Summary
The aryl hydrocarbon receptor, which has been central to studies in toxicology for years as the receptor for the toxicant dioxin, is rapidly gaining interest in immunology based on its ability to influence T cell differentiation. Multiple studies have documented that binding of this receptor with certain ligands favors T cell differentiation towards regulatory T cells, and paradoxically binding of this same receptor with different ligands enhances Th17 effector cell differentiation. This finding has been confirmed in both in vitro and in vivo models, where different ligands are able to either ameliorate or conversely aggravate autoimmunity in experimental autoimmune encephalomyelitis. The aryl hydrocarbon receptor has both an endogenous role that is important in development and normal physiology, and also has an exogenous role as a receptor for man-made toxicants, with their binding leading to transcription of cytochrome P450 enzymes that metabolize these same ligands. Based on recent reports that will be summarized in this overview, we will consider the role that the aryl hydrocarbon receptor might play as a sensor to the outside environment, leading to alteration of the acquired immune system that might have relevance in transplantation or other medical conditions. In addition to describing the data in normal physiology and T cell differentiation, we will present examples of the importance of this receptor in preclinical models of disease, and highlight specific ligands that target the aryl hydrocarbon receptor and will have efficacy in treating transplant rejection and in tolerance protocols.
Keywords: Aryl hydrocarbon receptor, environment, T cell differentiation, autoimmunity, immunomodulation
Introduction
Why is it that some transplant recipients have stable graft function for years and then suddenly present with severe rejection? And why can two seemingly similar patients that receive organs from the same donor have such disparate outcomes after transplant? Undoubtedly genetic differences may explain some of this, but perhaps the physical environment that patients live in plays a role as well. This overview will introduce the aryl hydrocarbon receptor (AHR), which has been central to the field of toxicology, as a sensor to the outside environment that modulates the acquired immune system in response to toxins. The role of this receptor in modulating T cell differentiation between regulatory cells and effector cells will be explored, and the importance of the AHR in models of disease will be discussed. Further interrogation of this receptor will help us better understand how exposures in the environment may already be disrupting the fine balance our patients are in after transplantation, and also will provide a novel target for immunomodulation in response to rejection.
Background of the AHR
The AHR is a cytosolic receptor (Figure 1). Ligand binding to the AHR results in chaperone shedding and translocation to the nucleus where it dimerizes with AHR nuclear translocator (ARNT) and becomes a transcription factor for various genes including the cytochrome P450 enzymes. The AHR is best known as the receptor for the man-made toxicant 2,3,7,8-Tetrachlorodibenzodioxin (TCDD) through which its pathologic consequences, such as thymic involution, immunosuppression, chloracne, and cancer are manifested. The AHR is also a primary receptor for numerous man-made environmental exposures, including cigarette smoke, diesel exhaust, urban dust, and their components (polycyclic aromatic hydrocarbons, polychlorinated biphenyls, and other halogenated aromatic hydrocarbons)(1–4). The AHR is so commonly activated after toxic exposures that evidence of binding of the AHR and resultant upregulation of cytochrome P450 enzymes is often used to assess levels of contamination of various environments(4, 5). The AHR has been evolutionarily conserved(6). There exist invertebrate forms that don’t bind TCDD(7), indicating that the function of this receptor evolved to respond to toxins, but may have initially had a different purpose.
Figure 1. AHR/ARNT signaling pathway.
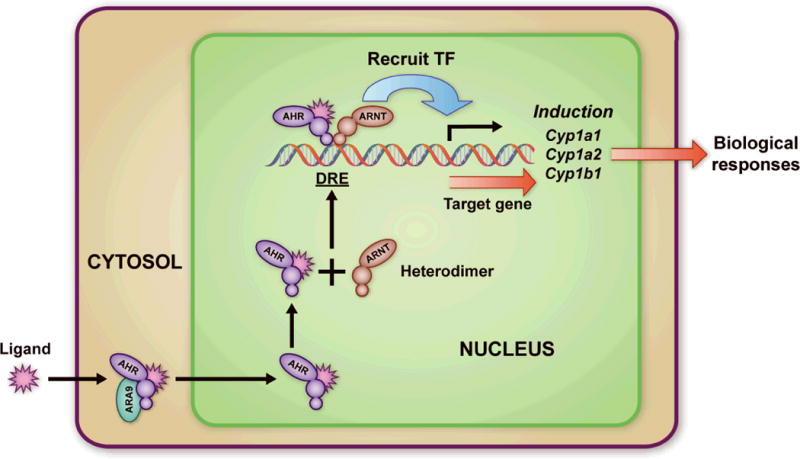
The AHR is a cytosolic receptor that is bound to a chaperone protein. When a ligand goes across the cell membrane and binds to the AHR, it sheds its chaperone and goes to the nucleus, where it associates with ARNT and binds to the Dioxin Response Element (DRE), becoming a transcription factor. It induces transcription of the cytochrome P450 enzymes including cyp1a1, cyp1a2, cyp1b1 and other metabolizing enzymes including glutathione S-transferase Ya (GSTYa) and aldehyde-3-dehydrogenase (ALDH-3). Reprinted with permission from (7). Copyright 2008 American Chemical Society.
The AHR and Normal Physiology
The strongest indication that the AHR has a role in embryology is the finding that AHR null (AHR–/–) mice have developmental abnormalities. The best defined is the patent ductus venosus (PDV), the embryologic connection between the portal system and venous drainage of the liver(8). Interestingly, the AHR hypomorph, which exhibits one-tenth the normal amount of AHR in hepatocytes, also has a PDV in 90% of recipients(9). However, if the hypomorphs are exposed to strong AHR ligands in utero, the PDV closes. This leads one to believe that presence and exposure of endogenous ligands is necessary for normal development. There continues to be an ongoing search for a true primary endogenous ligand of the receptor.
There are other associated abnormalities with the AHR–/– mouse, some of which involve the innate immune system (TABLE I). A recent report identifies the role of the AHR in the presence, location, and function of γδ-T cells in the gut and skin(10). γδ-T cells in AHR–/– mice are not able to generate IL-22, which binds to receptors on epithelial cells enhancing innate immune responses and promoting cell survival. In fact, IL-22 production is dependent on the presence of the AHR in a majority of cell types(11, 12). When characterizing related cell populations at epithelial barriers in skin and gut, intraepithelial lymphocytes (IEL) were not maintained in adequate numbers in the nulls, leading to increased bacterial burden in the gut and poor outcomes when infected with certain bacteria(13). Interestingly, adoptively transferred cells from wild-type into AHR–/– mice homed to the epithelium and repopulated the gut. Additionally, when wild-type mice were fed a diet lacking cruciferous proteins (that generate AHR ligands in the gut) or treated with gut-specific antibiotics, again IELs had significantly reduced number. Another population of cells in the intestine important in this gut-environment interface, intestinal lymphoid follicles, were found to depend on the presence of the AHR as well as activation of this receptor by ligands found in diet for their expansion and follicle formation(14, 15). Animals that lacked these cells were more susceptible to infection with certain gut bacteria (Citrobacter rodentium). These AHR-dependent cell populations at the interface of the gut are all part of the innate immune system, and through their secreted cytokines signal the acquired immune system in response to bacteria and dietary ligands. Loss of these cells leaves the host at risk to bacterial overgrowth and invasion.
Table I.
Differences between Aryl Hydrocarbon Receptor wild-type and null mice.
| Organ | AHR null response versus B6 /AHR het | Reference |
|---|---|---|
| Lung | Increased IL-6, TNF-α, and other inflammatory cytokines in BAL. | (62) |
| Increased neutrophillia following exposure to cigarette smoke and LPS. | (62) | |
| Increased edema following hyperoxia. | (63) | |
| GI | Increased development of colonic tumors | (64) |
| C. rodentium oral infection is 100% lethal. | (65) | |
| Reproduction | Abnormal ovarian follicles. | (66) |
| Altered seminal vesicle development. | (67) | |
| Vascular | Altered vascular structure in the eye, kidney and liver including patent ductus venosus. | (68) |
| Immune system | Reduced IL-22 expression. | (15, 18) |
| Enlarged spleen. | (69) | |
| Reduced γδ- T cell numbers in gut and skin. | (13, 70) | |
| Reduced Innate Lymphoid Cells in the gut. | (13, 14, 65) | |
| Delayed onset of EAE | (18) |
These observations indicate that the presence of the AHR is necessary to avoid phenotypic abnormalities in development, and also suggests the importance of activation of this receptor with one or more endogenous or gut/diet derived ligands early in development. In addition this receptor is central to maintaining elements of the innate immune system at interfaces with the outside environment.
Role in T Cell Differentiation
While it has long been known that TCDD toxicity causes immunosuppression and the generation of Regulatory T cells (Treg)(16), two landmark papers demonstrating a strong connection between the AHR and T cell differentiation were published in 2008. In the first, activation of the AHR with TCDD led to the differentiation of murine naïve CD4+ T cells into FoxP3+ Treg(17). In the second, activation of the AHR using a putative endogenous AHR ligand, 6-formylindolo[3,2-b]carbazole (FICZ), enhanced IL-17 and IL-22 expression in murine naïve CD4+ T cells cultured in Th17 conditions (stimulation with anti-CD3 and anti-CD28 antibodies in the presence of TGF-β and IL-6)(18). FICZ is a by-product of tryptophan breakdown following exposure to UVB light(19). In addition to roles in Th17 and Treg, the AHR is central to IL-22 expression by T cells(11) and for Th22 differentiation(20). IL-22, a member of the IL-10 cytokine family, is important in microbial immunity, and is considered to be protective to epithelial cells and hepatocytes(21–23).
Animal models have further supported the role of the AHR in both regulation and effector differentiation. Treatment of mice with TCDD or other AHR ligands was shown to enhance Treg numbers and activity in experimental autoimmune encephalomyelitis (EAE), resulting in less disease and prolonged graft survival respectively, while treatment with FICZ aggravated disease(17, 18).
The above data, along with our own experience that AHR-dependent Treg generation is enhanced by dendritic cells (DC), led us to consider the Indoleamine 2,3 Dioxygenase pathway (IDO) and its metabolites(24). IDO is produced by DCs in response to IFN-γ, is the rate-limiting enzyme in the breakdown of tryptophan, and plays a role in DC-derived Treg generation in vitro and in vivo(25). The mechanism for IDO-induced Treg has not been elucidated, although both tryptophan deprivation and activity of tryptophan metabolites have been proposed(26, 27). Because indole metabolites and tryptophan derivatives are known to be ligands of the AHR, we investigated a possible role for this receptor in the IDO pathway. We recently reported that kynurenine functions through the AHR on T cells to generate Treg directly(28). Additionally, ligands (including TCDD) can act through the AHR on DCs to upregulate IDO, which further leads to Treg generation. IFN-γ also leads to IDO generation in DCs in an AHR-independent manner.
We, and others, have identified in in vitro assays that multiple ligands including TCDD, kynurenine, ITE, and SU5416 (both discussed later) lead to an enhancement of Treg, and only the ligand FICZ, a tryptophan photoproduct present in the skin after light exposure, consistently enhances Th17 development(17, 18, 28, 29). Based on an idea that it may be beneficial for harmful toxicants to signal the acquired immune system to deviate towards an effector response, (FICZ, which is generated by UVB light, could function as a danger signal to UV exposure) we turned our attention to mixtures of polycyclic aromatic hydrocarbons (PAHs), which are known to activate the AHR and be broken down by cytochrome P450 enzymes(30). We focused on those that represent diesel fuel (diesel exhaust particles – DEP) and urban dust (urban dust particles – UDP), and we were able to duplicate the “effector” properties of FICZ in our assays. Addition of these PAH mixtures in Th17 conditions led to a strong enhancement of IL-17(31), which correlates with a couple of previous studies. In one, mice received urban dust particles intratracheally (IT) 3 times over the course of a week and were sacrificed a day later(32). The ensuing airway hyperresponsiveness was lymphocyte dependent, there was recruitment of CD4+ T cells to the airways, and high levels of Th17-associated cytokines were identified in BALs. In a second study, cigarette smoke extract (CSE) favored Th17 differentiation in naïve T-cells in an AHR-dependent manner(33). IT administration of CSE did induce Th17 cells in vivo, and ultimately caused emphysema in an IL-17 dependent manner. We have further duplicated the findings that mice exposed to urban dust IT displayed increased IL-17 in BALs in an AHR-dependent manner.
Collectively, these data support that there are identifiable ligands of the AHR that favor Treg generation in vitro and in vivo (“regulatory ligands”), and others seem to favor Th17 deviation (“effector ligands”). This fits into a concept that the AHR has a broad range of roles, both endogenous and exogenous. The endogenous roles, which pertain to embryology and regulation of physiologic functions of the immune system may be the primary, original functions of the AHR. Additionally, serving an exogenous (adaptive) role, the AHR has evolved as a sensor to the outside environment, in response to both natural toxins and man-made toxicants, to help metabolize pathogenic agents, as well as influence the acquired immune system to protect the organism from infectious insults (Figure 2). It is important to note that ligands of the AHR may not be strictly divided into “regulatory” or “effector” in all instances (at least in vitro), and in certain conditions those ligands associated with Treg can lead to increases in IL-17(34), and FICZ can lead to Treg(12). The importance of the ligand, dosing, culture conditions, inflammatory milieu, or in vivo model being tested needs to be considered in these analyses.
Figure 2. The AHR in T cell differentiation.
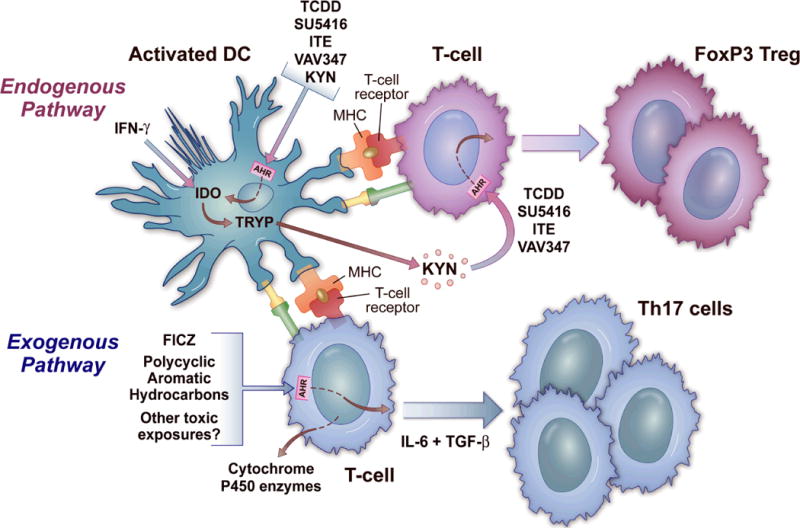
The AHR is represented in the cytosol of both DCs and T cells. In the top part of the figure, which represents the endogenous pathway, ligands including TCDD, SU5416, ITE, VAV347, and kynurenine bind to the AHR on DCs, leading to IDO generation. IFN-γ can generate IDO irrespective of the AHR. This leads to the metabolism of tryptophan to kynurenine, which itself is a ligand for the AHR that binds to the receptor on T cells to generate Regulatory T cells. The bottom part of the figure represents the exogenous pathway, where ligands like FICZ or polycyclic aromatic hydrocarbons bind directly to the AHR on T cells to favor Th17 differentiation under the right conditions. This AHR binding also leads to the transcription of metabolizing enzymes, most notably the cytochrome P450 enzymes. The two pathways potentially function together in a feedback loop, where the AHR metabolizes compounds that then themselves become ligands that can shift the T cell differentiation response when the inflammation abates. Modified and reprinted from (57) with permission from the publisher, Taylor & Francis Ltd, http://www.tand.co.uk/journals).
The AHR in Models of Disease
Given the above data, the potential to utilize the AHR as a target for immunomodulation in essentially contradictory diseases becomes a possibility. Animal models already exist that support this, and a few pharmaceuticals already on the market likely function through the AHR (TABLE II).
Table II.
The Aryl Hydrocarbon Receptor in models of disease.
| Disease | Summary of Selected Reference | Selected Reference | Other References |
|---|---|---|---|
| Cancer | Kynurenine is an endogenous AHR ligand that suppresses anti-tumor immune responses. | (35) | (64, 71–76) |
| Infection & Immunity | TCDD treatment reduces the severity of HSV-mediated inflammatory lesions in the cornea. | (39) | (77–82) |
| Allergy & Asthma | Food allergy is reduced by AHR activation in a murine peanut allergy model. Allergic lung inflammation is inhibited by pharmacological AHR activation. |
(83) (41) |
(84–89) |
| Gastrointestinal | TCDD treatment reduced colonic inflammation in a murine model of Crohn’s disease. | (90) | (42, 44, 91) |
| Autoimmunity | AHR expression is required in a murine model of rheumatoid arthritis. Treatment with FICZ accelerates while TCDD delays clinical onset of EAE |
(40) (17, 18) |
(92–94) |
| Transplantation | Allograft-acceptance promoted by pharmacologic-activation of the AHR. Treatment with FICZ accelerates while TCDD delays rejection of allogeneic skin grafts. |
(56) (57) |
Cancer
The connection between the AHR and cancer has been studied for many years, primarily because of the known role of TCDD as a tumor promoter. The focus on TCDD may overlook the role that this receptor plays as a modulator of the immune system that may also influence tumor growth and survival. A recent paper studying brain tumors identified that some produce the enzyme Tryptophan 2,3-dioxygenase (TDO), which leads to tryptophan breakdown and generation of kynurenine (35). In this study, kynurenine had both an autocrine effect, as well as a paracrine effect that was dependent on kynurenine binding to the AHR, leading to Treg generation and a decreased immune response allowing tumors to grow and metastasize. These findings further underscore a possible connection between the AHR and tumor survival, and may support targeting the AHR to allow the immune system to clear tumors before they become malignant/metastatic.
Infection
Evidence that the AHR can be modulated to combat infection remains circumstantial. AHR–/– mice fare worse when confronted with certain bacterial challenges. Some of this effect may be due to the importance of the AHR in appropriate function of γδ-T cells or other components of innate immunity(10). In addition, the AHR has been shown to be important for resistance to certain bacterial infections, such as listeria(36) and Citrobacter rodentium(15). Treatment of mice infected with influenza virus with TCDD has been shown to worsen outcomes after exposure due to suppression of an appropriate T cell and IFN-γ mediated response(37, 38). At the same time, TCDD reduces the inflammatory lesions seen after HSV infection in the cornea by increasing the ratio of Treg to T effector cells(39). The ability to improve clearance of bacteria or virus by utilizing effector ligands of the AHR will need to be investigated further.
Autoimmunity
A number of recent publications have highlighted the role for this receptor in animal models of immunity, most notably EAE and Collagen Induced Arthritis (CIA)(17, 18, 40). In both of these models, AHR–/– mice acquire a less severe form of the disease (EAE) or none at all (CIA). Additionally, in EAE, the disease can be aggravated or ameliorated simply by taking advantage of either “regulatory or “effector” ligands of the receptor. At least one low molecular weight compound with promise in treating lung inflammation in animals is known to function through the AHR(41). Another area of autoimmunity that seems promising in terms of a role for the AHR is colitis, given the papers cited above that show a role for this receptor in presence and function of immune cells of the gut. In humans, AHR RNA expression is downregulated in active lesions in patients with inflammatory bowel disease(42), and studies have shown modulation of severity of colitis in mice with different ligands and antagonists of the AHR(42–44).
Role of AHR in Transplantation
The above data indicate that the AHR can modulate the response of the acquired immune system, and play a role in development and function of the innate immune system where it interfaces with the outside environment. The observation that certain ligands can either aggravate or ameliorate autoimmune disease supports the potential to harness this receptor to influence an immune response. Another intriguing finding that may relate to transplantation is the relationship of the AHR and the thymus. It has long been known that treatment of animals with TCDD results in near total thymic involution within 10 days(45). The mechanism of this is not known, although data suggest it is due to a loss of hematopoietic cells. This is based on the findings that mice with constitutively activated AHR in T-cell lineages underwent thymic involution(46) and our own finding that VAV-Cre AHR/– (no AHR in hematopoietic and endothelial cells) mice do not undergo thymic involution when exposed to TCDD (unpublished data). Additionally at least one study suggests that TCDD causes dysregulation of KLF2 (Kruppel-like factor 2, which encodes an Sp1-like zinc finger transcription factor) in some developing thymocytes that is important in trafficking, leading to acute egress of these thymocytes from the thymus after exposure (47). This might point to another role of the AHR in trafficking of immune cells that could further be explored in regards to organ transplantation.
Transplantation and the environment
One area that has not received much attention in transplantation is the potential role that the environment might play in immunomodulation and disparate transplant outcomes. While the importance of the AHR in responding to environmental triggers and causing transplant rejection has not yet been documented, the finding that PAHs can shift the balance of Treg/Th17 cells in the lung is intriguing. In addition, a recent study showed that cigarette smoke exposure in mice decreased long-term islet allograft survival by inhibiting IDO in DCs(48), which would be consistent with the previously described role of the AHR in the IDO pathway. The importance of the AHR in responding to dietary ligands and bacterial products in both shaping gut immunity and leading to diseases like inflammatory bowel disease is becoming well-documented in numerous studies(49, 50), and further supports examining the potential for the AHR to influence the immune system after transplant. Perhaps the one organ system where the environment has been considered is lung transplantation. The fact that the lung is in a direct interface with the outside environment does make it unique amongst solid organ transplantation. A recent publication analyzing 281 patients after lung transplantation found that patients living in close proximity to major roads had a significantly higher risk of developing Bronchiolitis Obliterans Syndrome (BOS) after transplantation, with increased IL-6 and neutrophil content in their BALs(51). The mechanisms involved in the development of BOS after lung transplant are beginning to be characterized, and there is significant support for the role of IL-17(52–54). A recent article documented that lymphocytic bronchiolitis, which predisposed lung transplant patients to chronic rejection and BOS, was associated with increased air pollution measured as high particulate matter (PM) in the air, which would correlate with elevated PAHs(55). This correlation between pollution and exposure to PAHs, possibly leading to Th17 deviation and a trend towards BOS, may suggest a role for the AHR in the pathogenesis of environmentally-induced chronic rejection. Although anecdotal, we recently had a stable lung transplant recipient who was caught in a house fire months after transplant, and thereafter became dramatically sensitized to collagen V with a strong Th17 bias, rapidly developing BOS and needing retransplant (personal communication, William Burlingham). Clearly the role of the AHR in this case and other exposure-related outcomes will need to be explored in depth. If this relationship with environmental exposures and immune deviation proves to be valid, both avoidance of certain exposures and targeting the AHR with inhaled regulatory ligands could show promise for prevention of BOS.
Targeting the AHR for treatment of rejection
There are some published data that AHR ligands can alter transplant rejection. One report utilized a regulatory ligand of the AHR to induce tolerance to islet cell grafts in a murine transplant model(56). These investigators used a low molecular weight compound that activates the AHR (VAF347), and found that tolerance to MHC-mismatched islet grafts could be achieved in an AHR-dependent manner. The authors further showed that the mechanism of tolerance involved generation of Treg, and that the effects were mediated by CD11c+ DCs. In fact, tolerance could be achieved by transferring these DCs from VAF347-treated mice, which led to Treg generation and tolerance in recipients of DCs. In a second example, we have recently documented that FICZ can significantly hasten skin graft rejection across a full mismatch (Balb/c to C57BL/6), and TCDD can prolong graft survival(57).
An especially intriguing ligand relevant in transplant models is, 2-(1’H-indole-3’-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE). This regulatory ligand has been shown to lead to Treg via direct action on T cells as well as DCs. It suppresses EAE when given both IV or orally, in an AHR and Treg-dependent manner(29). Furthermore, utilizing a strategy of co-administration of ITE and a T-cell epitope from MOG35–55 (the epitope that causes EAE) Yeste et al reported the generation of Treg and suppression of EAE(58). This strategy is enticing for a tolerance protocol to known MHC mismatches in organ transplantation, where one could imagine co-administering mismatched MHC epitopes along with a regulatory ligand to develop donor-specific tolerance. Another ligand we have been interested in is [3-(3,5-dimethyl-1H-pyrrol-2-ylmethylene)-1,3-dihydro-indole-2-one] (SU5416). This experimental compound, originally designed as a VEGFR-2 inhibitor, has recently been identified as an AHR agonist with regulatory properties on both DCs and T cells(59). It has previously been shown to have efficacy in ameliorating allergic airway disease(60) and EAE(61) in mice, and our own experience has shown it can prolong MHC-mismatched skin graft survival in mice (data not published). The combined features of regulation through the AHR and anti-angiogenic properties give it two mechanisms through which it could inhibit transplant rejection. Another potential ligand for immunomodulation could be kynurenine.
Conclusions
Is it possible that exposures in the environment can explain why stable patients suddenly begin rejecting organs, or why similar patients achieve different outcomes after transplant? The answer is hard to know at this point, although further studies identifying activation of this receptor and correlating this with patient outcomes are warranted. The role of the environment in the pathogenesis of many disease states is well documented, and should be explored in transplantation as well. The importance of the AHR in the immune system is becoming clearer, and future exploration of how it might affect transplant immunology will be very exciting. The above data do identify the AHR as a novel target for immunomodulation in a number of disease states, and may hold great promise in the treatment of transplant rejection and achievement of tolerance.
Acknowledgments
The authors thank Christopher Bradfield, PhD, William Burlingham, PhD, and Dixon Kaufman, MD, PhD, for their collaboration.
The project described was supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- AHR
aryl hydrocarbon receptor
- ARNT
AHR nuclear translocator
- TCDD
2,3,7,8-Tetrachlorodibenzodioxin (also known as dioxin)
- AHR–/–
aryl hydrocarbon receptor null mouse
- γδ T cells
gamma delta T cells
- IEL
intraepithelial lymphocytes
- Treg
regulatory T cells
- EAE
experimental authoimmune encephalomyelitis
- FICZ
6-formylindolo[3,2-b]carbazole
- IDO
indoleamine 2,3 Dioxygenase
- TDO
tryptophan 2,3-dioxygenase
- ITE
2-(1’H-indole-3’-carbonyl)-thiazole-4-carboxylic acid methyl ester
- MOG35–55
myelin oligodendrocyte glycoproteins 35–55
- SU5416
[3-(3,5-dimethyl-1H-pyrrol-2-ylmethylene)-1,3-dihydro-indole-2-one]
- PAH
polycyclic aromatic hydrocarbons
- P450 enzymes
Cytochrome P450 enzymes
- DEP
diesel exhaust particles
- UDP
urban dust particles
- CSE
cigarette smoke extract
- CIA
collagen-induced arthritis
- VAV-Cre AHR–/–
AHR null in hematopoietic and endothelial cells
- KLF2
Kruppel-like factor 2, which encodes an Sp1-like zinc finger transcription factor
- BOS
bronchiolitis obliterans syndrome
- VAF347
4-(3-chlorophenyl)-N-[4-(trifluoromethyl)phenyl]pyrimidin-2-amine
- VEGFR-2 inhibitor
vascular endothelial growth factor receptor-2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors participated in writing the paper (as this is an overview, no new research was conducted).
There are no conflicts of interest for any of these authors.
References
- 1.Denison MS, Pandini A, Nagy SR, Baldwin EP, Bonati L. Ligand binding and activation of the Ah receptor. Chem Biol Interact. 2002;141(1–2):3. doi: 10.1016/s0009-2797(02)00063-7. [DOI] [PubMed] [Google Scholar]
- 2.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- 3.Kitamura M, Kasai A. Cigarette smoke as a trigger for the dioxin receptor-mediated signaling pathway. Cancer Lett. 2007;252(2):184. doi: 10.1016/j.canlet.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Mason GG. Dioxin-receptor ligands in urban air and vehicle exhaust. Environ Health Perspect. 1994;102(Suppl 4):111. doi: 10.1289/ehp.94102s4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hahn ME. Biomarkers and bioassays for detecting dioxin-like compounds in the marine environment. Sci Total Environ. 2002;289(1–3):49. doi: 10.1016/s0048-9697(01)01016-6. [DOI] [PubMed] [Google Scholar]
- 6.Walker MK, Heid SE, Smith SM, Swanson HI. Molecular characterization and developmental expression of the aryl hydrocarbon receptor from the chick embryo. Comp Biochem Physiol C Toxicol Pharmacol. 2000;126(3):305. doi: 10.1016/s0742-8413(00)00119-5. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol. 2008;21(1):102. doi: 10.1021/tx7001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walisser JA, Bunger MK, Glover E, Bradfield CA. Gestational exposure of Ahr and Arnt hypomorphs to dioxin rescues vascular development. Proc Natl Acad Sci U S A. 2004;101(47):16677. doi: 10.1073/pnas.0404379101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walisser JA, Bunger MK, Glover E, Harstad EB, Bradfield CA. Patent ductus venosus and dioxin resistance in mice harboring a hypomorphic Arnt allele. J Biol Chem. 2004;279(16):16326. doi: 10.1074/jbc.M400784200. [DOI] [PubMed] [Google Scholar]
- 10.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31(2):321. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 11.Alam MS, Maekawa Y, Kitamura A, et al. Notch signaling drives IL-22 secretion in CD4+ T cells by stimulating the aryl hydrocarbon receptor. Proc Natl Acad Sci U S A. 2010;107(13):5943. doi: 10.1073/pnas.0911755107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci U S A. 2008;105(28):9721. doi: 10.1073/pnas.0804231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Innocentin S, Withers DR, et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147(3):629. doi: 10.1016/j.cell.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 14.Kiss EA, Vonarbourg C, Kopfmann S, et al. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. 2011;334(6062):1561. doi: 10.1126/science.1214914. [DOI] [PubMed] [Google Scholar]
- 15.Lee JS, Cella M, McDonald KG, et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol. 2012;13(2):144. doi: 10.1038/ni.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Funatake CJ, Marshall NB, Steppan LB, Mourich DV, Kerkvliet NI. Cutting edge: activation of the aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin generates a population of CD4+ CD25+ cells with characteristics of regulatory T cells. J Immunol. 2005;175(7):4184. doi: 10.4049/jimmunol.175.7.4184. [DOI] [PubMed] [Google Scholar]
- 17.Quintana FJ, Basso AS, Iglesias AH, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453(7191):65. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 18.Veldhoen M, Hirota K, Westendorf AM, et al. The aryl hydrocarbon receptor links T(H)17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453(7191):106. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 19.Ruan Q, Kameswaran V, Zhang Y, et al. The Th17 immune response is controlled by the Rel-RORgamma-RORgamma T transcriptional axis. J Exp Med. 2011;208(11):2321. doi: 10.1084/jem.20110462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramirez JM, Brembilla NC, Sorg O, et al. Activation of the aryl hydrocarbon receptor reveals distinct requirements for IL-22 and IL-17 production by human T helper cells. Eur J Immunol. 2010;40(9):2450. doi: 10.1002/eji.201040461. [DOI] [PubMed] [Google Scholar]
- 21.Pan H, Hong F, Radaeva S, Gao B. Hydrodynamic gene delivery of interleukin-22 protects the mouse liver from concanavalin A-, carbon tetrachloride-, and Fas ligand-induced injury via activation of STAT3. Cell Mol Immunol. 2004;1(1):43. [PubMed] [Google Scholar]
- 22.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27(4):647. doi: 10.1016/j.immuni.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21(2):241. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281(5380):1191. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 25.Chen W, Liang X, Peterson AJ, Munn DH, Blazar BR. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J Immunol. 2008;181(8):5396. doi: 10.4049/jimmunol.181.8.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwu P, Du MX, Lapointe R, Do M, Taylor MW, Young HA. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immunol. 2000;164(7):3596. doi: 10.4049/jimmunol.164.7.3596. [DOI] [PubMed] [Google Scholar]
- 27.Fallarino F, Grohmann U, You S, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176(11):6752. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 28.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185(6):3190. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quintana FJ, Murugaiyan G, Farez MF, et al. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2010;107(48):20768. doi: 10.1073/pnas.1009201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt JV, Bradfield CA. Ah receptor signaling pathways. Annu Rev Cell Dev Biol. 1996;12:55. doi: 10.1146/annurev.cellbio.12.1.55. [DOI] [PubMed] [Google Scholar]
- 31.Mezrich J, Zhang XJ, Fechner J. Environmental exposures alter T cell differentiation and aggravate airway disease through the Aryl Hydrocarbon Receptor. Journal of Immunology. 2012;188 [Google Scholar]
- 32.Saunders V, Breysse P, Clark J, Sproles A, Davila M, Wills-Karp M. Particulate matter-induced airway hyperresponsiveness is lymphocyte dependent. Environ Health Perspect. 2010;118(5):640. doi: 10.1289/ehp.0901461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen K, Pociask DA, McAleer JP, et al. IL-17RA Is Required for CCL2 Expression, Macrophage Recruitment, and Emphysema in Response to Cigarette Smoke. PLoS One. 2011;6(5):e20333. doi: 10.1371/journal.pone.0020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishimaru N, Takagi A, Kohashi M, et al. Neonatal exposure to low-dose 2,3,7,8-tetrachlorodibenzo-p-dioxin causes autoimmunity due to the disruption of T cell tolerance. J Immunol. 2009;182(10):6576. doi: 10.4049/jimmunol.0802289. [DOI] [PubMed] [Google Scholar]
- 35.Opitz CA, Litzenburger UM, Sahm F, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478(7368):197. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 36.Shi LZ, Faith NG, Nakayama Y, Suresh M, Steinberg H, Czuprynski CJ. The aryl hydrocarbon receptor is required for optimal resistance to Listeria monocytogenes infection in mice. J Immunol. 2007;179(10):6952. doi: 10.4049/jimmunol.179.10.6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lawrence BP, Roberts AD, Neumiller JJ, Cundiff JA, Woodland DL. Aryl hydrocarbon receptor activation impairs the priming but not the recall of influenza virus-specific CD8+ T cells in the lung. J Immunol. 2006;177(9):5819. doi: 10.4049/jimmunol.177.9.5819. [DOI] [PubMed] [Google Scholar]
- 38.Neff-LaFord H, Teske S, Bushnell TP, Lawrence BP. Aryl hydrocarbon receptor activation during influenza virus infection unveils a novel pathway of IFN-gamma production by phagocytic cells. J Immunol. 2007;179(1):247. doi: 10.4049/jimmunol.179.1.247. [DOI] [PubMed] [Google Scholar]
- 39.Veiga-Parga T, Suryawanshi A, Rouse BT. Controlling viral immuno-inflammatory lesions by modulating aryl hydrocarbon receptor signaling. PLoS Pathog. 2011;7(12):e1002427. doi: 10.1371/journal.ppat.1002427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakahama T, Kimura A, Nguyen NT, et al. Aryl hydrocarbon receptor deficiency in T cells suppresses the development of collagen-induced arthritis. Proc Natl Acad Sci U S A. 2011;108(34):14222. doi: 10.1073/pnas.1111786108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lawrence BP, Denison MS, Novak H, et al. Activation of the aryl hydrocarbon receptor is essential for mediating the anti-inflammatory effects of a novel low-molecular-weight compound. Blood. 2008;112(4):1158. doi: 10.1182/blood-2007-08-109645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monteleone I, Rizzo A, Sarra M, et al. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology. 2011;141(1):237. doi: 10.1053/j.gastro.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 43.Furumatsu K, Nishiumi S, Kawano Y, et al. A role of the aryl hydrocarbon receptor in attenuation of colitis. Dig Dis Sci. 2011;56(9):2532. doi: 10.1007/s10620-011-1643-9. [DOI] [PubMed] [Google Scholar]
- 44.Takamura T, Harama D, Fukumoto S, et al. Lactobacillus bulgaricus OLL1181 activates the aryl hydrocarbon receptor pathway and inhibits colitis. Immunol Cell Biol. 2011;89(7):817. doi: 10.1038/icb.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dencker L. The role of receptors in 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) toxicity. Arch Toxicol Suppl. 1985;8:43. doi: 10.1007/978-3-642-69928-3_5. [DOI] [PubMed] [Google Scholar]
- 46.Nohara K, Pan X, Tsukumo S, et al. Constitutively active aryl hydrocarbon receptor expressed specifically in T-lineage cells causes thymus involution and suppresses the immunization-induced increase in splenocytes. J Immunol. 2005;174(5):2770. doi: 10.4049/jimmunol.174.5.2770. [DOI] [PubMed] [Google Scholar]
- 47.McMillan BJ, McMillan SN, Glover E, Bradfield CA. 2,3,7,8-Tetrachlorodibenzo-p-dioxin induces premature activation of the KLF2 regulon during thymocyte development. J Biol Chem. 2007;282(17):12590. doi: 10.1074/jbc.M611446200. [DOI] [PubMed] [Google Scholar]
- 48.Wan F, Dai H, Zhang S, Moore Y, Wan N, Dai Z. Cigarette smoke exposure hinders long-term allograft survival by suppressing indoleamine 2, 3-dioxygenase expression. Am J Transplant. 2012;12(3):610. doi: 10.1111/j.1600-6143.2011.03820.x. [DOI] [PubMed] [Google Scholar]
- 49.Tilg H. Diet and intestinal immunity. N Engl J Med. 2012;366(2):181. doi: 10.1056/NEJMcibr1113158. [DOI] [PubMed] [Google Scholar]
- 50.Monteleone I, MacDonald TT, Pallone F, Monteleone G. The aryl hydrocarbon receptor in inflammatory bowel disease: linking the environment to disease pathogenesis. Curr Opin Gastroenterol. 2012;28(4):310. doi: 10.1097/MOG.0b013e328352ad69. [DOI] [PubMed] [Google Scholar]
- 51.Nawrot TS, Vos R, Jacobs L, et al. The impact of traffic air pollution on bronchiolitis obliterans syndrome and mortality after lung transplantation. Thorax. 2011;66(9):748. doi: 10.1136/thx.2010.155192. [DOI] [PubMed] [Google Scholar]
- 52.Fan L, Benson HL, Vittal R, et al. Neutralizing IL-17 Prevents Obliterative Bronchiolitis in Murine Orthotopic Lung Transplantation. Am J Transplant. 2011;11(5):911. doi: 10.1111/j.1600-6143.2011.03482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vanaudenaerde BM, De Vleeschauwer SI, Vos R, et al. The role of the IL23/IL17 axis in bronchiolitis obliterans syndrome after lung transplantation. Am J Transplant. 2008;8(9):1911. doi: 10.1111/j.1600-6143.2008.02321.x. [DOI] [PubMed] [Google Scholar]
- 54.Burlingham WJ, Love RB, Jankowska-Gan E, et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117(11):3498. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verleden SE, Scheers H, Nawrot TS, et al. Lymphocytic bronchiolitis after lung transplantation is associated with daily changes in air pollution. Am J Transplant. 2012;12(7):1831. doi: 10.1111/j.1600-6143.2012.04134.x. [DOI] [PubMed] [Google Scholar]
- 56.Hauben E, Gregori S, Draghici E, et al. Activation of the aryl hydrocarbon receptor promotes allograft-specific tolerance through direct and dendritic cell-mediated effects on regulatory T cells. Blood. 2008;112(4):1214. doi: 10.1182/blood-2007-08-109843. [DOI] [PubMed] [Google Scholar]
- 57.Pauly SK, Fechner JH, Zhang XJ, Torrealba J, Bradfield CA, Mezrich JD. The aryl hydrocarbon receptor influences transplant outcomes in response to environmental signals. Toxicological and Environmental Chemistry. 2012;94(6):1175. doi: 10.1080/02772248.2012.688546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yeste A, Nadeau M, Burns EJ, Weiner HL, Quintana FJ. Nanoparticle-mediated codelivery of myelin antigen and a tolerogenic small molecule suppresses experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2012;109(28):11270. doi: 10.1073/pnas.1120611109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mezrich JD, Nguyen LP, Kennedy G, et al. SU5416, a VEGF Receptor Inhibitor and Ligand of the AHR, Represents a New Alternative for Immunomodulation. PLoS One. 2012;7(9):e44547. doi: 10.1371/journal.pone.0044547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim YS, Hong SW, Choi JP, et al. Vascular endothelial growth factor is a key mediator in the development of T cell priming and its polarization to type 1 and type 17 T helper cells in the airways. J Immunol. 2009;183(8):5113. doi: 10.4049/jimmunol.0901566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roscoe WA, Welsh ME, Carter DE, Karlik SJ. VEGF and angiogenesis in acute and chronic MOG((35–55)) peptide induced EAE. J Neuroimmunol. 2009;209(1–2):6. doi: 10.1016/j.jneuroim.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 62.Thatcher TH, Maggirwar SB, Baglole CJ, et al. Aryl hydrocarbon receptor-deficient mice develop heightened inflammatory responses to cigarette smoke and endotoxin associated with rapid loss of the nuclear factor-kappaB component RelB. Am J Pathol. 2007;170(3):855. doi: 10.2353/ajpath.2007.060391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jiang W, Welty SE, Couroucli XI, et al. Disruption of the Ah receptor gene alters the susceptibility of mice to oxygen-mediated regulation of pulmonary and hepatic cytochromes P4501A expression and exacerbates hyperoxic lung injury. J Pharmacol Exp Ther. 2004;310(2):512. doi: 10.1124/jpet.103.059766. [DOI] [PubMed] [Google Scholar]
- 64.Kawajiri K, Kobayashi Y, Ohtake F, et al. Aryl hydrocarbon receptor suppresses intestinal carcinogenesis in ApcMin/+ mice with natural ligands. Proc Natl Acad Sci U S A. 2009;106(32):13481. doi: 10.1073/pnas.0902132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qiu J, Heller JJ, Guo X, et al. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36(1):92. doi: 10.1016/j.immuni.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Robles R, Morita Y, Mann KK, et al. The aryl hydrocarbon receptor, a basic helix-loop-helix transcription factor of the PAS gene family, is required for normal ovarian germ cell dynamics in the mouse. Endocrinology. 2000;141(1):450. doi: 10.1210/endo.141.1.7374. [DOI] [PubMed] [Google Scholar]
- 67.Lin TM, Ko K, Moore RW, Simanainen U, Oberley TD, Peterson RE. Effects of aryl hydrocarbon receptor null mutation and in utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure on prostate and seminal vesicle development in C57BL/6 mice. Toxicol Sci. 2002;68(2):479. doi: 10.1093/toxsci/68.2.479. [DOI] [PubMed] [Google Scholar]
- 68.Lahvis GP, Lindell SL, Thomas RS, et al. Portosystemic shunting and persistent fetal vascular structures in aryl hydrocarbon receptor-deficient mice. Proc Natl Acad Sci U S A. 2000;97(19):10442. doi: 10.1073/pnas.190256997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci U S A. 1996;93(13):6731. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kadow S, Jux B, Zahner SP, et al. Aryl hydrocarbon receptor is critical for homeostasis of invariant gammadelta T cells in the murine epidermis. J Immunol. 2011;187(6):3104. doi: 10.4049/jimmunol.1100912. [DOI] [PubMed] [Google Scholar]
- 71.Wang T, Wyrick KL, Meadows GG, Wills TB, Vorderstrasse BA. Activation of the aryl hydrocarbon receptor by TCDD inhibits mammary tumor metastasis in a syngeneic mouse model of breast cancer. Toxicol Sci. 2011;124(2):291. doi: 10.1093/toxsci/kfr247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koliopanos A, Kleeff J, Xiao Y, et al. Increased arylhydrocarbon receptor expression offers a potential therapeutic target for pancreatic cancer. Oncogene. 2002;21(39):6059. doi: 10.1038/sj.onc.1205633. [DOI] [PubMed] [Google Scholar]
- 73.O’Donnell EF, Kopparapu PR, Koch DC, et al. The aryl hydrocarbon receptor mediates leflunomide-induced growth inhibition of melanoma cells. PLoS One. 2012;7(7):e40926. doi: 10.1371/journal.pone.0040926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caruso JA, Mathieu PA, Joiakim A, Zhang H, Reiners JJ., Jr Aryl hydrocarbon receptor modulation of tumor necrosis factor-alpha-induced apoptosis and lysosomal disruption in a hepatoma model that is caspase-8-independent. J Biol Chem. 2006;281(16):10954. doi: 10.1074/jbc.M508383200. [DOI] [PubMed] [Google Scholar]
- 75.Ray S, Swanson HI. Activation of the aryl hydrocarbon receptor by TCDD inhibits senescence: a tumor promoting event? Biochem Pharmacol. 2009;77(4):681. doi: 10.1016/j.bcp.2008.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nebert DW, Dalton TP, Okey AB, Gonzalez FJ. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol Chem. 2004;279(23):23847. doi: 10.1074/jbc.R400004200. [DOI] [PubMed] [Google Scholar]
- 77.Vorderstrasse BA, Lawrence BP. Protection against lethal challenge with Streptococcus pneumoniae is conferred by aryl hydrocarbon receptor activation but is not associated with an enhanced inflammatory response. Infect Immun. 2006;74(10):5679. doi: 10.1128/IAI.00837-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang T, Wyrick KL, Pecka MR, Wills TB, Vorderstrasse BA. Mechanistic exploration of AhR-mediated host protection against Streptococcus pneumoniae infection. Int Immunopharmacol. 2012;13(4):490. doi: 10.1016/j.intimp.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Teske S, Bohn AA, Regal JF, Neumiller JJ, Lawrence BP. Activation of the aryl hydrocarbon receptor increases pulmonary neutrophilia and diminishes host resistance to influenza A virus. Am J Physiol Lung Cell Mol Physiol. 2005;289(1):L111. doi: 10.1152/ajplung.00318.2004. [DOI] [PubMed] [Google Scholar]
- 80.Yoshida T, Katsuya K, Oka T, et al. Effects of AhR ligands on the production of immunoglobulins in purified mouse B cells. Biomed Res. 2012;33(2):67. doi: 10.2220/biomedres.33.67. [DOI] [PubMed] [Google Scholar]
- 81.Inoue H, Mishima K, Yamamoto-Yoshida S, et al. Aryl hydrocarbon receptor-mediated induction of EBV reactivation as a risk factor for Sjogren’s syndrome. J Immunol. 2012;188(9):4654. doi: 10.4049/jimmunol.1101575. [DOI] [PubMed] [Google Scholar]
- 82.Elizondo G, Rodriguez-Sosa M, Estrada-Muniz E, Gonzalez FJ, Vega L. Deletion of the aryl hydrocarbon receptor enhances the inflammatory response to Leishmania major infection. Int J Biol Sci. 2011;7(9):1220. doi: 10.7150/ijbs.7.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schulz VJ, Smit JJ, Willemsen KJ, et al. Activation of the aryl hydrocarbon receptor suppresses sensitization in a mouse peanut allergy model. Toxicol Sci. 2011;123(2):491. doi: 10.1093/toxsci/kfr175. [DOI] [PubMed] [Google Scholar]
- 84.Wong PS, Vogel CF, Kokosinski K, Matsumura F. Arylhydrocarbon receptor activation in NCI-H441 cells and C57BL/6 mice: possible mechanisms for lung dysfunction. Am J Respir Cell Mol Biol. 2010;42(2):210. doi: 10.1165/rcmb.2008-0228OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chmill S, Kadow S, Winter M, Weighardt H, Esser C. 2,3,7,8-Tetrachlorodibenzo-p-dioxin impairs stable establishment of oral tolerance in mice. Toxicol Sci. 2010;118(1):98. doi: 10.1093/toxsci/kfq232. [DOI] [PubMed] [Google Scholar]
- 86.Chiba T, Uchi H, Tsuji G, Gondo H, Moroi Y, Furue M. Arylhydrocarbon receptor (AhR) activation in airway epithelial cells induces MUC5AC via reactive oxygen species (ROS) production. Pulm Pharmacol Ther. 2011;24(1):133. doi: 10.1016/j.pupt.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 87.Zhu J, Cao Y, Li K, et al. Increased expression of aryl hydrocarbon receptor and interleukin 22 in patients with allergic asthma. Asian Pac J Allergy Immunol. 2011;29(3):266. [PubMed] [Google Scholar]
- 88.Masuda K, Kimura A, Hanieh H, et al. Aryl hydrocarbon receptor negatively regulates LPS-induced IL-6 production through suppression of histamine production in macrophages. Int Immunol. 2011;23(10):637. doi: 10.1093/intimm/dxr072. [DOI] [PubMed] [Google Scholar]
- 89.Podechard N, Lecureur V, Le Ferrec E, et al. Interleukin-8 induction by the environmental contaminant benzo(a)pyrene is aryl hydrocarbon receptor-dependent and leads to lung inflammation. Toxicol Lett. 2008;177(2):130. doi: 10.1016/j.toxlet.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 90.Benson JM, Shepherd DM. Aryl hydrocarbon receptor activation by TCDD reduces inflammation associated with Crohn’s disease. Toxicol Sci. 2011;120(1):68. doi: 10.1093/toxsci/kfq360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Singh NP, Singh UP, Singh B, Price RL, Nagarkatti M, Nagarkatti PS. Activation of aryl hydrocarbon receptor (AhR) leads to reciprocal epigenetic regulation of FoxP3 and IL-17 expression and amelioration of experimental colitis. PLoS One. 2011;6(8):e23522. doi: 10.1371/journal.pone.0023522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu D, Nishimura N, Kuo V, et al. Activation of aryl hydrocarbon receptor induces vascular inflammation and promotes atherosclerosis in apolipoprotein E-/- mice. Arterioscler Thromb Vasc Biol. 2011;31(6):1260. doi: 10.1161/ATVBAHA.110.220202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Holladay SD, Mustafa A, Gogal RM., Jr Prenatal TCDD in mice increases adult autoimmunity. Reprod Toxicol. 2011;31(3):312. doi: 10.1016/j.reprotox.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kerkvliet NI, Steppan LB, Vorachek W, et al. Activation of aryl hydrocarbon receptor by TCDD prevents diabetes in NOD mice and increases Foxp3+ T cells in pancreatic lymph nodes. Immunotherapy. 2009;1(4):539. doi: 10.2217/imt.09.24. [DOI] [PMC free article] [PubMed] [Google Scholar]


