Abstract
Background
A phase II trial was performed to evaluate the efficacy and safety of gefitinib in patients with persistent/recurrent endometrial cancer.
Methods
Women with histologically confirmed persistent/recurrent endometrial cancer were treated with 500 mg oral gefitinib daily until progression or severe toxicity, with progression-free survival (PFS) at six months as the primary endpoint. Tumor expression of total epidermal growth factor receptor (EGFR), estrogen receptor (ER), progesterone receptor A (PRA) and B (PRB), Ki67, pEGFR and activated extracellular signal-regulated kinase (pERK) were examined pre- and post-treatment. EGFR was sequenced, and serum concentrations of soluble EGFR (sEGFR) at baseline also were examined.
Results
Of 29 patients enrolled, 26 were evaluable for efficacy and toxicity. Four patients experienced PFS ≥6 months, and one had a complete response which was not associated with an EGFR mutation. The concentration of sEGFR in pretreatment serum was positively correlated with overall survival (OS), but not with responsiveness to gefitinib in this small patient cohort. Expression of tumor biomarkers was not associated with PFS or OS. Co-expression of ER with PRA in primary and recurrent tumors, and pEGFR with pERK in primary tumors was observed.
Conclusions
This treatment regimen was tolerable but lacked sufficient efficacy to warrant further evaluation in this setting. The possible association between serum sEGFR concentrations and OS, and temporal changes in expression of pEGFR and pERK and the documented CR of one patient are interesting and warrant additional investigation.
Keywords: gefitinib, endometrial cancer, epidermal growth factor receptor (EGFR), soluble EGFR, estrogen receptor, progesterone receptor
INTRODUCTION
Endometrial cancer is the most common gynecologic malignancy in the United States, with an estimated 47,130 cases and 8,010 deaths expected in 2012 [1]. While most patients present with early stage disease and are cured by treatment, the prognosis for patients who relapse is poor, and traditional chemotherapeutic regimens for relapsed patients result in low response rates [2–4]. For these patients, biologically targeted therapeutics are enticing experimental regimens.
The epidermal growth factor receptor (EGFR) is a transmembrane receptor tyrosine kinase that regulates many basic facets of cell and tissue function including cellular growth, survival, differentiation, and migration [5]. EGFR is often overexpressed or mutated in adult solid tumors. Efforts over the last two decades to design EGFR tyrosine kinase inhibitors culminated with FDA approval of the orally active drugs gefitinib and erlotinib for treatment of non small cell lung cancer (NSCLC) and erlotinib for treatment of pancreatic cancer. While methods for stratifying patients most likely to benefit from gefitinib treatment are still being optimized, mutations in EGFR to date appear to be the best predictive marker of responsiveness for NSCLC patients [6]. Moreover, an alternate isoform of EGFR, designated sEGFR, present in both tumor tissue and in circulation, also has been shown to have clinical utility in cancer patients [7–14] and is being studied as a predictive marker of responsiveness to treatment in cancer patients [15].
In vitro and in vivo studies of endometrial cancer have implicated EGFR as an important regulator of cell proliferation and survival [16–21]. However, tumor EGFR expression has been associated with adverse outcomes in endometrial cancer only in some studies [19, 22–24], whereas in others, EGFR is not a significant marker of survival [25–28]. Serum sEGFR concentrations have not previously been examined in endometrial cancer patients.
Gefitinib has substantial growth inhibitory and apoptotic inductive activity in a number of in vitro and in vivo studies using tumor cell lines and xenografts, including those of endometrial origin [17, 29–33]. Only one study thus far has reported on the efficacy of an EGFR tyrosine kinase inhibitor (i.e. erlotinib) for the treatment of patients with endometrial cancer [34]. Gefitinib is safe and well tolerated with some associated dermatological and gastrointestinal adverse events.
The primary endpoint of this phase II clinical trial was progression-free survival (PFS) at six months for daily oral gefitinib (500 mg) as a treatment for recurrent or persistent endometrial cancer. Overall survival (OS) was included as a secondary endpoint. The potential prognostic and predictive clinical utility of several candidate biomarkers previously associated with steroid receptor and EGFR signal transduction pathways in endometrial cancer were evaluated.
MATERIALS AND METHODS
This was a Gynecologic Oncology Group (GOG) sponsored non-randomized, multicenter phase II open-label trial, designated GOG 229C, which evaluated the efficacy and safety of gefitinib (supplied by AstraZeneca, Cheshire, UK) in 26 evaluable patients with endometrial carcinoma who had persistent or recurrent disease following front-line chemotherapy and higher priority protocols. Clinical and laboratory toxicities were monitored and graded according to the National Cancer Institute Common Toxicity Criteria (CTC) Version 2.0. All adverse events were recorded and graded according to the CTC, Version 2.0 (http://ctep.info.nih.gov). Radiographic studies were performed at two-month intervals. All patients who progressed were followed to assess OS.
Eligibility
Patients with histologically confirmed, recurrent or persistent endometrial carcinoma after at least one chemotherapeutic regimen, and with at least one measurable lesion (at least 20 mm by palpation, x-ray, CT scan, or MRI, or at least 10 mm by spiral CT scan) were eligible for this trial. Each patient provided written consent for the protocol including the translational research component with annual Institution Review Board approval at each of the participating institutions and laboratories in accordance with local, state, and federal regulations and guidelines.
Study Design and Treatment Plan
Gefitinib was administered at a dose of 500 mg per day orally. Each 28 day period was considered a cycle. If side effects were not severe and requirements for monitoring toxicity were met, patients were eligible to remain on the study agent until progression.
Management of Toxicity
In general, gefitinib was withheld in patients with grade 2 or greater toxicities until resolution, and patients were then restarted on a reduced dose of 250 mg/day. No dose reductions below 250 mg were allowed. If toxicities did not resolve to grade ≤1 or baseline after two weeks of withholding gefitinib (≥15 days) for any toxicity, the patient was to be removed from study.
On-Study Evaluation
Details are provided in the Supplemental Methods.
Biological samples
Archived formalin-fixed, paraffin-embedded (FFPE) primary tumor tissue from the initial hysterectomy, and serial pre- and post-treatment biopsies (core biopsies or final needle aspirates) of recurrent or persistent tumor were required for this protocol. Patients also were asked to provide serum samples prior to gefitinib treatment. See Supplemental Methods for additional details.
Analysis of EGFR Mutation Status
Genomic DNA was extracted from FFPE tumor tissue using a TrimGen DNA purification kit (TrimGen Corp, Sparks, MD) according to the kit instructions. EGFR exons 18–21 were amplified by polymerase chain reaction (PCR) as published previously and the amplicons sequenced as described in Supplemental Methods [35].
Analysis of Serum sEGFR Concentrations
Twenty-four (of 26 evaluable) patients provided baseline serum samples prior to gefitinib treatment for sEGFR quantitation. Serum sEGFR was quantitated by acridinium-linked immunosorbent assay as previously described [36–37].
Analysis of Tumor Biomarker Expression
FFPE archival tumor tissue specimens, either from the primary or the recurrent endometrial tumors, were tested for expression of selected biomarkers in the GOG Core Laboratory for Receptors using previously published immunohistochemical (IHC) methods, as described in Supplemental Methods [38].
Design, End Points, and Statistical Considerations
The primary endpoints of this study included the frequency of patients with progression free survival (PFS) for at least six months and the frequency and severity of adverse events. Overall survival (OS), PFS, and response were evaluated as secondary endpoints. Demographical and clinicopathological covariates included patient age, race, performance status, and tumor cell histology. Biomarker covariates included tumor EGFR mutation status, tumor expression of EGFR, phospho-EGFR (pEGFR), estrogen receptor (ER), progesterone receptor A (PRA), progesterone receptor B (PRB), activated (phosphor extracellular-regulated kinase (pERK), and the nuclear cell proliferation protein Ki67, and baseline serum soluble EGFR (sEGFR) concentrations. Primary vs. tumor versus recurrent tumors were compared for changes in EGFR, pEGFR, ER, PRA, PRB, pERK, and Ki67 expression. Pretreatment baseline sEGFR concentrations were evaluated for associations with PFS and OS.
The study was designed to detect cytostatic activity of gefitinib by examining the proportion of patients with PFS for a period of six months. Historical controls of a similar population of endometrial cancer patients were used to determine an uninteresting proportion of patients with PFS at six months. Based on this analysis, agents that yield a true probability of 15% or less in patients with PFS at six months should be considered unpromising whereas agents capable of inducing 30% or more patients with PFS at six months should be investigated further in phase III studies. A flexible, two-stage group sequential study design by Chen and Ng [39] which had an average 10% level of significance with 90% average power when the true probability was 30% was used to compare the control and gefitinib-treated groups. The specific features of this study design are more fully explained in Schilder et al. [40]. This design required more than four patients with PFS at six months to proceed to the second stage when 26 patients were recruited to the first stage. Had the study proceeded to the second stage, the trial would have targeted 56 patients cumulatively and required at least 12 patients to be PFS at six months before classifying gefitinib as clinically interesting. Potential associations between biomarkers, patient demographics, and clinical outcome (response, PFS at six months, PFS, and OS) were explored using Kendall’s or Spearman’s correlation coefficient, Fisher’s Exact Test, exact Chi-square tests, or Cox proportional hazards models. Any test yielding an unadjusted (for multiple testing) p-value <0.05 was deemed suggestive or notable for the purpose of hypothesis generation in future studies. Any test yielding a p-value between 0.05 and 0.1 was considered a “possible trend.” Since the sample size was small with a high degree of missingness, these analyses were unpowered with potential bias; therefore, negative results cannot be interpreted as conclusive.
RESULTS
This phase II study enrolled 29 patients with recurrent or persistent endometrial cancer from 16 participating institutions across the United States from July 2002 to November 2003. Two patients were excluded due to inadequate pathology, and one was excluded because she was misclassified as having endometrial cancer. All of the remaining 26 patients were eligible and evaluable.
Patient Characteristics and Treatment Administration
Table 1 summarizes the patient’s demographical and clinicopathological characteristics, therapy, and primary endpoints. Of the eligible and evaluable patients, eight had prior hormonal therapy. Eighteen patients had received prior radiation therapy. Twenty of the 26 patients received more than one cycle of gefitinib monotherapy, and three patients received six or more cycles of gefitinib monotherapy before disease progression. The median number of gefitinib cycles was two.
Table 1.
Patient characteristics and outcomes
| Characteristic | Number of Cases |
|---|---|
| Age | |
| 30–39 | 1 |
| 40–49 | 2 |
| 50–59 | 7 |
| 60–69 | 9 |
| 70–79 | 4 |
| 80–89 | 3 |
| Race | |
| White | 24 |
| American Indian | 1 |
| Black | 1 |
| Performance Status (GOG) | |
| 0 | 14 |
| 1 | 9 |
| 2 | 3 |
| Cell Type | |
| Undifferentiated Carcinoma | 1 |
| Endometrioid Adenocarcinoma | 16 |
| Mixed Epithelial Carcinoma | 3 |
| Serous Adenocarcinoma | 6 |
| Prior Chemotherapy Regimens | |
| 1 | 19 |
| 2 | 7 |
| Prior Hormone therapy | 8 |
| Prior Radiotherapy | 18 |
| Cycles of Treatment | |
| 1 | 6 |
| 2 | 13 |
| 3 | 1 |
| 4 | 3 |
| 6–20 | 3 |
| Progression-free ≥ 6 months | 4 (15.4) |
| Objective Tumor Response | |
| Complete Response | 1 (3.8) |
| Stable Disease | 7 (26.9) |
| Progressive Disease | 16 (61.5) |
| Not Evaluable | 2 (7.7) |
Toxicity
All eligible patients were evaluable for adverse effects. Table 2 shows the adverse events experienced by patients during the course of gefitinib treatment. Toxicities associated with gefitinib treatment were not excessive within this patient population, with the majority of patients experiencing grade 1 and 2 toxicities. However, several patients experienced grade 3 and grade 4 toxicities. Grade 3 toxicities included the following adverse event categories: gastrointestinal (5), constitutional (5), dermatologic (4), other hematologic (3), pain (3), neurologic (2) anemia (1), cardiovascular (1), metabolic (1), ocular (1), and pulmonary (1). Grade 4 toxicities included the following adverse event categories: anemia (1), neurologic (1), and pain (1). One patient died while on study (cardiopulmonary arrest). This death was not attributable to treatment. No patients were removed from study therapy for toxicity, but two patients refused further therapy.
Table 2.
Adverse effects of gefitinib using Common Toxicity Criteria (CTC) Version 2.
| Adverse Event | Grade | Total | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
|
| ||||||
| Leukopenia | 24 | 2 | 0 | 0 | 0 | 26 |
| Thrombocytopenia | 20 | 6 | 0 | 0 | 0 | 26 |
| Neutropenia | 25 | 1 | 0 | 0 | 0 | 26 |
| Anemia | 7 | 7 | 10 | 1 | 1 | 26 |
| Hematologic-other | 23 | 0 | 0 | 3 | 0 | 26 |
| Cardiovascular | 22 | 2 | 1 | 1 | 0 | 26 |
| Fatigue | 8 | 4 | 9 | 5 | 0 | 26 |
| Dermatologic | 9 | 11 | 2 | 4 | 0 | 26 |
| Gastrointestinal | 6 | 6 | 9 | 5 | 0 | 26 |
| Hemorrhage | 22 | 3 | 1 | 0 | 0 | 26 |
| Hepatic | 20 | 5 | 1 | 0 | 0 | 26 |
| Infection | 25 | 0 | 1 | 0 | 0 | 26 |
| Musculoskeletal | 24 | 2 | 0 | 0 | 0 | 26 |
| Metabolic | 20 | 4 | 1 | 1 | 0 | 26 |
| Neurologic | 19 | 3 | 1 | 2 | 1 | 26 |
| Ocular | 21 | 2 | 2 | 1 | 0 | 26 |
| Pain | 17 | 4 | 1 | 3 | 1 | 26 |
| Pulmonary | 24 | 0 | 1 | 1 | 0 | 26 |
Objective Tumor Response
Of the 26 patients who were evaluable for response to gefitinib, two patients were not assessed post-treatment for reasons unrelated to their cancer diagnosis. One patient with a 2 cm vaginal recurrent lesion achieved a complete response (CR) lasting 10.6 months (3.8% with 90% 2-sided CI 0.1–17.0%). Seven patients had disease stabilization. The clinical benefit rate (responders + disease stabilization) was 31% among evaluable patients.
Four of 26 patients (15.3% with 90% 2-sided CI 5.4–31.8%) had PFS at six months following initiation of therapy (see Figure 1A). At 24 months, three patients survived, each of whom had progressive disease. Median PFS and OS were 1.8 and 7.1 months, respectively.
Figure 1.
Progression-free survival and overall survival of endometrial cancer patients receiving single agent gefitinib (A). Kaplan-Meier plot of progression-free survival (solid line) and overall survival (dashed line) is depicted above. By the end of 24 months on the study, all patients had progressed, and three patients were still alive. Overall survival distribution for women with low vs. high pre-treatment serum sEGFR (B).
Mutation Analysis of the EGFR Tyrosine Kinase Domain
Twenty patients had archival primary tumor samples positive for EGFR by immunohistochemistry. Genomic sequencing of EGFR exons 18–21, which encode the tyrosine kinase domain, was performed. In one patient, an E709K mutation was observed. This mutation has been described previously in non small cell lung cancer patients treated with gefitinib but not associated with response in this study [41]. The patient with the complete response did not demonstrate an EGFR mutation.
Serum sEGFR Biomarker Analysis
Serum was obtained from 24 patients prior to initiating gefitinib treatment. In these patients, the median serum sEGFR concentration was 1.960 fmoles/ml. Patients were dichotomized using the median concentration as a cutoff (i.e. “low sEGFR” <1.960 fmoles/ml, “high sEGFR” ≥1.960 fmoles/ml) and assessed for association with response and survival (see Table 3). High vs. low serum sEGFR was not associated with response or PFS in gefitinib-treated patients. Only one patient in the trial had a complete response to gefitinib monotherapy; 0 of 12 patients with low serum sEGFR responded to gefitinib, and 1 of 12 patients with high sEGFR responded to gefitinib. One of 12 patients with low serum sEGFR demonstrated PFS >6 months, and 3 of 12 patients with high serum sEGFR demonstrated PFS >6 months. Patients with high vs. low sEGFR had an indeterminate risk of disease progression (PFS hazard ratio = 0.574, 95% CI 0.245 – 1.344). However, the hazard ratio of death for patients with high vs. low sEGFR was 0.320 (95% CI: 0.128 – 0.796), suggesting that patients with high sEGFR had a 68% lower risk of death at a given time point than patients with low sEGFR (Figure 1B). Patients with high vs. low sEGFR had a median survival of 11.0 months vs. 4.1 months for patients with low sEGFR respectively (Table 3).
Table 3.
Association between pre-treatment serum sEGFR concentration and objective tumor response, proportion progression-free ≥ 6 months, progression-free survival (PFS) and overall survival (OS). sEGFR levels were categorized as low or high.
| Cases | Response | PFS >6 Mon. | Median Survival (Mon.) | ||||
|---|---|---|---|---|---|---|---|
| No | Yes | No | Yes | PFS | OS | ||
| sEGFR | |||||||
| Low | 12 | 12 | 0 | 11 | 1 | 1.6 | 4.1 |
| High | 12 | 11 | 1 | 9 | 3 | 1.9 | 11.0 |
| Total | 24 | 23 | 1 | 20 | 4 | 1.8 | 6.8 |
| Cases | Cox Modeling | ||||
|---|---|---|---|---|---|
| PFS | OS | ||||
|
| |||||
| sEGFR | HR | 95% CI | HR | 95% CI | |
| Low | 12 | 1.00 | Referent | 1.00 | Referent |
| High | 12 | 0.57 | (0.25–1.34) | 0.32 | (0.13–0.80) |
Tumor Biomarker Analyses
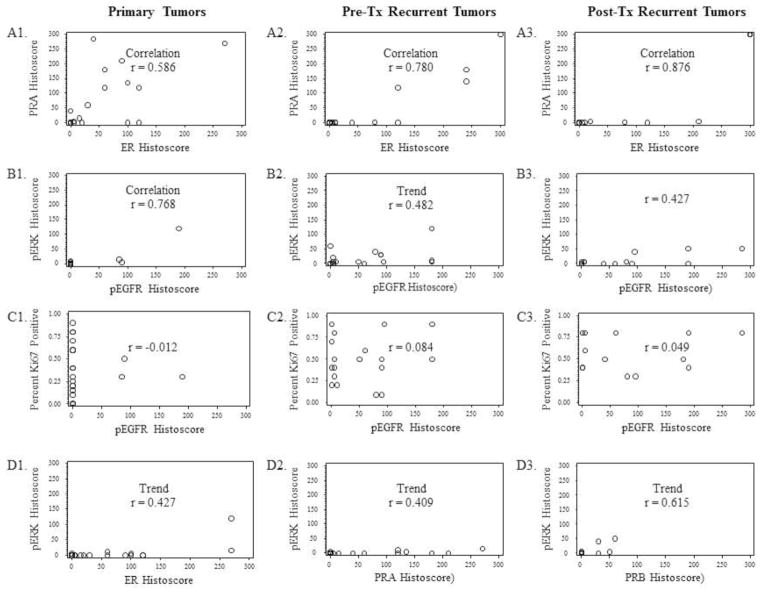
Primary and recurrent tumor samples were assessed by immunohistochemistry for expression of EGFR, Ki67, ER, PRA, PRB, activated (phosphorylated) EGFR (pEGFR) and ERK (pERK). Response, PFS, and OS were not associated with expression for any of the tested biomarkers. However, we did note a number of interesting patterns of co-expression (Figures 2 and 3). Correlations were observed between ER with PRA in primary tumors (Figure 2A, panel 1; r=0.586), pre-treatment recurrent tumors (Figure 2A2; r=0.780), and post-treatment tumors (Figure 2A3; r=0.876). This finding underscores the functional link between ER and PRA and is expected as PR is induced by ER. The positive correlation between ER and PRA in all specimens (original hysterectomy, pre-treatment recurrent and post-treatment recurrent) demonstrates the consistent biologic mechanism underlying the association. A strong correlation between pEGFR with pERK was demonstrated in primary tumors (Figure 2B1; r=0.768), which is expected in cases where EGFR is a primary growth factor receptor controlling downstream ERK phosphorylation. A lower correlation coefficient in pre-treatment recurrent cases (r=0.482) and post-treatment recurrent tumors (r=0.427) was found. The biological explanation for this is not fully known; however, it could be speculated that in recurrent cases, ERK signaling may be dependent upon other growth factor receptors in addition to EGFR
Figure 2.
Relationship between expression of ER and PR (A1–A3), pEGFR and pERK (B1–B3), pEGFR and Ki67 (C1–C3), and pERK and steroid receptors (D1–D3) in primary tumors (A1, B1, C1, D1, D2), pre-treatment (Pre-Tx) recurrent tumors (A2, B2, C2), and post-treatment (Post-Tx) recurrent tumors (A3, B3, C3, D3). ER, PRA, PRB, pEGFR, and pERK were expressed as a histoscore (aggregate of percent of positive tumor cells and staining intensity). Ki67 was expressed as a percent of Ki67 positive tumor cells.
Figure 3.
Ranked difference between pEGFR (A1, A2), EGFR (B1, B2), pERK (C1, C2), Ki67 (D1, D2), ER (E1, E2), or PRA (F1, F2) in recurrent tumors and primary tumors (A1, B1, C1, D1, E1, F1) and in post-treatment and pre-treatment recurrent tumors (A2, B2, C2, D2, E2, F2). ER, PRA, PRB, pEGFR, and pERK were expressed as a histoscore (aggregate of percent of positive tumor cells and staining intensity). Ki67 was expressed as a percent of Ki67 positive tumor cells.
Interesting temporal changes in the expression of selected markers were also noted in primary hysterectomy vs. recurrent pre-treatment tumors (Figures 3 and 4). Specifically, expression of pEGFR and pERK appeared to be higher in many matched recurrent vs. primary tumors (Figure 3A1 and Figure 3C1, respectively). Only two of 17 (12%) primary tumors expressed high levels of pEGFR. However, in matched recurrent tumors, 12 of the 15 (80%) tumors expressed high levels of pEGFR. Similarly, four of 17 (24%) primary tumors expressed high levels of pERK, but in matched recurrent samples, nine of these 13 (69%) tumors expressed high levels of pERK. These findings suggest that recurrent tumors may activate this pathway to a greater degree than primary tumors. However, somewhat surprisingly, higher expression of pEGFR was observed in several post- vs. pre-treatment recurrent tumors in which gefitinib treatment would be expected to block pEGFR (Figure 3A2, Figure 4).
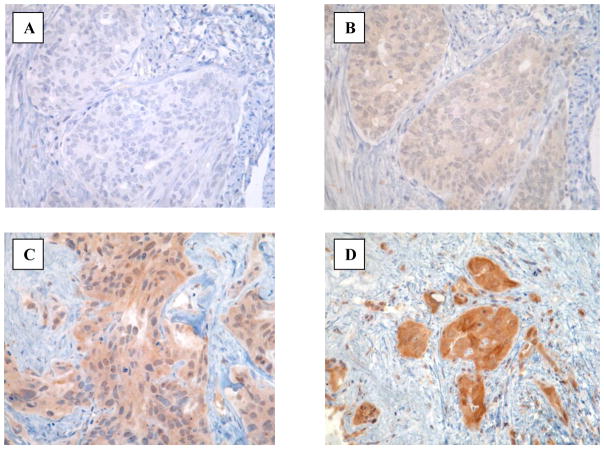
Figure 4.
Expression of pEGFR as determined by IHC in primary and recurrent endometrial tumors. Negative control staining in primary tumor is shown in panel A. pEGFR staining is preferentially localized in primary tumor cells (panel B), pre-treatment recurrent tumor cells (panel C), or post-treatment recurrent tumor cells (panel D).
DISCUSSION
Daily 500 mg gefitinib resulted in a low proportion responding among patients with persistent or recurrent endometrial cancer in this phase II trial. Response rates were lower than those observed in a recent phase II trial of another EGFR tyrosine kinase inhibitor, erlotinib, but the small sample size of both trials makes a direct comparison between these two studies challenging [34]. Though only one patient had a CR to gefitinib monotherapy, it is worth noting that this was an unselected patient population with regard to EGFR status. Four patients exhibited PFS ≥ six months, and seven patients experienced disease stabilization.
Correlations were observed between ER vs. PRA (r=0.586), and pEGFR vs. pERK (r=0.768) in primary tumors, and between ER vs. PRA in both pre-treatment (r=0.780) and post-treatment (r=0.876) recurrent tumors. While others have demonstrated correlations between ER vs. PR status in endometrial cancer [42–47], correlations between pEGFR vs. pERK have not been previously reported. This study also compared pEGFR and pERK expression in the original hysterectomy specimen vs. pre-treatment and post-treatment tumor specimens. Increased expression of pEGFR and pERK was noted in pre-treatment recurrent vs. primary hysterectomy tumor specimens, supporting the primary hypothesis that EGFR/ERK signaling is activated during disease progression. However, treatment with gefitinib did not block EGFR phosphorylation or ERK phosphorylation in all cases. Whether these results can be attributed to suboptimal dosing, primary tumor resistance to gefitinib, or the activation of alternative signaling pathways is not known, and deserves further study. It is important to note that there is an increased risk of obtaining biased results from post-treatment samples due to data missingness, especially if the biomarker is related to treatment efficacy. Five patients progressed before the first scheduled post-treatment sample, so considerable caution needs to be exercised.
The aforementioned tumor markers analyzed by immunohistochemistry also were tested for associations with survival. Previous studies have demonstrated an association (positive or negative) between survival and expression of ER [42–44, 48–67], PR [42–44, 48–49, 51–54, 57–64], and Ki67 in primary endometrial carcinoma [65–66]. Our analyses did not find associations between the immuno-detection of these selected biomarkers and patient survival; however, it must be noted that the patient population was specifically selected for progression to an advanced disease stage following chemotherapy, as opposed to an unselected population.
Notably, baseline serum concentrations of sEGFR were associated with OS in this small patient population. Serum concentrations of sEGFR have been reported to vary in post-menopausal within the range of 519–31,465 fmol/ml [68]. In tumors, while the range remains broad, the sEGFR quartile level is prognostic for outcome. In most cancers, including ovarian, lung and colorectal cancer, reduced concentrations of serum sEGFR have been correlated with poor prognosis [15], consistent with the findings in this study where elevated sEGFR predicted positively for OS. Women with high concentrations of serum sEGFR had an estimated 68% reduced risk of death (HR=0.320; 95% CI: 0.128 – 0.796). The patient who achieved a CR and three of four patients with PFS ≥6 months had high baseline serum sEGFR concentrations. Since only one of 26 patients had a CR to gefitinib treatment, and only seven patients had stable disease, the size of this study was underpowered to observe statistically significant associations between serum sEGFR and CR or PFS. Nevertheless, these results suggest that high vs. low serum sEGFR concentrations may be useful in predicting PFS and OS among patients with persistent or recurrent endometrial cancer.
While the biological basis for the association between serum sEGFR and OS is not yet known, the potential of sEGFR to function as a decoy receptor, thereby regulating ligand bio-availability, as well as its role in cell cohesion and survival signaling may contribute to this phenomenon [69]. Given the paucity of prognostic and theragnostic biomarkers predictive of survival and treatment responsiveness, respectively, in endometrial cancer, these results warrant validation as well as further study regarding the contribution of this novel alternate EGFR isoform to the biology of endometrial cancer. In this regard, sEGFR expression also has been detected in endometrial cancer-derived cell lines [33]. Since our previous studies have shown a correlation between serum sEGFR and gonadotropin concentrations [68], as well as responsiveness to the aromatase inhibitor letrozole [13], future studies examining the interplay between steroid hormones, gonadotropins, and the EGFR/HER receptor growth regulatory axis in endometrial cancer cells may shed further light on the interrelationships among these important endometrial growth regulatory factors.
Although only one patient responded to gefitinib monotherapy, it is interesting that this patient achieved a CR and did not harbor any detectable EGFR mutations that could be associated with sensitivity to gefitinib, such as those observed in NSCLC. Though the preponderance of evidence thus far suggests limited efficacy of EGFR inhibitors for endometrial cancer, this CR demonstrates that some patient subpopulations will respond to gefitinib. A better understanding of the phenotypes of responsive endometrial cancer subpopulations, including the development of methods to identify those patients most likely to respond to gefitinib, will be required for this drug to become a viable treatment option for patients with endometrial cancer.
Supplementary Material
RESEARCH HIGHLIGHTS.
Gefitinib was evaluated in a phase II trial of advanced endometrial cancer.
One patient achieved a complete response, though gefitinib did not demonstrate significant clinical activity overall.
The levels of a soluble truncated form of EGFR, sEGFR, positively correlated with overall survival.
Acknowledgments
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group (GOG) Administrative Office, the GOG Core Laboratory for Receptors and Targets and the GOG Tissue Bank (CA 27469), the GOG Statistical and Data Center (CA 37517), K. Leslie (R01-CA099908), A.K. Godwin (R01-CA140323), N. Maihle (R01-CA79808), as well as support from Susan G Komen for the Cure and the Marsha Rivkin Foundation for Ovarian Cancer Research (to J. Wilken). We also thank and acknowledge the Barbara Beach Fund to support endometrial cancer research (to K. Leslie).
The following GOG member institutions participated in this protocol: University of Alabama at Birmingham, Abington Memorial Hospital, University of North Carolina School of Medicine, University of Iowa Hospitals and Clinics, Indiana University School of Medicine, University of California Medical Center at Irvine, SUNY Downstate Medical Center, The Cleveland Clinic Foundation, State University of New York at Stony Brook, Washington University School of Medicine, Cooper Hospital/University Medical Center, University of Oklahoma, Tacoma General Hospital, Case Western Reserve University, and Tampa Bay Cancer Consortium.
Footnotes
CONFLICT OF INTEREST
Andre Baron serves on the Board of Directors and holds shares in Tumor Biology Investment Group, Inc., a biotechnology company that owns patents for soluble epidermal growth factor receptor (sEGRF). Nita Maihle is co-investor on patents related to sEGFR and is co-founder of a Biotech start up company. Of note, these investigators were blinded to all clinical data and were not involved in the statistical analysis or interpretation of the results from these studies.
All other co-authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Calero F, Asins-Codoner E, Jimeno J, Rodriguez Escudero F, Mendana J, Iglesias J, et al. Epirubicin in advanced endometrial adenocarcinoma: a phase II study of the Grupo Ginecologico Espanol para el Tratamiento Oncologico (GGETO) Eur J Cancer. 1991;27:864–6. doi: 10.1016/0277-5379(91)90135-z. [DOI] [PubMed] [Google Scholar]
- 3.Thigpen JT, Buchsbaum HJ, Mangan C, Blessing JA. Phase II trial of adriamycin in the treatment of advanced or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. Cancer Treat Rep. 1979;63:21–7. [PubMed] [Google Scholar]
- 4.Tropé C, Grundsell H, Johnsson JE, Cavallin-Stahl E. A phase II study of Cis-platinum for recurrent corpus cancer. Eur J Cancer. 1980;16:1025–6. doi: 10.1016/0014-2964(80)90248-0. [DOI] [PubMed] [Google Scholar]
- 5.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–54. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 6.Sequist LV, Lynch TJ. EGFR tyrosine kinase inhibitors in lung cancer: an evolving story. Annu Rev Med. 2008;59:429–42. doi: 10.1146/annurev.med.59.090506.202405. [DOI] [PubMed] [Google Scholar]
- 7.Baron AT, Cora EM, Lafky JM, Boardman CH, Buenafe MC, Rademaker A, et al. Soluble epidermal growth factor receptor (sEGFR/sErbB1) as a potential risk, screening, and diagnostic serum biomarker of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:103–13. [PubMed] [Google Scholar]
- 8.Baron AT, Lafky JM, Boardman CH, Balasubramaniam S, Suman VJ, Podratz KC, et al. Serum sErbB1 and epidermal growth factor levels as tumor biomarkers in women with stage III or IV epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 1999;8:129–37. [PubMed] [Google Scholar]
- 9.Baron AT, Boardman CH, Lafky JM, Rademaker A, Liu D, Fishman DA, et al. Soluble epidermal growth factor receptor (sEGFR) [corrected] and cancer antigen 125 (CA 125) as screening and diagnostic tests for epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:306–18. doi: 10.1158/1055-9965.EPI-04-0423. [DOI] [PubMed] [Google Scholar]
- 10.Baron AT, Lafky JM, Boardman CH, Cora EM, Buenafe MC, Liu D, et al. Soluble epidemal growth factor receptor: A biomarker of epithelial ovarian caner. Cancer Treat Res. 2009;149:189–202. doi: 10.1007/978-0-387-98094-2_9. [DOI] [PubMed] [Google Scholar]
- 11.Halle C, Lando M, Svendsrud DH, Clancy T, Holden M, Sundfor K, et al. Membranous expression of ectodomain isoforms of the epidermal growth factor receptor predicts outcome after chemoradiotherapy of lymph node-negative cervical cancer. Clin Cancer Res. 2011;17:5501–12. doi: 10.1158/1078-0432.CCR-11-0297. [DOI] [PubMed] [Google Scholar]
- 12.Lafky JM, Greenwood TM, Baron AT, Boardman CH, Cora EM, Maihle NJ. Soluble ErbB receptors (sEGFR/sErbBs): serum biomarker in breast and ovarian cancer. In: Diamandis EP, et al., editors. Tumor Markers: Physiology, Pathobiology, Technology and Clinical Applications. American Association for Clinical Chemistry, Inc; Washington D.C: 2002. pp. 427–431. [Google Scholar]
- 13.Lafky JM, Baron AT, Cora EM, Hillman DW, Suman VJ, Perez EA, et al. Serum soluble epidermal growth factor receptor concentrations decrease in postmenopausal metastatic breast cancer patients treated with letrozole. Cancer Res. 2005;65:3059–62. doi: 10.1158/0008-5472.CAN-05-0067. [DOI] [PubMed] [Google Scholar]
- 14.Lafky JM, Wilken JA, Baron AT, Maihle NJ. Clinical implications of the ErbB/epidermal growth factor (EGF) receptor family and its ligands in ovarian cancer. Biochim Biophys Acta. 2008;1785:232–65. doi: 10.1016/j.bbcan.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Baron AT, Wilken JA, Haggstrom DE, Goodrich ST, Maihle NJ. Clinical implementation of soluble EGFR (sEGFR) as a theragnostic serum biomarker of breast, lung and ovarian cancer. IDrugs. 2009;12:302–8. [PubMed] [Google Scholar]
- 16.Takahashi K, Saga Y, Mizukami H, Takei Y, Machida S, Fujiwara H, et al. Cetuximab inhibits growth, peritoneal dissemination, and lymph node and lung metastasis of endometrial cancer, and prolongs host survival. Int J Oncol. 2009;35:725–9. doi: 10.3892/ijo_00000385. [DOI] [PubMed] [Google Scholar]
- 17.Gaikwad A, Wolf JK, Brown J, Ramondetta LM, Smith JA. In vitro evaluation of the effects of gefitinib on the cytotoxic activity of selected anticancer agents in a panel of human endometrial cancer cell lines. J Oncol Pharm Pract. 2009;15:35–44. doi: 10.1177/1078155208095141. [DOI] [PubMed] [Google Scholar]
- 18.Konecny GE, Santos L, Winterhoff B, Hatmal M, Keeney GL, Mariani A, et al. HER2 gene amplification and EGFR expression in a large cohort of surgically staged patients with nonendometrioid (type II) endometrial cancer. Br J Cancer. 2009;100:89–95. doi: 10.1038/sj.bjc.6604814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albitar L, Carter MB, Davies S, Leslie KK. Consequences of the loss of p53, RB1, and PTEN: relationship to gefitinib resistance in endometrial cancer. Gynecol Oncol. 2007;106:94–104. doi: 10.1016/j.ygyno.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Zhao S, Chen X, Lu X, Yu Y, Feng Y. Epidermal growth factor receptor signaling enhanced by long-term medroxyprogesterone acetate treatment in endometrial carcinoma. Gynecol Oncol. 2007;105:45–54. doi: 10.1016/j.ygyno.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Tang LL, Yokoyama Y, Wan X, Iwagaki S, Niwa K, Tamaya T. PTEN sensitizes epidermal growth factor-mediated proliferation in endometrial carcinoma cells. Oncol Rep. 2006;15:855–9. [PubMed] [Google Scholar]
- 22.Scambia G, Benedetti Panici P, Ferrandina G, Battaglia F, Distefano M, D’Andrea G, et al. Significance of epidermal growth factor receptor expression in primary human endometrial cancer. Int J Cancer. 1994;56:26–30. doi: 10.1002/ijc.2910560106. [DOI] [PubMed] [Google Scholar]
- 23.Khalifa MA, Abdoh AA, Mannel RS, Haraway SD, Walker JL, Min KW. Prognostic utility of epidermal growth factor receptor overexpression in endometrial adenocarcinoma. Cancer. 1994;73:370–6. doi: 10.1002/1097-0142(19940115)73:2<370::aid-cncr2820730222>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 24.Khalifa MA, Mannel RS, Haraway SD, Walker J, Min KW. Expression of EGFR, HER-2/neu, P53, and PCNA in endometrioid, serous papillary, and clear cell endometrial adenocarcinomas. Gynecol Oncol. 1994;53:84–92. doi: 10.1006/gyno.1994.1092. [DOI] [PubMed] [Google Scholar]
- 25.Sato S, Ito K, Ozawa N, Yajima A, Sasano H. Expression of c-myc, epidermal growth factor receptor and c-erbB-2 in human endometrial carcinoma and cervical adenocarcinoma. Tohoku J Exp Med. 1991;165:137–45. doi: 10.1620/tjem.165.137. [DOI] [PubMed] [Google Scholar]
- 26.Reinartz JJ, George E, Lindgren BR, Niehans GA. Expression of p53 transforming growth factor alpha-epidermal growth factor receptor, and c-erbB-2 in endometrial carcinoma and correlation with survival and known predictors of survival. Human Pathol. 1994;25:1075–83. doi: 10.1016/0046-8177(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 27.Athanassiadou P, Petrakakou E, Liossi A, Nakopoulou L, Zerva C, Dimopoulos A, et al. Prognostic significance of p53, bcl-2 and EGFR in carcinoma of the endometrium. Acta Cytol. 1999;43:1039–44. doi: 10.1159/000331351. [DOI] [PubMed] [Google Scholar]
- 28.Engelsen IB, Stefansson IM, Beroukhim R, Sellers WR, Meyerson M, Akslen LA, et al. HER-2/neu expression is associated with high tumor cell proliferation and aggressive phenotype in a population based patient series of endometrial carcinomas. Int J Oncol. 2008;32:307–16. [PubMed] [Google Scholar]
- 29.Albitar L, Pickett G, Morgan M, Davies S, Leslie KK. Models representing type I and type II human endometrial cancers: Ishikawa H and Hec50co cells. Gynecol Oncol. 2007;106:52–64. doi: 10.1016/j.ygyno.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 30.Albitar L, Laidler LL, Abdallah R, Leslie KK. Regulation of signaling phosphoproteins by epidermal growth factor and Iressa (ZD1839) in human endometrial cancer cells that model type I and II tumors. Mol Cancer Ther. 2005;4:1891–9. doi: 10.1158/1535-7163.MCT-05-0274. [DOI] [PubMed] [Google Scholar]
- 31.Gadducci A, Cosio S, Genazzani AR. Old and new perspectives in the pharmacological treatment of advanced or recurrent endometrial cancer: Hormonal therapy, chemotherapy and molecularly targeted therapies. Crit Rev Oncol Hematol. 2006;58:242–56. doi: 10.1016/j.critrevonc.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Leslie KK, Laidler LL, Albitar L, Davies S, Holmes A, Nguyen T, et al. Tyrosine kinase inhibitors in endometrial cancer. Int J Gynecol Cancer. 2005;15:409–11. [Google Scholar]
- 33.Albitar L, Pickett G, Morgan M, Wilken JA, Maihle NJ, Leslie KK. EGFR isoforms and gene regulation in human endometrial cancer cells. Mol Cancer. 2010;9:166. doi: 10.1186/1476-4598-9-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oza AM, Eisenhauer EA, Elit L, Cutz JC, Sakurada A, Tsao MS, et al. Phase II study of erlotinib in recurrent or metastatic endometrial cancer: NCIC IND-148. J Clin Oncol. 2008;26:4319–25. doi: 10.1200/JCO.2007.15.8808. [DOI] [PubMed] [Google Scholar]
- 35.Garcia AA, Sill MW, Lankes HA, Godwin AK, Mannel RS, Armstrong DK, et al. A phase II evaluation of lapatinib in the treatment of persistent or recurrent epithelial ovarian or primary peritoneal carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol. 2012;124:569–74. doi: 10.1016/j.ygyno.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baron AT, Lafky JM, Connolly DC, Peoples J, O’Kane DJ, Suman VJ, et al. A sandwich type acridinium-linked immunosorbent assay (ALISA) detects soluble ErbB1 (sErbB1) in normal human sera. J Immunol Methods. 1998;219:23–43. doi: 10.1016/s0022-1759(98)00129-x. [DOI] [PubMed] [Google Scholar]
- 37.Lafky JM, Baron AT, Maihle NJ. Soluble epidermal growth factor receptor acridinium-linked immunosorbent assay. Methods Mol Biol. 2006;327:39–47. doi: 10.1385/1-59745-012-X:39. [DOI] [PubMed] [Google Scholar]
- 38.Singh M, Zaino RJ, Filiaci VJ, Leslie KK. Relationship of estrogen and progesterone receptors to clinical outcome in metastatic endometrial carcinoma: a Gynecologic Oncology Group Study. Gynecol Oncol. 2007;106:325–33. doi: 10.1016/j.ygyno.2007.03.042. [DOI] [PubMed] [Google Scholar]
- 39.Chen TT, Ng TH. Optimal flexible designs in phase II clinical trials. Stat Med. 1998;17:2301–12. doi: 10.1002/(sici)1097-0258(19981030)17:20<2301::aid-sim927>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 40.Schilder RJ, Sill MW, Chen X, Darcy KM, Decesare SL, Lewandowski G, et al. Phase II study of gefitinib in patients with relapsed or persistent ovarian or primary peritoneal carcinoma and evaluation of epidermal growth factor receptor mutations and immunohistochemical expression: a Gynecologic Oncology Group Study. Clin Cancer Res. 2005;11:5539–48. doi: 10.1158/1078-0432.CCR-05-0462. [DOI] [PubMed] [Google Scholar]
- 41.Han SW, Kim TY, Hwang PG, Jeong S, Kim J, Choi IS, et al. Predictive and prognostic impact of epidermal growth factor receptor mutation in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol. 2005;23:2493–501. doi: 10.1200/JCO.2005.01.388. [DOI] [PubMed] [Google Scholar]
- 42.Fukuda K, Mori M, Uchiyama M, Iwai K, Iwasaka T, Sugimori H. Prognostic significance of progesterone receptor immunohistochemistry in endometrial carcinoma. Gynecol Oncol. 1998;69:220–5. doi: 10.1006/gyno.1998.5023. [DOI] [PubMed] [Google Scholar]
- 43.Shabani N, Kuhn C, Kunze S, Schulze S, Mayr D, Dian D, et al. Prognostic significance of oestrogen receptor alpha (ERalpha) and beta (ERbeta), progesterone receptor A (PR-A) and B (PR-B) in endometrial carcinomas. Eur J Cancer. 2007;43:2434–44. doi: 10.1016/j.ejca.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 44.Miyamoto T, Kakizawa T, Hashizume K. Inhibition of nuclear receptor signalling by poly(ADP-ribose) polymerase. Mol Cell Biol. 1999;19:2644–9. doi: 10.1128/mcb.19.4.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chakravarty D, Gupta N, Goda JS, Srinivasan R, Patel FD, Dhaliwal L. Steroid receptors, HER2/neu and Ki-67, in endometrioid type of endometrial carcinoma: Correlation with conventional histomorphological features of prognosis. Acta Histochem. 2010;112:355–63. doi: 10.1016/j.acthis.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 46.Nunobiki O, Taniguchi E, Ishii A, Tang W, Utsunomiya H, Nakamura Y, et al. Significance of hormone receptor status and tumor vessels in normal, hyperplastic and neoplastic endometrium. Pathol Int. 2003;53:846–52. doi: 10.1046/j.1440-1827.2003.01565.x. [DOI] [PubMed] [Google Scholar]
- 47.Sivridis E, Giatromanolaki A, Koukourakis M, Anastasiadis P. Endometrial carcinoma: association of steroid hormone receptor expression with low angiogenesis and bcl-2 expression. Virchows Arch. 2001;438:470–7. doi: 10.1007/s004280000361. [DOI] [PubMed] [Google Scholar]
- 48.Acs G, Xu X, Chu C, Acs P, Verma A. Prognostic significance of erythropoietin expression in human endometrial carcinoma. Cancer. 2004;100:2376–86. doi: 10.1002/cncr.20244. [DOI] [PubMed] [Google Scholar]
- 49.Falcon O, Chirino R, Leon L, Lopez-Bonilla A, Torres S, Fernandez L, et al. Low levels of cathepsin D are associated with a poor prognosis in endometrial cancer. Br J Cancer. 1999;79:570–6. doi: 10.1038/sj.bjc.6690090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gates EJ, Hirschfield L, Matthews RP, Yap OW. Body mass index as a prognostic factor in endometrioid adenocarcinoma of the endometrium. J Natl Med Assoc. 2006;98:1814–22. [PMC free article] [PubMed] [Google Scholar]
- 51.Gehrig PA, Van Le L, Olatidoye B, Geradts J. Estrogen receptor status, determined by immunohistochemistry, as a predictor of the recurrence of stage I endometrial carcinoma. Cancer. 1999;86:2083–9. [PubMed] [Google Scholar]
- 52.Hoshimoto K, Yamauchi N, Takazawa Y, Onda T, Taketani Y, Fukayama M. CD44 variant 6 in endometrioid carcinoma of the uterus: its expression in the adenocarcinoma component is an independent prognostic marker. Pathol Res Pract. 2003;199:71–7. doi: 10.1078/0344-0338-00357. [DOI] [PubMed] [Google Scholar]
- 53.Jongen V, Briet J, de Jong R, ten Hoor K, Boezen M, van der Zee A, et al. Expression of estrogen receptor-alpha and -beta and progesterone receptor-A and -B in a large cohort of patients with endometrioid endometrial cancer. Gynecol Oncol. 2009;112:537–42. doi: 10.1016/j.ygyno.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 54.Nyholm HC, Christensen IJ, Nielsen AL. Progesterone receptor levels independently predict survival in endometrial adenocarcinoma. Gynecol Oncol. 1995;59:347–51. doi: 10.1006/gyno.1995.9964. [DOI] [PubMed] [Google Scholar]
- 55.Oreskovic S, Babic D, Kalafatic D, Barisic D, Beketic-Oreskovic L. A significance of immunohistochemical determination of steroid receptors, cell proliferation factor Ki-67 and protein p53 in endometrial carcinoma. Gynecol Oncol. 2004;93:34–40. doi: 10.1016/j.ygyno.2003.12.038. [DOI] [PubMed] [Google Scholar]
- 56.Pertschuk LP, Masood S, Simone J, Feldman JG, Fruchter RG, Axiotis CA, et al. Estrogen receptor immunocytochemistry in endometrial carcinoma: A prognostic marker for survival. Gynecol Oncol. 1996;63:28–33. doi: 10.1006/gyno.1996.0273. [DOI] [PubMed] [Google Scholar]
- 57.Saito S, Ito K, Nagase S, Suzuki T, Akahira J, Okamura K, et al. Progesterone receptor isoforms as a prognostic marker in human endometrial carcinoma. Cancer Sci. 2006;97:1308–14. doi: 10.1111/j.1349-7006.2006.00332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Creasman WT. Prognostic significance of hormone receptors in endometrial cancer. Cancer. 1993;71:1467–70. doi: 10.1002/cncr.2820710411. [DOI] [PubMed] [Google Scholar]
- 59.Chambers JT, MacLusky N, Eisenfield A, Kohorn EI, Lawrence R, Schwartz PE. Estrogen and progestin receptor levels as prognosticators for survival in endometrial cancer. Gynecol Oncol. 1988;31:65–81. doi: 10.1016/0090-8258(88)90270-3. [DOI] [PubMed] [Google Scholar]
- 60.Kleine W, Maier T, Geyer H, Pfleiderer A. Estrogen and progesterone receptors in endometrial cancer and their prognostic relevance. Gynecol Oncol. 1990;38:59–65. doi: 10.1016/0090-8258(90)90012-a. [DOI] [PubMed] [Google Scholar]
- 61.Nan F, Lu Q, Zhou J, Cheng L, Popov VM, Wei S, et al. Altered expression of DACH1 and cyclin D1 in endometrial cancer. Cancer Biol Ther. 2009;8:1534–9. doi: 10.4161/cbt.8.16.8963. [DOI] [PubMed] [Google Scholar]
- 62.Sakaguchi H, Fujimoto J, Hong BL, Nakagawa Y, Tamaya T. Drastic decrease of progesterone receptor form B but not A mRNA reflects poor patient prognosis in endometrial cancers. Gynecol Oncol. 2004;93:394–9. doi: 10.1016/j.ygyno.2004.01.042. [DOI] [PubMed] [Google Scholar]
- 63.Utaaker E, Iversen OE, Skaarland E. The distribution and prognostic implications of steroid receptors in endometrial carcinomas. Gynecol Oncol. 1987;28:89–100. doi: 10.1016/s0090-8258(87)80013-6. [DOI] [PubMed] [Google Scholar]
- 64.Benevolo M, Vocaturo A, Novelli F, Mariani L, Vocaturo G, Cianciulli AM, et al. Prognostic value of HER2 and progesterone receptor expression in endometrial carcinoma with positive peritoneal washing. Anticancer Res. 2007;27:2839–44. [PubMed] [Google Scholar]
- 65.Giuffre G, Arena F, Scarfi R, Simone A, Todaro P, Tuccari G. Lactoferrin immunoexpression in endometrial carcinomas: relationships with sex steroid hormone receptors (ER and PR), proliferation indices (Ki-67 and AgNOR) and survival. Oncol Rep. 2006;16:257–63. [PubMed] [Google Scholar]
- 66.Stefansson IM, Salvesen HB, Immervoll H, Akslen LA. Prognostic impact of histological grade and vascular invasion compared with tumour cell proliferation in endometrial carcinoma of endometrioid type. Histopathology. 2004;44:472–9. doi: 10.1111/j.1365-2559.2004.01882.x. [DOI] [PubMed] [Google Scholar]
- 67.Mizumoto Y, Kyo S, Mori N, Sakaguchi J, Ohno S, Maida Y, et al. Activation of ERK1/2 occurs independently of KRAS or BRAF status in endometrial cancer and is associated with favorable prognosis. Cancer Sci. 2007;98:652–8. doi: 10.1111/j.1349-7006.2007.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baron AT, Lafky JM, Suman VJ, Hillman DW, Buenafe MC, Boardman CH, et al. A preliminary study of serum concentrations of soluble epidermal growth factor receptor (sErbB1), gonadotropins, and steroid hormones in healthy men and women. Cancer Epidemiol Biomarkers Prev. 2001;10:1175–85. [PubMed] [Google Scholar]
- 69.Wilken JA, Baron AT, Foty RA, McCormick DJ, Maihle NJ. Identification of immunoreactive regions of homology between soluble epidermal growth factor receptor and alpha5-integrin. Biochemistry. 2011;50:4309–21. doi: 10.1021/bi200126j. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.