal and diurnal mammals. In particular, REM sleep of diurnal and nocturnal mammals is under strong unimodal circadian modulation, with the acrophase coinciding with core body temperature nadir.12,13 The opponent process model proposes that circadian modulation of the sleep-wake cycle is explained by an active promotion of arousal by the circadian system that opposes the homeostatic sleep pressure accumulated during wakefulness.14 If this is true, diurnal and nocturnal anticrepuscular sleep-pre-dominant rest intervals observed in O. degus occur permissively, mirroring the bimodal crepuscular promotion of arousal.
However, some evidence suggests that REM sleep may be actively promoted by the circadian system, as homeostatic REM sleep recovery after sleep deprivation is facilitated during the corresponding rest phase and impaired during the active phase.15,16 To explore phase-dependent REM sleep modulation in the crepuscular O. degus, in this report we take advantage of a property of REM sleep: REM sleep quickly compensates for state debts after selective REM sleep deprivation during the rest phase, as has been observed in the rat.15,17,18 If REM sleep during the anticrepuscular rest phases occurs permissively, without circadian REM sleep promotion, as predicted by the opponent process model, REM sleep deprivation during those intervals would be compensated for by an equivalent homeostatic response. Alternatively, asymmetric diurnal versus nocturnal REM sleep homeostatic responses must be interpreted as the manifestation of phase-specific REM sleep promotion, supporting the notion that sleep states are actively promoted by the circadian system.
METHODS
Animal handling and experimentation was performed according to local institutional animal care guidelines and authorization of the Servicio Agrícola y Ganadero of the Ministry of Agriculture of Chile. Nine adult male O. degus animals (190-230 g) were captured in their natural environment using mediumsized baited Sherman traps (H.B. Sherman traps, Tallahassee, FL).19 After at least a 2-month period of quarantine and adaptation to captivity under a stable 12:12 light-dark cycle, the animals were installed in recording cages (30 × 40 × 30 cm) contained in individual isolation chambers (65 × 60 × 60 cm) for another 3 weeks. Light intensity was set at 300 lux at floor level during light hours. Ambient temperature was maintained at 21-23°C, with food and water ad libitum.
Animals were implanted with two epidural (coordinates as by Kas and Edgar10) and two neck muscle stainless steel electrodes for EEG and electromyographic (EMG) recordings under xylazine (8 mg/kg) / ketamine (40 mg/kg) intramuscular anesthesia. After 10 days of postoperative recovery, animals were connected to a flexible counterbalanced cable attached to a slip ring. A piezoelectric device placed at the base of the animal's cage detected locomotor activity.19 Recording sessions began after at least 2 days of adaptation to recording conditions.
Data Collection and State Scoring
A computer-based data acquisition system sampled, displayed, and stored EEG, EMG, and locomotor activity signals.20 Sampling was performed at 250 Hz after analog filtering and signal conditioning, and online display allowed for quality control. Three channels amplified EEG, EMG, and locomotor activity (LMA) signals, respectively (Grass model 12 amplifier, Grass Instruments, Quincy, MA). The EEG channel was set at band-pass 1.0-30.0 Hz. Band-pass for EMG and LMA signals were set at 30-90 Hz. Two independent scorers assigned epochs to wakefulness (W), NREM, or REM by offline polysomnographic visual analysis at a 15-sec time resolution (epochs). Epochs of wakefulness were defined by a desynchronized EEG associated with elevated muscle tonus or muscle spikes and LMA. NREM sleep was defined by delta (1-4 Hz) waves and sleep spindles (10-15 Hz) in the absence of LMA and muscle spikes, and REM sleep by the presence of tonic theta (4-8 Hz) activity associated with low neck muscle tonus. State scoring procedures assigned each 15-sec epoch to the state that occupied more than 50% of that epoch. Each day of recording was summarized into a state-by-epoch array containing 5,760 assignments. Epochs with artifacts were excluded from analysis. Hours containing more than 10% of damaged data were excluded from statistics. Additionally, 1-h polysomnographic samples of baseline and deprivation days were compared visually with behavioral profiles to evaluate the robustness of offline visual scoring.
Selective REM Sleep Deprivation Procedure and Deprivation Protocols
Ten days after surgery, O. degus adapted for at least 2 days to the recording environment. Four baseline recordings were then obtained on consecutive days, to be followed by deprivation sessions. Two 3-h selective REM sleep deprivation sessions were semirandomly applied on nonconsecutive days (four animals started with diurnal and five animals with nocturnal selective deprivations). The diurnal deprivation started at zeitgeber time (ZT) 06 (RH06 protocol), and nocturnal deprivation at ZT18 (RH18 protocol). The intervention to interrupt REM sleep was the gentle movement of the cage by means of a manually operated mechanism connected to a lever outside the isolation box.20 Interventions were prompted by the appearance of the first unequivocal REM sleep signs (decreasing amplitude of cortical EEG, sustained midline theta activity, and muscle atonia). REM sleep transitions were counted as such whether they were spontaneously or externally interrupted. The transitions were counted as distinct events only if separated by at least 15 sec. Any ongoing REM sleep episode occurring at the time in which a deprivation window was to start was immediately interrupted.
Data Analysis and Statistics
Statistical analysis was carried out using Intercooled Stata 9.2 for Windows (StataCorp, College Station, TX). The values for results presented throughout this manuscript are arithmetic averages (± standard errors).
Hours are denominated according to zeitgeber time, ZT00 being the first hour after lightson. When we refer to a given hour, we mean the 1-h interval that starts at that time; e.g., “ZT06” refers to the interval from 06:00 to 06:59. Variables were also collapsed into eight consecutive 3-h blocks called octants, starting at octant 0 (ZT00 to 02:59 h). Deprivation sessions performed during the RH06 protocol correspond to octant 2 and those of the RH18 protocol to octant 6 (see Tables 1 and 2).
Table 1.
Sleep-wake cycle: Deprivation versus baseline comparisons
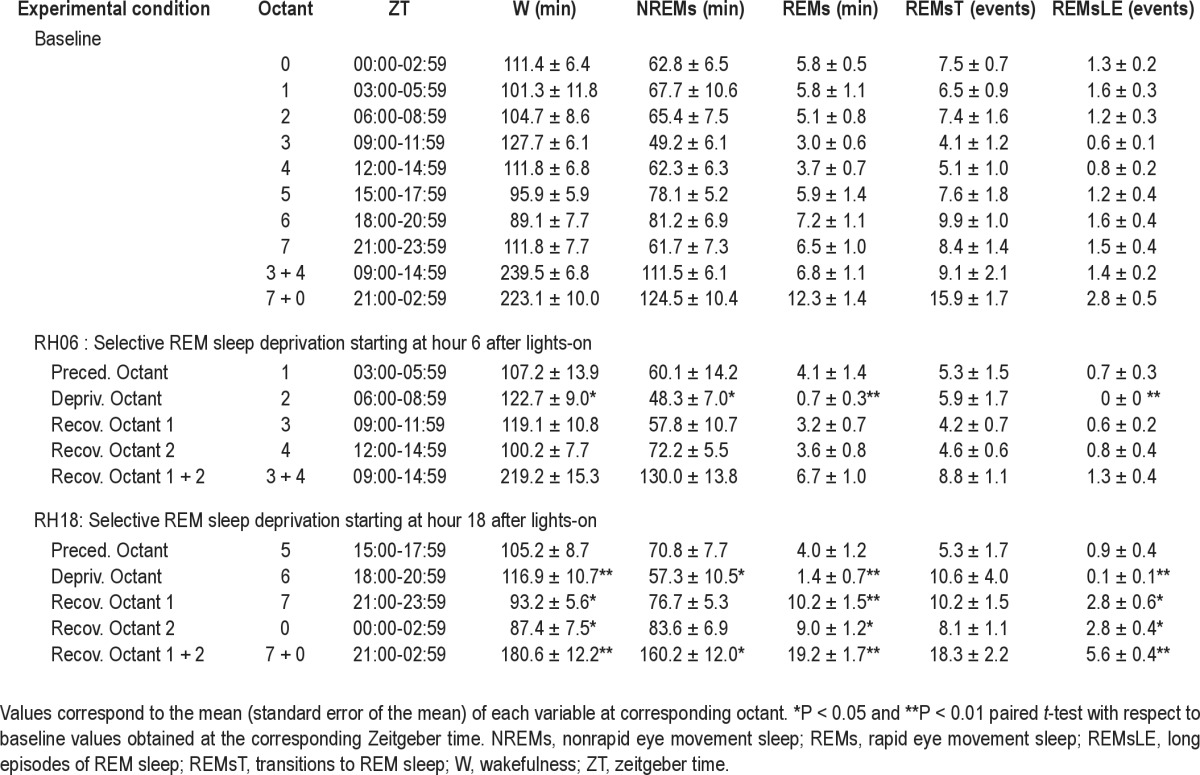
Table 2.
Sleep-wake cycle parameters: RH06 versus RH18 comparisons
The variables analyzed were: amount of W, NREM, and REM sleep; REM/total sleep time (REM/TST) ratio; REM sleep debt; REM sleep transitions; number of long REM sleep episodes; REM sleep transition index; and consolidation rate of REM sleep. Amount of W, NREM, and REM sleep corresponds to the number of 15-sec epochs observed at the corresponding time window expressed in min. REM/TST ratio is expressed as a percentage. REM sleep debt corresponds to the difference between the cumulative amount of REM sleep observed during RH06 or RH18 protocols and the expected value of cumulative amount of REM sleep in the corresponding baseline interval.21 REM sleep transitions correspond to the number of NREM to REM sleep transitions in the given recording time, a value that under selective deprivation included the number of REM sleep bouts that were experimentally interrupted. Long REM sleep episode frequency is the number of REM sleep episodes lasting at least six consecutive epochs scored as REM sleep (1.5 min) per unit of time. The duration threshold was set at the limit of the fourth and fifth longest quintile of the pool of REM sleep episodes obtained under baseline conditions. REM sleep transition index corresponds to the number of NREM to REM sleep transitions expressed as events per 10 min of NREM sleep. Consolidation rate corresponds to the long REM sleep episodes rate over total number of REM sleep transitions at a given time window expressed as percentage. Baseline values of studied variables were obtained by averaging the 4 undisturbed days at the corresponding time resolution (hour or octant). Hourly baseline values of sleep state amounts were subjected to a one-way repeated-measures analysis of variance (rANOVA) for factor ZT (24 levels). Linear orthogonal planned contrasts were performed between selected intervals of baseline values of sleep-wake states (W, NREM, and REM sleep amounts in min). 22 Intervals were selected to account for the bimodal pattern of the sleep-wake cycle modulation in this crepuscular specie. Selected segments coincided with the evening an morning peaks of W (at ZT12 and ZT23 respectively) and 4-h intervals centered at midday and mid-night (ZT4-7 and ZT16-19, respectively) anticrepuscular sleep predominant rest phases. A three-way rANOVA was performed for repeated factors phase (two levels: diurnal and nocturnal); protocol (two levels: baseline and REM sleep deprivation); and experimental octant (four levels: preceding octant, deprivation octant, recovery octant 1, and recovery octant 2) (P values of rANOVA with Huynh-Feldt correction are presented). A two-way rANOVA was also computed to compare values obtained in collapsed recovery octants 1 + 2, corresponding to the 6-h interval after deprivation, for repeated factors phase (two levels: diurnal and nocturnal) and protocol (two levels: baseline and REM sleep deprivation). Two-tailed paired t- tests were performed as post hoc comparisons for significant factors or factor interactions found in rANOVAs. Paired t-tests were also performed between nocturnal and diurnal values expressed as differences with respect to baseline values.
RESULTS
Modulation of States in the 12:12 Light-Dark Schedule
REM sleep accounted for 3.0% of total recording time, NREM sleep for 37.1%, and W for 59.8%. Diurnal and nocturnal amounts (in min) of W, NREM sleep, and REM sleep were 450.4 and 411.5; 247.8 and 284.9; and 20.1 and 23.6, respectively. A dominant characteristic in the sleep-wake cycle of this species is the strong bimodal modulation of W (Figure 1). The modes exhibited crepuscular timing, one occurring 1-h after dusk (ZT12) and the other 1-h before dawn (ZT23) (One-way rANOVA for factor ZT, F(23,184) = 3.06, P = 0.036). The mode at ZT23 corresponded to an ephemeral bout of W before dawn, whereas the mode at ZT12 was the highest value of a broad peak of W beginning during the last third of the light phase. Values of W at ZT12 and ZT23 were higher than those at ZT4-7 and the ZT16-19 intervals (Table 3).
Figure 1.
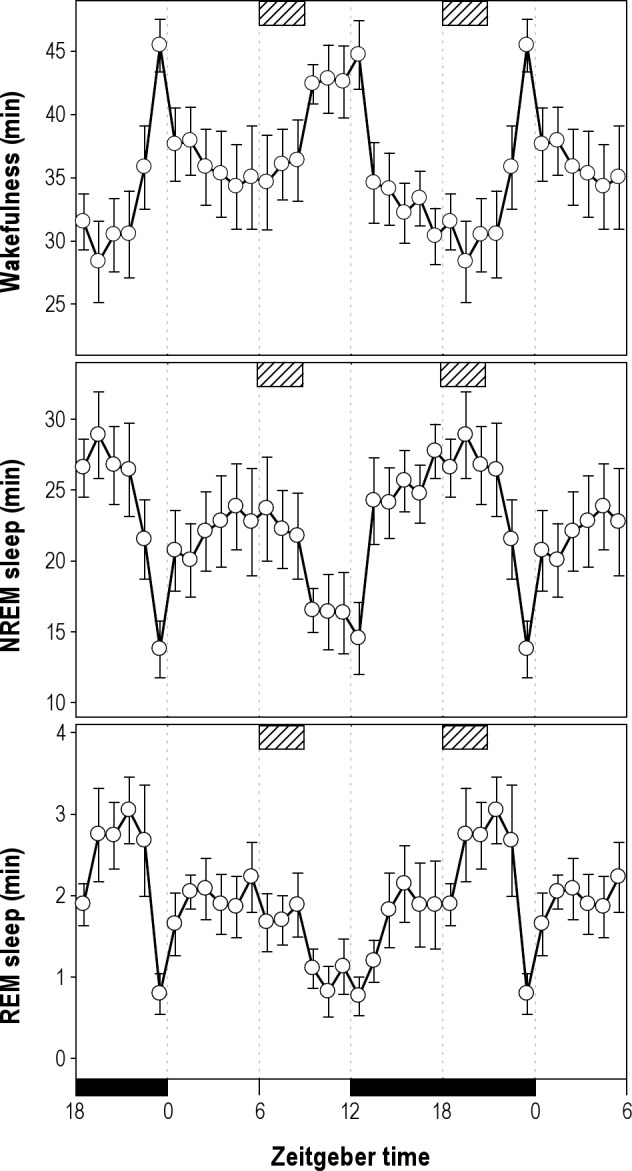
Mean curve of sleep-wake states. Circles correspond to the hourly mean time of each state (± standard error of the mean) obtained during 4 undisturbed days in nine male O. degus. For clarity, the initial and final 6 h are double-plotted. Horizontal black bars indicate dark phase. Hatcheds bar in upper abscissa indicate diurnal (RH06) and nocturnal (RH18) deprivation time. NREM, nonrapid eye movement; REM, rapid eye movement.
Table 3.
Statistics of planned contrast between selected intervals of baseline mean curves
The bimodal profile of W delimited two sleep predominant rest phases, centered at midday and midnight, respectively (Figure 1). The NREM sleep profile exhibited a diurnal plateau starting at ZT0 and declining after midday, in concurrence with the evening activity peak, and a nocturnal plateau starting at ZT13 and declining during the second half of the dark phase (one-way rANOVA for factor ZT, F(23,184) = 2.88, P = 0.0418). NREM sleep amounts observed at ZT12 were lower than those observed at ZT4-7 and ZT16-19 intervals, and NREM sleep at ZT23 was lower than that at ZT4-7 and ZT16-19 (Table 3). REM sleep modulation exhibited a similar pattern to that of NREM sleep (Figure 1), with two crepuscular dips, whose minima mirrored the W peaks (One-way rANOVA for factor ZT, F(23,184) = 3.04, P = 0.0128). A diurnal mode of REM sleep occurred at ZT5, and the nocturnal mode was shifted toward the second half of the dark phase. The amounts of REM sleep at ZT4-7 and ZT16-19 intervals were higher than REM sleep at ZT12 and ZT23 (Table 3). No differences are detected between REM sleep amounts of midday and midnight anticrepuscular rest phases.
Response of Sleep States to REM Sleep Deprivation
Three-hour deprivation sessions were scheduled to coincide with the diurnal and nocturnal rest phases, corresponding to octant 2 in the case of the RH06 protocol and to octant 6 for RH18 (see Table 1 for details on the octants corresponding to a given ZT). An almost complete suppression of consolidated REM sleep episodes was achieved during diurnal and nocturnal deprivation sessions. REM sleep obtained during deprivation octants represented 13% and 19.6% of baseline values (three-way rANOVA, experimental octant*protocol interaction, F(3,18) = 17.7, P < 0.0001, Table 1), corresponding to a net loss of 4.4 and 5.8 min of the state during RH06 and RH18 deprivation intervals with respect to corresponding baseline values (paired t-test, P < 0.0005). It became evident during deprivation sessions that our wild-captured O. degus animals were highly reactive to gentle moving. W increased by 21.7 and 29.6 min during diurnal and nocturnal deprivation, corresponding to a 20.3% (paired t-test, P < 0.0084) and 29.6% (paired t-test, P < 0.0086) increase with respect to baseline (three-way rANOVA, experimental octant*protocol interaction, F(3,18) = 8.06, P = 0.0001). As a consequence, NREM during RH06 and RH18 deprivation octants was reduced by 23.4% (paired t-test, P < 0.0299) and 28.8% (paired t-test, P < 0.0211) of baseline values (three-way rANOVA, experimental octant*protocol interaction, F(3,18) = 6.04, P < 0.0009; Table 1).
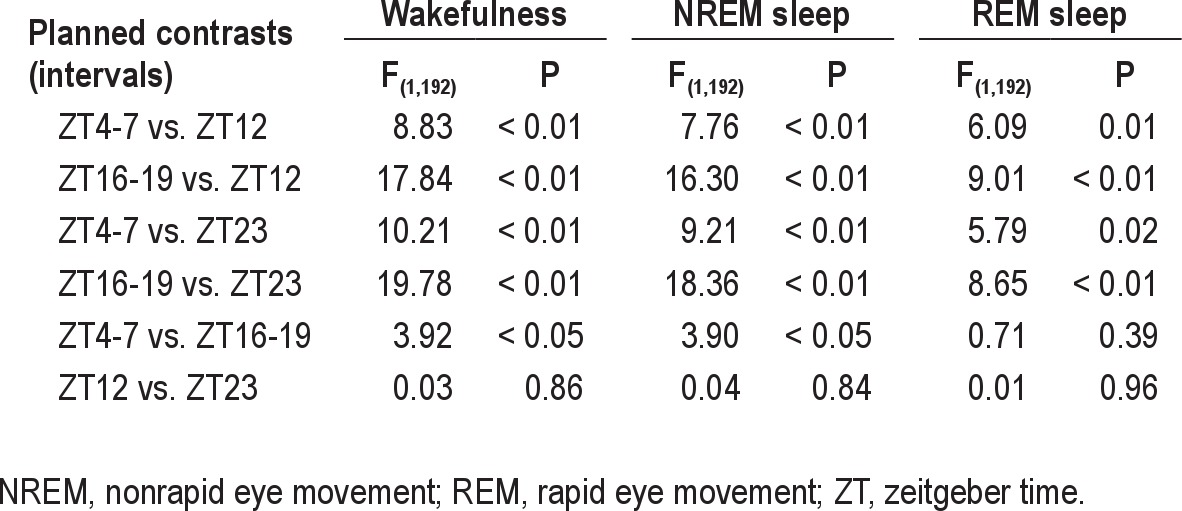
REM sleep propensity increased during the nocturnal deprivation session, as the REM sleep transition index for the third hour of deprivation was four times higher than the first hour (2.26 versus 0.65 respectively, two-tailed paired t-test, P = 0.038, Figure 2). No trend was detected during diurnal deprivation.
Figure 2.
REM sleep transition index during diurnal (closed circles) and nocturnal (closed squares) deprivation. Symbols correspond to hourly means (± standard error of the mean). Horizontal dashed lines represent mean baseline values for the corresponding deprivation octant (aP = 0.0375, bP = 0.0875, two-tailed paired t-test with respect to the first and second hour of RH18 deprivation interval, respectively). NREM, nonrapid eye movement; REM, rapid eye movement.
REM Sleep Homeostatic Response
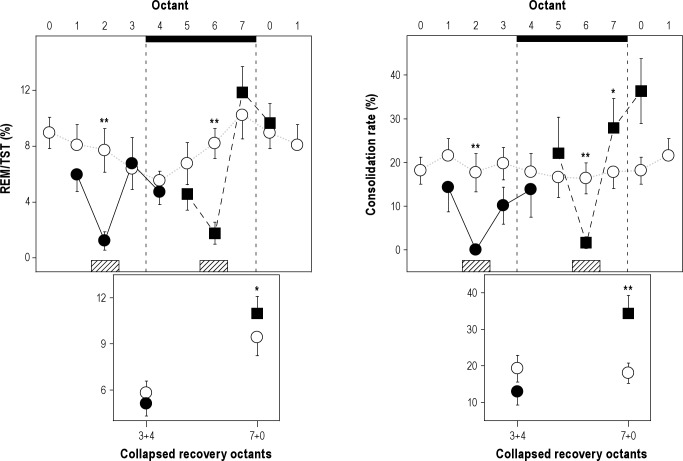
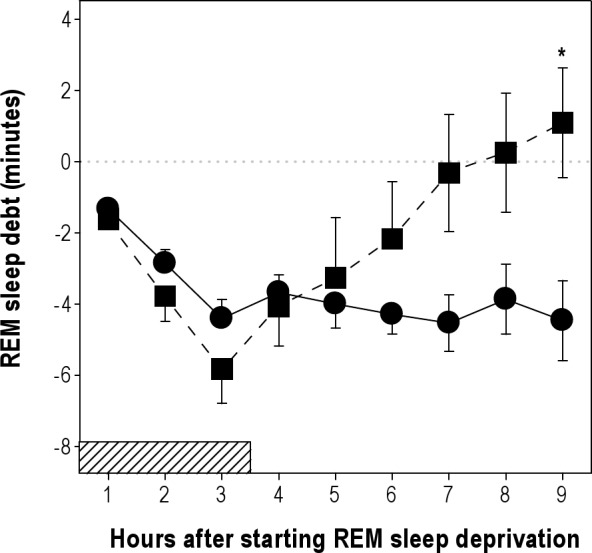
The homeostatic response of REM sleep was evaluated during the 6 -h interval following selective deprivation. Consistent with the increased propensity for REM sleep during nocturnal deprivation (Figure 2), a 56.3% increase in REM sleep amount with respect to baseline (paired t-test, P < 0.0001; two-way rANOVA, phase * protocol interaction, F(1,8) = 15.28, P = 0.0045, Table 1) was obtained during recovery octants 1 + 2 (corresponding to octants 7 and 0). In the same interval, NREM sleep time increased by 28.6% with respect to baseline (paired t-test, P = 0.0010; two-way rANOVA for factor protocol F(1,8) = 27.7, P = 0.0008). The specific increase in REM sleep was evaluated by means of the REM/TST ratio. REM/TST observed during recovery octants 1 + 2 of the nocturnal deprivation protocol was higher than baseline (11.0 and 9.6 respectively, paired t-test P = 0.0194; two-way rANOVA, phase*protocol interaction, F(1,8) = 9.64, P = 0.0146; Figure 3). The REM sleep increase observed during RH18 recovery octants 1 + 2 was associated with a 100% increment in the number of long REM sleep episodes as compared to the corresponding baseline interval (paired t-test, P < 0.0001; two-way rANOVA, phase*protocol interaction, F(1,8) = 44.46, P = 0.0002; Table 1). The REM sleep consolidation rate increased from 18.0 during baseline to 34.3 during RH18 recovery octants (paired t-test, P = 0.0059; two- way rANOVA, phase*protocol interaction, F(1,8) = 12.31, P = 0.0080; Figure 3). Increases in sleep states after nocturnal deprivation occurred at the expense of wake-fulness, which diminished by 19.1% with respect to baseline (two-way rANOVA for factor protocol, F(1,8) = 32.6, P = 0.0004, Table 1). In contrast to the robust nocturnal REM sleep response, no signs of REM sleep rebound were found during recovery octants 1 + 2 after diurnal deprivation. Total amount of REM sleep, number of REM sleep transitions, and long REM sleep episodes were equivalent to baseline values. Furthermore, W and NREM sleep amounts did not differ with respect to baseline (Table 1 and Figure 3).
Figure 3.
REM sleep homeostasis in response to 3-h diurnal and nocturnal deprivation. The panel compares REM/TST (left) and consolidation rate (right) baseline values (open circles) to diurnal (closed circles) and nocturnal (closed squares) deprivation protocols. The entire day is presented at 3-h time resolution (upper graphs). Collapsed data for the two recovery octants and respective baseline after diurnal and nocturnal deprivation are also shown (lower graphs). Values correspond to the means (± standard error of the mean) obtained in nine O. degus animals in the corresponding octant. *P < 0.05; **P < 0.01, posthoc two-tailed paired t-test with respect to baseline. A hatched bar on the abscissa identifies the diurnal and nocturnal deprivation octants. Horizontal black bars indicate dark phase. REM, rapid eye movement; TST, total sleep time.
Evaluation of REM Sleep Homeostatic Process and Phase of Day
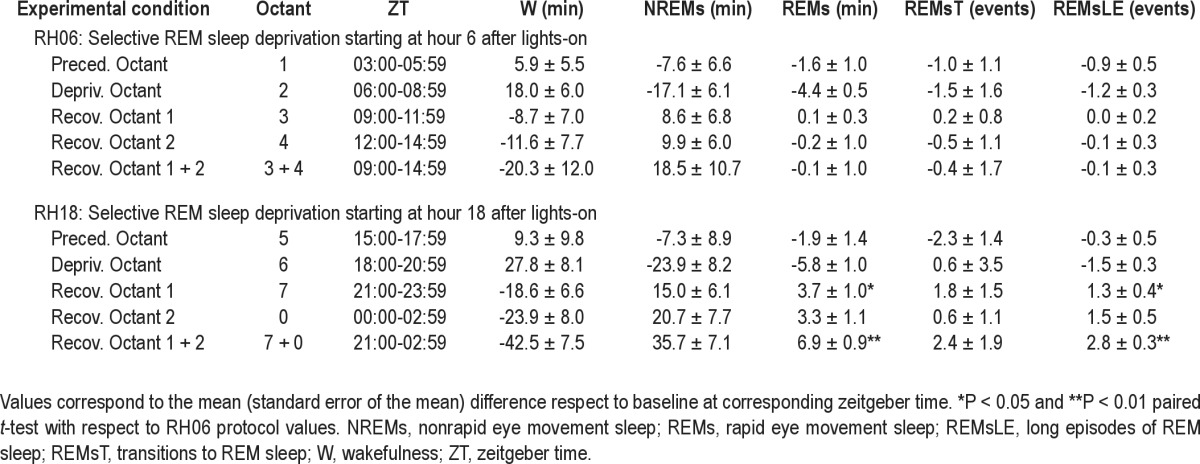
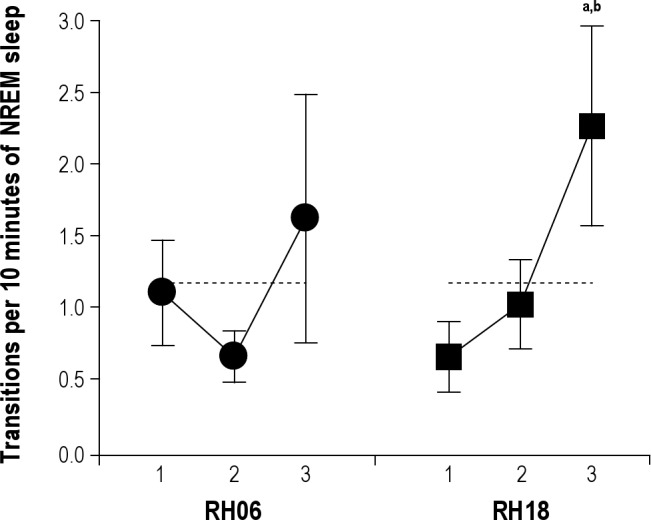
Equivalent cumulative REM sleep debt at the third hour of deprivation was obtained during diurnal and nocturnal deprivations (Table 2). The increase in wakefulness obtained during deprivation did not differ between the diurnal and nocturnal condition. The diurnal and nocturnal decreases in NREM sleep during deprivation octants were also similar (Table 2). Figure 4 depicts the evolution of REM sleep debt during deprivation and recovery intervals for RH06 and RH18 protocols. REM sleep debt after nocturnal deprivation was fully compensated for at the end of the recovery interval, and the recovered amount was greater than that of RH06 at the sixth hour of recovery (two-way rANOVA, hour after starting deprivation*phase interaction, F(8,64) = 6.2, P = 0.0022, Figure 4). REM sleep amount and the number of long REM sleep episodes obtained during recovery octants of the RH18 protocol more than doubled the amounts documented after diurnal deprivation (Table 1). REM sleep expression during RH18 recovery octants 1 + 2 accumulated an excess of 6.9 min (paired t-test, P < 0.0001) and 2.8 long REM sleep episodes with respect to baseline (paired t-test, P < 0.0001) as compared to 0.1 min REM sleep excess and -0.1 long episodes after diurnal deprivation (Table 2).
Figure 4.
Compensation for REM sleep debt after diurnal and nocturnal deprivation. Values correspond to the mean (± standard error of the mean) cumulative difference with respect to baseline values starting at the first hour of REM sleep deprivation and including 6 h of recovery. Diurnal deprivation: closed circles; nocturnal deprivation: closed squares. *P = 0.0435, two-tailed paired t-test of RH18 versus RH06 protocol. Horizontal hatched bars represent deprivation intervals. REM, rapid eye movement.
To further demonstrate the phase dependence of REM sleep propensity measures, we expressed REM/TST ratio and consolidation rate as differences respect to baseline (Figure 5). Two-way rANOVA for factors phase and experimental octant was performed obtaining a significant effect of experimental octant on REM/TST (F(2,16) = 4.54, p = 0.0275), and a significant interaction of phase*experimental octant on consolidation rate (F(2,13) = 4.99, P = 0.0280). Two-tailed paired t-tests demonstrated that REM/TST ratio (P = 0.0141) and consolidation rate (P = 0.0059) observed during RH18 recovery octants 1 + 2 were significantly and consistently higher than those of RH06.
Figure 5.
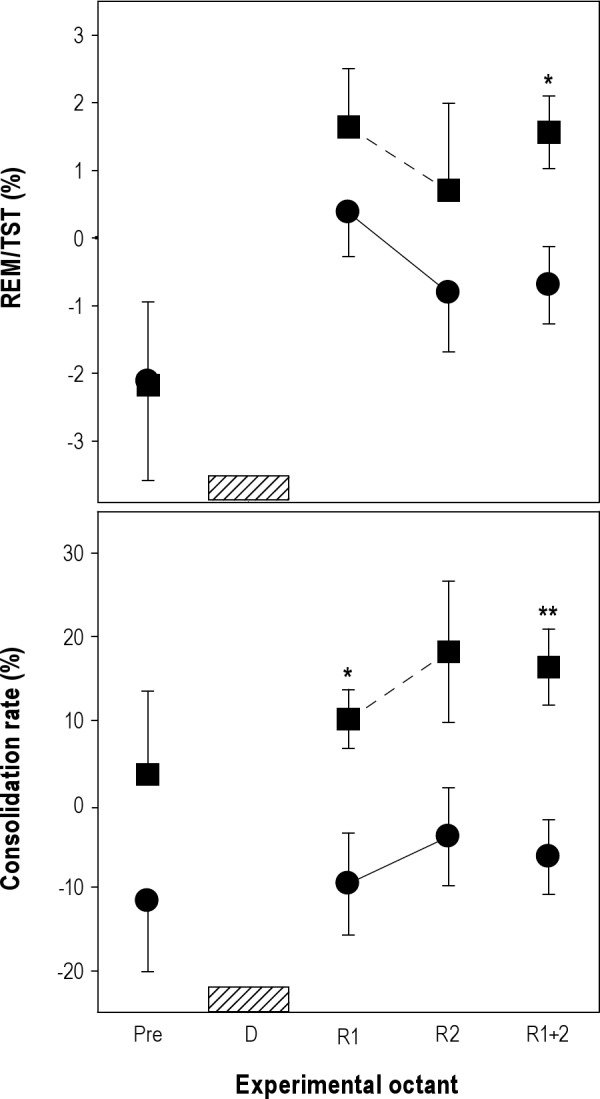
Comparisons of the REM sleep homeostatic response after diurnal and nocturnal deprivations. Values correspond to the mean (± standard error of the mean) obtained in nine O. degus and are expressed as differences with respect to baseline. Diurnal (closed circles) and nocturnal (closed squares) data were aligned to deprivation interval (hatched bar): preceding octant (Pre), deprivation octant (D, hatched bar), and recovery octants (R1, R2, and collapsed recovery octants R1 + 2). *P < 0.05, **P < 0.01 two-tailed paired t-test of nocturnal versus diurnal differences. REM, rapid eye movement; TST, total sleep time.
DISCUSSION
We present evidence that REM sleep is actively promoted during the nocturnal rest phase in crepuscular O. degus animals. Equivalent amounts of REM sleep debt were differentially compensated for at dawn and dusk, consistent with the notion that REM sleep is not only permitted at the nadir of the circadian modulation of arousal, as proposed by the opponent-process model, but also actively promoted by the circadian system during nocturnal rest.
Crepuscular Organization of the Sleep-Wake Cycle in O. degus
Our results in wild-captured O. degus are consistent with those reported by Kas and Edgar10,11 obtained in captive-bred individuals, suggesting that the crepuscular pattern of the sleep-wake cycle is a constitutive trait of the species. The sleep-wake cycle of wild-captured and captive-bred O. degus recorded under isolation exhibited three main features: high sleep state fragmentation with relatively low REM sleep content, no significant light-dark differences for sleep states, and crepuscular consolidated bouts of W just before dusk and dawn. Under a 12:12 light-dark cycle, a relatively brief (1-h) and sustained activity bout occurring at ZT23 occurs before dawn, occupying 75% of that hour, whereas a broader evening peak begins about 3-h before dusk and ends 1-h after lights-off (Figure 1). Particularly relevant to our study is the fact that sleep-predominant rest intervals are found around midday and midnight, with similar amounts of NREM and REM sleep.
REM Sleep Homeostasis in the Crepuscular O. degus
The analytic perspective adopted in this study assumes that the buildup of the homeostatic REM sleep propensity is determined primarily by the REM sleep debt, where the absence of REM sleep is connected causally with its compensation process.23–25 Alternatively, it has been proposed that REM sleep propensity is a consequence of NREM sleep expression, so that the magnitude of the REM sleep rebound after a deprivation interval will be directly correlated to the amount of NREM sleep cumulated during deprivation window.26 This hypothesis has been directly tested in the rat (1) by estimating the REM sleep homeostatic process under combined total and REM selective sleep deprivation protocols,18,27 (2) by comparing REM sleep rebound after long-term total or selective REM sleep deprivation protocols,28 (3) by modeling the timing of REM sleep homeostatic response after total sleep deprivations of different durations,29 and (4) by estimating the completeness of REM sleep compensation after REM sleep deprivations.21,30 Most results support the notion that NREM sleep is critical in the buildup of short-term REM sleep homeostatic process,31 that determines the dynamics of the NREM-REM sleep cycle,29 but do not support a role of NREM sleep in the long-term REM sleep homeostatic process that determines REM sleep rebound.18,27–29 Our results are consistent with a model where the REM sleep homeostatic response is determined primarily by the REM sleep debt and not by cumulated NREM sleep. REM sleep after nocturnal deprivation was fully compensated despite a 28.8% fall in cumulated NREM sleep during deprivation window. In addition, the 23.4% reduction in NREM sleep during diurnal deprivation protocol was followed by a minimal and nonsignificant (1.5%) reduction of REM sleep during recovery respect to baseline. Finally, no significant correlations were obtained between NREM sleep cumulated during deprivation window and REM sleep during recovery interval for diurnal and nocturnal protocols (data not shown).
Kas and Edgar11 explored sleep homeostasis in captive-bred O. degus animals. They performed 12-h total sleep deprivation ending at dusk or shortly before dawn. Increments in TST, mean sleep bout duration, and NREM sleep amounts were observed within the hour immediately consecutive to nocturnal and diurnal deprivation. A minor increment in REM sleep was documented only after nocturnal deprivation. The lack of REM sleep increment after diurnal deprivation and the minor response of REM sleep after nocturnal deprivation could be interpreted as the interference of the enhanced NREM sleep homeostatic process in response to the prolonged 12-h wake bout, interfering with the diurnal and nocturnal REM sleep rebound as described in the rat.27 Whether the REM sleep increment observed after nocturnal deprivation corresponded to a REM sleep homeostatic process or to an unspecific increment in TST was left undetermined because neither REM sleep propensity measures nor estimates of the ongoing REM sleep compensation was reported.
Selective REM sleep deprivation is the procedure of choice to explore REM sleep homeostatic processes as it minimizes NREM sleep interference on REM sleep expression. REM sleep propensity may be estimated by evaluating the NREM-REM sleep transition probability during selective deprivation intervals.16,18,20,25 During the recovery interval, REM sleep rebound,23 REM sleep compensation,21,24 REM/TST ratio, and REM sleep episode consolidation29 are confident reporters of the REM sleep homeostatic process.
Particularly relevant to our study are short-term sleep deprivation studies performed in the rat, where fast REM sleep rebounds are observed only when the deprivation interval coincides with the highest REM sleep circadian-driven propensity. Whereas 3- to 6-h total sleep deprivation during the active (dark) phase is not followed by a consistent REM sleep compensation in the rat,32 a 2- to 3-h sleep deprivation during the rest phase triggers a robust REM sleep rebound.17,18,20,30 We took advantage of this property of REM sleep regulation to determine the REM sleep phase preference in O. degus.
Our results suggest that the observed nocturnal versus diurnal differences in REM sleep compensation were not a consequence of a purely homeostatic phenomenon: (1) the sleep deprivation procedure was effective causing a REM sleep loss of 4.4 min during the day and 5.8 min during the night, corresponding to a relative loss of 87% and 83%, respectively (Table 2); (2) mean residual amounts of REM sleep during deprivation session were 0.7 min of REM sleep during diurnal deprivation and 1.4 min during nocturnal deprivation (Table 1), with a paired nocturnal-diurnal difference of 0.8 min (paired t-test, P = 0.2545); and (3) REM sleep losses were acquired at expenses of equivalent diurnal and nocturnal increases in wakefulness and decreases in NREM sleep with respect to baseline (Tables 1 and 2). The complete compensation of REM sleep after nocturnal deprivation contrasts with the absolute lack of REM sleep response after diurnal deprivation (Table 1 and Figure 4).
Phase Dependence of REM Sleep Promotion
Among diurnal and nocturnal mammals the timing of REM sleep rebound depends on circadian phase, as REM-selective deprivation ending during the rest phase results in a faster compensatory rebound than deprivation ending during active phase.15,16 The fact that our wild-captured O. degus compensate for REM sleep debts cumulated during nocturnal deprivation and not during diurnal deprivation suggest that a circadian process that promotes REM sleep during the second half of the night and early morning may be in operation.
One finding argues against a phase-dependent REM sleeppromoting process. The arousal drive occurring during evening octants 3 + 4 appears to be stronger than that of morning octants 7 + 0 (241 versus 223 min of wakefulness, respectively, paired t-test, P = 0.0687). A weaker morning arousal drive may favor in part the REM sleep recovery after nocturnal deprivation (Figure 4) without necessarily accompanied by a concurrent increase in REM sleep promotion according to the opponent process model. Nevertheless, REM sleep propensity measures support that REM sleep compensation after nocturnal deprivation occurs as a result of a state specific REM sleep promotion. First, the nocturnal deprivation interval was accompanied by an increasing trend toward REM sleep propensity (Figure 2) and was followed by a REM sleep rebound that occurred at the expense of wake time during the morning arousal bout (Table 1). Second, the REM sleep consolidation rate observed after nocturnal deprivation almost doubled that observed at the corresponding baseline interval, and third, the increase in the REM/TST ratio during recovery octants confirms that the increased REM sleep is a specific response of the state (Figure 3). In contrast, REM sleep propensity measures were unaffected during or after the diurnal deprivation (Figures 2 and 3), and were significantly lower than that of the nocturnal deprivation (Table 2 and Figure 5).
The results presented here were obtained under photic entrainment to a 12:12 light-dark cycle. Photic input to the retina influences the activity of neural networks involved in sleep and wake generation, a phenomenon known as photic masking. As photic masking could interfere in the circadian and ho-meostatic-driven sleep propensity,33,34 we cannot exclude that light availability may, at least in part, explain the observed nocturnal-diurnal asymmetry in the REM sleep homeostatic response. Nevertheless, our results are consistent with minor photic masking on the observed REM sleep rebound, as the robust REM sleep response observed after nocturnal deprivation started immediately during the last octant of the dark phase and continued during the first of the light phase. In addition, REM sleep parameters following diurnal deprivation exhibit no increasing or decreasing trend after the light-to-dark transition. Research is needed to further elucidate the role of photic masking in the diel organization of the sleep-wake cycle in this crepuscular species.
A Choral Model of the Crepuscular Organization of the Sleep-Wake Cycle
Mistlberger35 discussed the incompleteness of the opponent process model, noting that there is evidence to support the notion that sleep is actively promoted by the circadian system. We propose that the sleep-wake cycle of O. degus is modulated by a chorus of circadian outputs that account for arousal and REM sleep promotion (Figure 6): under steady-state photic entrainment, a bimodal arousal promoting (A) process with modes at dawn (M-peak) and dusk (E-peak) separated by anticrepuscular midday and midnight nadirs coexist with a unimodal REM sleep promoting process (R). The acrophase of the R process is centered at the second half of the dark phase with a nadir at the second half of the light phase. The bimodal arousing process may correspond to the output of two coupled M and E circadian oscillators, as proposed by Pittendrigh and Daan.36 In our qualitative model, A and R circadian processes delimit four windows or phases of the crepuscular sleep-wake cycle:
Figure 6.
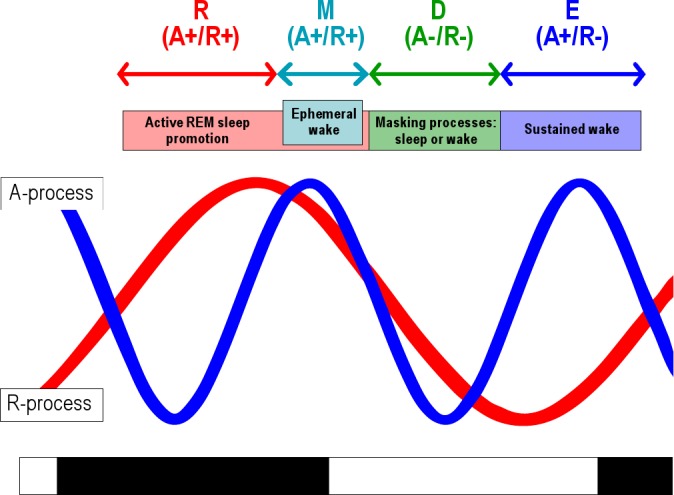
Choral model of the sleep-wake cycle (explanation in Discussion section). REM, rapid eye movement.
(1) The R-window coincides with the acrophase of the R process and the nocturnal nadir of the circadian arousing output, so that only REM sleep is favored (“R+/A-” condition). It corresponds to the second half of the night in coincidence with the core body temperature minimum as has been reported for O. degus by several authors.1,37,38 It is during this phase that total sleep deprivation11 or selective REM sleep deprivation triggers REM sleep homeostatic compensation.
(2) An ephemeral and intense M-window occurs just before dawn at ZT23 and coincides with the M-peak of the A process and the initial falling limb of the R process, so that REM sleep and arousal are favored (“R+/A+” condition). The M arousal peak has been found to interfere with NREM sleep rebound at circadian time 23.11 The coincidence of a strong R process at the M-window is supported by the persistence of the REM sleep homeostatic response in the hours immediately following the arousal peak, as has been described in our report and under constant darkness.11 The ephemeral nature of the M arousal bout of wakefulness may be the consequence of the competitive influence of R and A modulation on sleep-wake state generators.
(3) A default (D) window is centered on midday where the arousal process attains its diurnal nadir in coincidence with a low R process (“R-/A-” condition). Our estimation of the R process rests on the evidence that selective REM sleep deprivation was ineffective in triggering a REM sleep homeostatic rebound, in both 6 or 12 total sleep deprivation protocols performed during the subjective day.11 Because of the minimal circadian arousal and sleep promotion during the D-window, we expect a predominance of masking processes, so that a sleep-or wake-predominant D-interval may be the result of environmental or housing conditions. Vivanco et al.39 reported a strong masking of wheel running under a 1:1 light-dark protocol in a time interval consistent with our D-interval. As Horne40 and Hut et al.41 discussed, more or less naturalistic environments may deeply affect phase preferences and expression of sleep states or activity. Our undisturbed O. degus animals displayed a sleep predominant D-interval (Figure 1), which may be explained by a negative masking of W or positive masking of sleep states, given the arbitrary confinement settings. O. degus living in its natural environment exhibits midday-centered activity peaks or valleys depending on season and ambient temperature.9
(4) Finally, an E-window coincides with the sustained evening (E) bout of wakefulness before dusk and may last until the first half of the night. We propose that the robustness of the evening arousal interval is explained by the coincidence of the E-peak of the A process and the nadir of the R process (“R-/ A+” condition). The absence of sleep promotion may facilitate the positive masking of activity and wheel-running during the first half of the night as can be observed in the diurnal-nocturnal transposition of degus.19,42
The nocturnal REM sleep phase preference proposed here is in line with other biological traits of our studied specie such as the nocturnal core body temperature nadir,37,38 cortisol increases at dawn,43 several hematological and biochemical variables described by Otalora et al.,38 and the diurnal characteristic of the retina, with a relatively high proportion of cones.44,45 Positive and/or negative masking processes occurring in coincidence with the D-window may explain the facultative nature of the midday activity peak observed during winter under natural conditions,9 the unstable diurnal preference observed in captive individuals,6,39 and the rapid diurnal-nocturnal phase preference inversion in response to nonphotic stimuli.19,42
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by Grant FONDECYT 3010028. During this project, Mr. Felipe Hernández was a fellow of the Fundación Guillermo Puelma. Animals were captured with the help of Dr. Rodrigo Vásquez (Faculty of Sciences, University of Chile). Institution at which the work was performed: Programa de Fisiología y Biofísica, Instituto de Ciencias Biomédicas, Facultad de Medicina, Universidad de Chile, Santiago, Chile
REFERENCES
- 1.Labyak SE, Lee TM, Goel N. Rhythm chronotypes in a diurnal rodent, Octodon degus. Am J Physiol. 1997;273:R1058–66. doi: 10.1152/ajpregu.1997.273.3.R1058. [DOI] [PubMed] [Google Scholar]
- 2.Cuesta M, Clesse D, Pévet P, Challet E. From daily behavior to hormonal and neurotransmitters rhythms: comparison between diurnal and nocturnal rat species. Horm Behav. 2009;55:338–47. doi: 10.1016/j.yhbeh.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Fernández-Duque E, de la Iglesia H, Erkert HG. Moonstruck primates: owl monkeys (Aotus) need moonlight for nocturnal activity in their natural environment. PLoS One. 2010;5:e12572. doi: 10.1371/journal.pone.0012572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McElhinny TL, Smale L, Holekamp KE. Patterns of body temperature, activity, and reproductive behavior in a tropical murid rodent, Arvicanthis niloticus. Physiol Behav. 1997;62:91–6. doi: 10.1016/s0031-9384(97)00146-7. [DOI] [PubMed] [Google Scholar]
- 5.Katona C, Smale L. Wheel-running rhythms in Arvicanthis niloticus. Physiol Behav. 1997;61:365–72. doi: 10.1016/s0031-9384(96)00407-6. [DOI] [PubMed] [Google Scholar]
- 6.García-Allegue R, Lax P, Madariaga AM, Madrid JA. Locomotor and feeding activity rhythms in a light-entrained diurnal rodent, Octodon degus. Am J Physiol. 1999;277:R523–31. doi: 10.1152/ajpregu.1999.277.2.R523. [DOI] [PubMed] [Google Scholar]
- 7.Verhagen LA, Pévet P, Saboureau M, et al. Temporal organization of the 24-h corticosterone rhythm in the diurnal murid rodent Arvicanthis ansorgei Thomas 1910. Brain Res. 2004;995:197–204. doi: 10.1016/j.brainres.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Rattenborg NC, Voirin B, Vyssotski AL, et al. Sleeping outside the box: electroencephalographic measures of sleep in sloths inhabiting a rainforest. Biol Lett. 2008;4:402–5. doi: 10.1098/rsbl.2008.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kenagy GJ, Nespolo RF, Vasquez RA, Bozinovic F. Daily and seasonal limits of time and temperature to activity of degus. Rev Chil Hist Nat. 2002;75:567–81. [Google Scholar]
- 10.Kas MJ, Edgar DM. Crepuscular rhythms of EEG sleep-wake in a hystri-comorph rodent, Octodon degus. J Biol Rhythms. 1998;13:9–17. doi: 10.1177/074873098128999871. [DOI] [PubMed] [Google Scholar]
- 11.Kas MJ, Edgar DM. Circadian timed wakefulness at dawn opposes compensatory sleep responses after sleep deprivation in Octodon degus. Sleep. 1999;22:1045–53. doi: 10.1093/sleep/22.8.1045. [DOI] [PubMed] [Google Scholar]
- 12.Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalo-graphic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15:3526–38. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cambras T, Weller JR, Anglès-Pujoràs M, et al. Circadian desynchronization of core body temperature and sleep stages in the rat. Proc Natl Acad Sci U S A. 2007;104:7634–9. doi: 10.1073/pnas.0702424104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edgar DM, Dement WC, Fuller CA. Effect of SCN lesions on sleep in squirrel monkeys: evidence for opponent processes in sleep-wake regulation. J Neurosci. 1993;13:1065–79. doi: 10.1523/JNEUROSCI.13-03-01065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wurts SW, Edgar DM. Circadian and homeostatic control of rapid eye movement (REM) sleep: promotion of REM tendency by the suprachiasmatic nucleus. J Neurosci. 2000;20:4300–10. doi: 10.1523/JNEUROSCI.20-11-04300.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Werth E, Cote KA, Gallmann E, Borbély AA, Achermann P. Selective REM sleep deprivation during daytime I. Time course of interventions and recovery sleep. Am J Physiol. 2002;283:R521–6. doi: 10.1152/ajpregu.00462.2001. [DOI] [PubMed] [Google Scholar]
- 17.Benington JH, Woudenberg MC, Heller HC. REM-sleep propensity accumulates during 2-h REM-sleep deprivation in the rest period in rats. Neurosci Lett. 1994;180:76–80. doi: 10.1016/0304-3940(94)90917-2. [DOI] [PubMed] [Google Scholar]
- 18.Ocampo-Garcés A, Molina E, Rodríguez A, Vivaldi EA. Homeostasis of REM sleep after total and selective sleep deprivation in the rat. J Neurophysiol. 2000;84:2699–702. doi: 10.1152/jn.2000.84.5.2699. [DOI] [PubMed] [Google Scholar]
- 19.Ocampo-Garcés A, Hernández F, Mena W, Palacios AG. Wheel-running and rest activity pattern interaction in two octodontids (Octodon degus, Octodon bridgesi) Biol Res. 2005;38:299–305. doi: 10.4067/s0716-97602005000200019. [DOI] [PubMed] [Google Scholar]
- 20.Ocampo-Garcés A, Vivaldi EA. Short-term homeostasis of REM sleep assessed in an intermittent REM sleep deprivation protocol in the rat. J Sleep Res. 2002;11:81–9. doi: 10.1046/j.1365-2869.2002.00281.x. [DOI] [PubMed] [Google Scholar]
- 21.Amici R, Cerri M, Ocampo-Garcés A, et al. Cold exposure and sleep in the rat: REM sleep homeostasis and body size. Sleep. 2008;31:708–15. doi: 10.1093/sleep/31.5.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sokal R, Rohlf FJ. New York: W. H. Freeman and Company; 1981. Biometry. [Google Scholar]
- 23.Dement W, Greenberg S, Klein R. The effect of partial REM sleep deprivation and delayed recovery. J Psychiatr Res. 1966;4:141–52. doi: 10.1016/0022-3956(66)90003-3. [DOI] [PubMed] [Google Scholar]
- 24.Parmeggiani PL, Cianci T, Calasso M, Zamboni G, Perez E. Quantitative analysis of short term deprivation and recovery of desynchronized sleep in cats. Electroencephalogr Clin Neurophysiol. 1980;50:293–302. doi: 10.1016/0013-4694(80)90157-1. [DOI] [PubMed] [Google Scholar]
- 25.Endo T, Roth C, Landolt HP, et al. Selective REM sleep deprivation in humans: effects on sleep and sleep EEG. Am J Physiol. 1998;274:R1186–94. doi: 10.1152/ajpregu.1998.274.4.R1186. [DOI] [PubMed] [Google Scholar]
- 26.Benington JH, Heller HC. Does the function of REM sleep concern non-REM sleep or waking? Prog Neurobiol. 1994;44:433–49. doi: 10.1016/0301-0082(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 27.Endo T, Schwierin B, Borbély AA, Tobler I. Selective and total sleep deprivation: effect on the sleep EEG in the rat. Psychiatry Res. 1997;66:97–110. doi: 10.1016/s0165-1781(96)03029-6. [DOI] [PubMed] [Google Scholar]
- 28.Rechtschaffen A, Bergmann BM, Gilliland MA, Bauer K. Effect of method, duration, and sleep stage on rebounds from sleep deprivation in the rat. Sleep. 1999;22:11–31. doi: 10.1093/sleep/22.1.11. [DOI] [PubMed] [Google Scholar]
- 29.Franken P. Long-term vs. short-term processes regulating REM sleep. J Sleep Res. 2002;11:17–28. doi: 10.1046/j.1365-2869.2002.00275.x. [DOI] [PubMed] [Google Scholar]
- 30.Shea JL, Mochizuki T, Sagvaag V, Aspevik T, Bjorkum AA, Datta S. Rapid eye movement (REM) sleep homeostatic regulatory processes in the rat: changes in the sleep-wake stages and electroencephalographic power spectra. Brain Res. 2008;1213:48–56. doi: 10.1016/j.brainres.2008.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vivaldi EA, Ocampo A, Wyneken U, Roncagliolo M, Zapata AM. Short-term homeostasis of active sleep and the architecture of sleep in the rat. J Neurophysiol. 1994;72:1745–55. doi: 10.1152/jn.1994.72.4.1745. [DOI] [PubMed] [Google Scholar]
- 32.Tobler I, Borbély AA. The effect of 3-h and 6-h sleep deprivation on sleep and EEG spectra of the rat. Behav Brain Res. 1990;36:73–8. doi: 10.1016/0166-4328(90)90161-7. [DOI] [PubMed] [Google Scholar]
- 33.Benca RM, Gilliland MA, Obermeyer WH. Effects of lighting conditions on sleep and wakefulness in albino Lewis and pigmented Brown Norway rats. Sleep. 1998;21:451–60. doi: 10.1093/sleep/21.5.451. [DOI] [PubMed] [Google Scholar]
- 34.Deboer T, Ruijgrok G, Meijer JH. Short light-dark cycles affect sleep in mice. Eur J Neurosci. 2007;26:3518–23. doi: 10.1111/j.1460-9568.2007.05964.x. [DOI] [PubMed] [Google Scholar]
- 35.Mistlberger RE. Circadian regulation of sleep in mammals: role of the suprachiasmatic nucleus. Brain Res Brain Res Rev. 2005;49:429–54. doi: 10.1016/j.brainresrev.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. V. Pacemaker structure: a clock for all seasons. J Comp Physiol. 1976;106:333–55. [Google Scholar]
- 37.Refinetti R. Rhythms of body temperature and temperature selection are out of phase in a diurnal rodent, Octodon degus. Physiol Behav. 1996;60:959–61. doi: 10.1016/0031-9384(96)00147-3. [DOI] [PubMed] [Google Scholar]
- 38.Otalora BB, Vivanco P, Madariaga AM, Madrid JA, Rol MA. Internal temporal order in the circadian system of a dual-phasing rodent, the Octodon degus. Chronobiol Int. 2010;27:1564–79. doi: 10.3109/07420528.2010.503294. [DOI] [PubMed] [Google Scholar]
- 39.Vivanco P, Rol MA, Madrid JA. Pacemaker phase control versus masking by light: setting the circadian chronotype in dual Octodon degus. Chronobiol Int. 2010;27:1365–79. doi: 10.3109/07420528.2010.502984. [DOI] [PubMed] [Google Scholar]
- 40.Horne J. REM sleep, energy balance and ‘optimal foraging’. Neurosci Biobehav Rev. 2009;33:466–74. doi: 10.1016/j.neubiorev.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 41.Hut RA, Pilorz V, Boerema AS, Strijkstra AM, Daan S. Working for food shifts nocturnal mouse activity into the day. PLoS One. 2011;6:e17527. doi: 10.1371/journal.pone.0017527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kas MJ, Edgar DM. A nonphotic stimulus inverts the diurnal-nocturnal phase preference in Octodon degus. J Neurosci. 1999;19:328–33. doi: 10.1523/JNEUROSCI.19-01-00328.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohawk JA, Lee TM. Restraint stress delays reentrainment in male and female diurnal and nocturnal rodents. J Biol Rhythms. 2005;20:245–56. doi: 10.1177/0748730405276323. [DOI] [PubMed] [Google Scholar]
- 44.Jacobs GH, Calderone JB, Fenwick JA, Krogh K, Williams GA. Visual adaptations in a diurnal rodent, Octodon degus. J Comp Physiol A. 2003;189:347–61. doi: 10.1007/s00359-003-0408-0. [DOI] [PubMed] [Google Scholar]
- 45.Chávez AE, Bozinovic F, Peichl L, Palacios AG. Retinal spectral sensitivity, fur coloration, and urine reflectance in the genus octodon (rodentia): implications for visual ecology. Invest Ophthalmol Vis Sci. 2005;44:2290–6. doi: 10.1167/iovs.02-0670. [DOI] [PubMed] [Google Scholar]