Abstract
Background
2-8% of all children aged between 6 months and 5 years have febrile seizures. Often these seizures cease spontaneously, however depending on different national guidelines, 20-40% of the patients would need therapeutic intervention. For seizures longer than 3-5 minutes application of rectal diazepam, buccal midazolam or sublingual lorazepam is recommended. Benzodiazepines may be ineffective in some patients or cause prolonged sedation and fatigue. Preclinical investigations in a rat model provided evidence that febrile seizures may be triggered by respiratory alkalosis, which was subsequently confirmed by a retrospective clinical observation. Further, individual therapeutic interventions demonstrated that a pCO2-elevation via re-breathing or inhalation of 5% CO2 instantly stopped the febrile seizures. Here, we present the protocol for an interventional clinical trial to test the hypothesis that the application of 5% CO2 is effective and safe to suppress febrile seizures in children.
Methods
The CARDIF (CARbon DIoxide against Febrile seizures) trial is a monocentric, prospective, double-blind, placebo-controlled, randomized study. A total of 288 patients with a life history of at least one febrile seizure will be randomized to receive either carbogen (5% CO2 plus 95% O2) or placebo (100% O2). As recurrences of febrile seizures mainly occur at home, the study medication will be administered by the parents through a low-pressure can fitted with a respiratory mask. The primary outcome measure is the efficacy of carbogen to interrupt febrile seizures. As secondary outcome parameters we assess safety, practicability to use the can, quality of life, contentedness, anxiousness and mobility of the parents.
Prospect
The CARDIF trial has the potential to develop a new therapy for the suppression of febrile seizures by redressing the normal physiological state. This would offer an alternative to the currently suggested treatment with benzodiazepines. This study is an example of academic translational research from the study of animal physiology to a new therapy.
Trial registration
ClinicalTrials.gov identifier: NCT01370044
Keywords: Febrile Seizure, Alkalosis, Carbogen, Carbon Dioxide, Clinical Trial, Intervention, Translation
Background
Febrile seizures are defined as cerebral seizures occurring in children between 6 months and 5 years of age that are solely associated with fever and not with CNS infection or previous neonatal or unprovoked seizures, and do not meet defining criteria for acute symptomatic seizures [1]. Febrile seizures are the most common type of cerebral seizures in childhood and affect between 2-8 % of all children, depending on geographical and ethnic factors, as well as on ascertainment definitions and methods [2-4]. The peak incidence of febrile seizures is around 16-18 months-of-age [5-7]. Regarding the duration of febrile seizures, Hesdorffer et al. investigated 158 children with a first febrile seizure and distinguished two groups, one with an average duration of 3.8 minutes (82% of the study population) and a second one with 39.8 minutes (18%). In 38-54% of the children the duration of febrile seizure was above 5 and 3 minutes, respectively, and would thus have triggered pharmacological intervention according to the guidelines [8-10]. In about 30% of affected children febrile seizures are recurrent [11]. Most febrile seizures have a benign prognosis, but their occurrence during childhood is associated with an increased risk of 2.4-8.0% to develop epilepsy later in life [12,13]. In contrast to simple febrile seizures, prolonged febrile seizures or febrile status epilepticus (seizure duration > 30 min) have a substantial higher risk to cause short- and long-term brain abnormalities. For example, it has been shown that complex febrile seizures are associated with an increased T2-signal intensity on cranial MRI, most likely reflecting acute injury of the hippocampal formation [14,15]. “Simple” febrile seizures mainly occur within the first 24 hours of a febrile illness and only once during a fever period, last less than 15 minutes and do not show a focal component. “Complex” febrile seizures last longer than 15 minutes, occur several times during one fever episode, and may have focal features. The duration of recurrent febrile seizures is often longer than that of single ones [11].
In Germany, no commonly agreed and published guidelines exist regarding the treatment of febrile seizures, but a published Italian guideline recommends waiting for the febrile seizure to cease spontaneously within the first 3 minutes. Only after this “waiting period” benzodiazepines (rectal diazepam, buccal midazolam, or sublingual lorazepam) should be administered as the “standard therapy”, which will reach pharmacologically active concentrations in the CNS after 3-5 minutes [9]. However, benzodiazepines are effective in only 60-80% of children with seizures [16,17]. Furthermore, due to its sedative effect, children may sleep for the rest of the day. Undesirable strong sedation and fatigue, headache, ataxia, confusion, anterograde amnesia, and paradoxical stimulatory effects are potential side effects of benzodiazepines. The incidence of respiratory depression after the rectal application of diazepam is 5-8% with some patients even requiring mechanical ventilation [18-20]. Additionally, the sedative effect may impede a precise pediatric assessment and possibly lead to unnecessary invasive diagnostic procedures such as a lumbar puncture. As children with febrile seizures are mainly treated at home, these potential side effects should be taken into consideration and an alternative to the currently used pharmacological therapy would be welcome.
Schuchmann et al. reported an animal model, in which they raised to 40-41°C the body temperature of young rats aged P8-11 (corresponding to a human developmental age of 1-5 years) and P22-23 (≈ human 12-18 years) in a heated chamber [21]. Only all the young rats (P8-11, n = 29) had cerebral seizures, but none of the older ones. The authors demonstrated a disproportional increase of the breathing frequency (hyperventilation) in young rats along with the rise of body temperature. Hyperventilation led to hypocapnia, subsequent respiratory alkalosis, elevation of the intracranial pH, and thus an increase of the excitability of cortical neurons. To restore the normal physiological state, the authors increased the ambient CO2 concentration to 5%. Indeed, the blood pCO2 normalized and the seizures stopped within 15-20 seconds, thus verifying the antiepileptic effect of CO2 under these conditions.
These findings prompted us to investigate, whether febrile seizures might also be associated with and triggered by respiratory alkalosis in human patients. In a retrospective study Schuchmann et al. analyzed the blood gas measurements of 433 children who were admitted to the pediatric emergency department of the Charité with various conditions. They discovered that up to 2 hours after the febrile seizure the blood pH in children was significantly higher and the pCO2 levels lower than in children admitted for other reasons (e.g. gastroenteritis, upper respiratory tract infection) [22]. Beyond that, individual uncontrolled therapeutic interventions have been reported, by which seizures could be stopped through elevation of the bood-pCO2 either via re-breathing [23] or by carbogen inhalation [24]. Thus, there is preclinical evidence from experimental febrile seizures and case reports supporting the idea that fever-related respiratory alkalosis plays a role in the generation of febrile seizures [25]. These findings and the uncritical safety profile of carbogen, which has been used for long in various diagnostic procedures, encouraged us to translate these findings into a controlled interventional clinical trial in a domestic setting that combines phases I and II – the CARDIF study.
Methods
Study objective
We hypothesize that carbogen administered through a low-pressure can fitted with a respiratory mask is safe and interrupts recurrences of febrile seizure more effectively than placebo (pure oxygen) within 3 minutes.
Application device
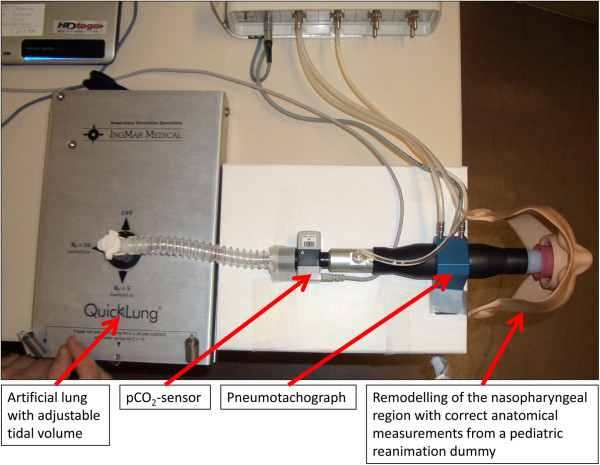
In order to verify that inhalation of 6 liters carbogen over 3 minutes through a loosely fitting mask from a low-pressure can (Figure 1) achieves the required increase of endtidal pCO2, we tested the device in a respiratory model and in a human adult volunteer (Figures 2 &3). The respiratory model was composed of an artificial test lung (QuickLung®, IngMar Medical, Pittsburgh, PA, USA), airways with adjusted dead space, a pneumotachograph (Metabo, Epalinges, Switzerland), sidestream capnography (Microstream® and IntelliVue® MP30, Philips, Hamburg, Germany) and an anatomical model of the infantile nasopharynx. The tidal volume, dead space and the respiratory frequency were set to several age-specific physiological values. Breaths were generated by manually pumping the test lung. During the investigations predefined tidal-volumes were kept constant by fixing the hub of the test lung to a certain volume. Frequency was given by a metronome. Resulting respiratory minute-volumes, breathing rates, air flow, airway pressure and pCO2 before, during and after carbogen inflation were continuously recorded with a sampling rate of 50 Hz (ICU-Lab, KleisTEK Engineering, Bari, Italy and ICM+, University of Cambridge, England). The in vivo test was done with a healthy adult volunteer, whose endtidal pCO2 was continuously measured with same sidestream pCO2 device as before, during and after carbogen inflation. Finally, in order to verify that breathing from the mask, which always would imply some degree of re-breathing, did not unduly increase the endtidal pCO2, the volunteer breathed into the mask with stopped gas flow while inspiratory and endtidal pCO2 as well as SaO2 were simultaneously measured.
Figure 1.
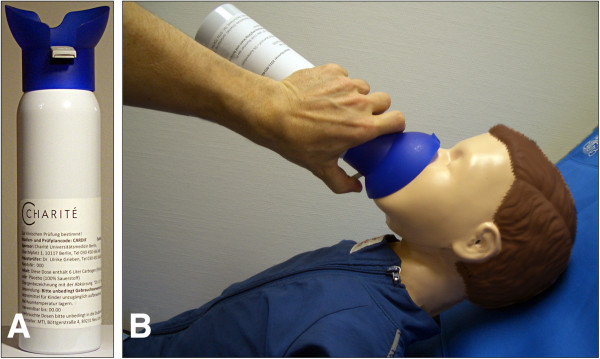
The application device. (A) Low-pressure can containing 6 liter of the study medication with attached, loosely fitting, “one-size for all” respiratory mask. Pressing on the grey cantilever initiates the gas flow. (B) Application of the study medication to a child. The parents are advised to press on the grey cantilever until the audible gas-flow has stopped (after ≈3 minutes).
Figure 2.
Experimental setting. Assessment of inspiratory & endtidal pCO2, respiratory volume & rates, air flow velocity & pressure was done using an artificial lung, a pCO2-sensor, a pneumotychograph, and an anatomical facsimile of a face with nasopharynx and oral cavity of a 2 year-old child. Accordingly, anatomical dead space was adjusted to 40 ml.
Figure 3.
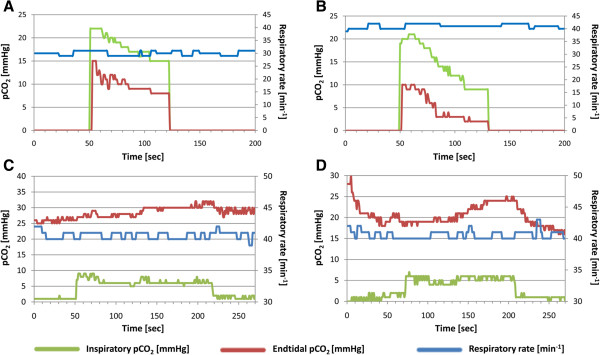
Inspiratory and endtidal pCO2 concentrations. (A-B) Course of the inspiratory and endtidal pCO2 using the experimental setting described in Figure 2. The gas flow was started after 50 seconds and continued for 60 seconds. The experiment was repeated with two different respiratory rates and tidal volumes: (A) 30/min | 90 ml (corresponding to 2-year-old child) and (B) 40/min | 65 ml (corresponding to a 9-months-old infant). In both cases we observed an increase of the inspiratory pCO2 by a maximum of ≈20 mmHg. (C-D)In vivo respiration of 6 liter CO2 over 3 minutes: the gas flow was again started at 50 seconds. The panels depict two different experimental conditions: (C) The test person tried to maintain a respiratory rate of 40/min in synchrony with a metronome over the entire period. Under these conditions the endtidal pCO2 rose by 7 mmHg. In setting (D) the test person had hyperventilated before the start of the gas flow. Starting from a lower pCO2 of 17 mmHg, the pCO2 again rose by 7 mmHg.
Study design
The CARDIF study is a monocentric, prospective, double-blind, placebo-controlled, randomized study. It has been approved by the Ethical Review Board of the State of Berlin and the Competent Regulatory Authority, and is registered at the U.S. Clinical Trials Registration System (NCT01370044). The detailed study design according to the revised CONSORT statement [26,27] is described below (see also Figure 4). The study design had to meet the following challenges:
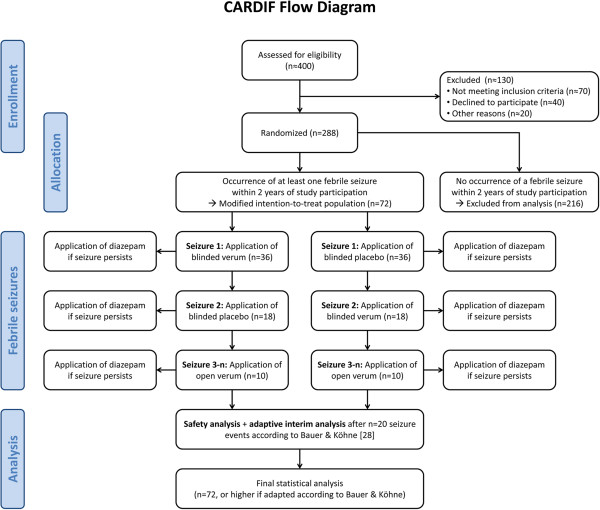
Figure 4.
Flow diagram of the CARDIF-trial.
Route and method of CO2 application
As febrile seizures usually occur at home, safety and practicability aspects had to be specifically addressed while selecting the appropriate method for CO2 delivery. The pCO2 in human blood may be increased by two methods: one is to re-breathe exhaled air through a plastic bag (the re-breathing technique), which is commonly used to treat acute hyperventilation syndrome and panic disorders [25,28]. In young infants, however, this technique requires special expertise as prolonged re-breathing might reduce inspiratory oxygen concentration and induce hypoxia. Re-breathing must thus not be applied without the possibility to measure endtidal pO2 and pCO2[23]. A second, much safer method is the application of CO2-enriched air or of medical carbogen (5% CO2 plus 95% O2), which ensures that the blood pCO2 does not increase and the pO2 does not drop beyond certain limits. The added oxygen even improves oxygenation and prevents hypoxia. As febrile seizures usually occur at home, where no blood gas monitoring is possible, we opted for carbogen to be used in our clinical trial. For this procedure we developed the concept of a special low-pressure can with an attached mask (Figure 1). The pressurized gas secures high enough flow rates to obtain the desired intra-tracheal pCO2 (Figure 3).
Biometric design
As the preexisting clinical evidence was limited, we chose a two phase adaptive design according to Bauer & Köhne [29], which comprises an adaptive interim analysis to allow for sample size modifications without compromising the overall significance level to assure the overall power of the study.
Randomization
Due to procedural constraints, the randomization cannot take place while the child has a seizure, but has to be done immediately after inclusion into the study. As there is only a 25-30% chance that the included patients will indeed have a febrile seizure recurrence during the 24 months study period, thrice as many individuals have to be randomized than will be finally included into the analysis. The individuals for the analysis will thus be chosen according to the principle of the “modified intention-to-treat population” [30]. Accordingly, only patients with a febrile seizure recurrence will be analyzed in the intention-to-treat analysis. A total of 288 patients will be included into the study, 80 for the first and 208 for the second study phase. We expect to include 20+52 = 72 patients with febrile seizure recurrences into the final analysis. This number could be corrected upwards after completion of the first phase, but not downwards (see Statistical analysis).
“Crossover” design
After the first seizure recurrence a crossover occurs. With this, regardless of the result of the first treatment, patients who received placebo for the first seizure recurrence receive verum for the second one or vice versa. From the third seizure recurrence onwards, all patients receive open label verum. Since it is anticipated that only a minority of patients will suffer from a second seizure recurrence and thus enter the crossover arm, the study is not a true crossover study. Data from the “crossover” and the open label extension phase will thus only be considered for secondary analyses. The primary analysis will only include the first seizure recurrence, while the secondary analyses consider all seizure recurrences per patient.
Primary and secondary outcome
The primary outcome is the efficacy of a 3 minute application of carbogen to stop a febrile seizure recurrence. Secondary outcome parameters are safety, practicability to use the can, quality of life, contentedness, and anxiousness and mobility of the parents.
Treatment failure is defined as a febrile seizure recurrence, which has been treated with the study medication but did not cease within the first 3 minutes, so that the “standard therapy” (rectal diazepam) has to be applied. Safety is defined as the number of adverse events. The practicability to handle the can and mask are assessed by a questionnaire. Further, parents are asked to report on their quality-of-life, contentedness, anxiousness and mobility. These data are gathered in an open interview following standardized study-related questions.
Patient population
Patients will be recruited from the Children’s University Hospital of the Charité in Berlin. Parents or custodians of potential participants will be thoroughly informed about the study rationale, procedures, potential risks and benefits. After written informed consent, children will be included into the study if all study selection criteria (see Table 1) are met. The study begins with the randomization into one of the two arms. Parents are trained hands-on by the study personnel to assemble the mask and use the can (Figure 1). This training is complemented by written instructions.
Table 1.
In - and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| 1. Written informed consent from the parents or custodians of the study participant. |
1. Suspected or proven meningitis or encephalitis as a possible cause for a past cerebral seizure associated with fever. |
| 2. At least one febrile seizure in the past medical history of the study participant. |
2. Presence of a neurological disease or a brain malformation. |
| 3. Age 12 months—5 years in phase 1 and age 6 months—5 years in phase 2 of the adaptive design (after observation of 20 seizures, approximately patients number 81-288). |
4. Cerebral seizures without fever in the past medical history. |
| |
5. Proof of spike-wave discharges or of a focus in the in the interval EEG. |
| 6. Presence of a respiratory illness (e.g. bronchial asthma or bronchopulmonary dysplasia, BPD). | |
| 7. Presence of other severe organ or chronic diseases. | |
| 8. Current participation in another clinical trial. | |
| 9. Failure to provide written informed consent on part of the parents or custodians with regard to the storage and dissemination of pseudonymized study results. | |
| 10. Known adverse reactions against carbogen. | |
| 11. Placement of either the child or a parent/custodian in an institution, based on judicial orders. | |
| 12. Lack of understanding of the study procedures on part of the parents or custodians (e.g. lack of language knowledge or of general intellectual capacity). |
Study medication
Carbogen is a gas mixture composed of 5% CO2 and 95% O2, which is stored in compressed gas cylinders (Figure 1A). These low-pressure cans are manufactured and filled by the company MTI Industriegase AG (in cooperation with the Westphalen AG, Germany) and contain 6 liters carbogen gas. A breathing mask with attached application cantilever and a folded instruction sheet are shrink-wrapped in plastic foil together with the can. The amount of gas and the size of the valve are adjusted to allow an easily audible gas flow of 3 minutes duration. It is important that the parents realize when the can is empty in order to apply rectal diazepam (“standard therapy”) if the seizure recurrence has not stopped within these 3 minutes of gas application. Carbogen is known to be tolerated very well with only little side effects. Some discomfort has been reported by patients breathing 5% CO2 for a prolonged period of time [31,32], which was also seen in healthy volunteers [33]. However, due to the short application time, effects on normal brain function and other side effects are expected to be minimal. Patients suffering from febrile seizures tend to hyperventilate during fever, which leads to a pathological reduction of pCO2 in the blood (respiratory alkalosis) [22]. In these patients, carbogen inhalation would thus not lead to a pathological pCO2 increase, but restore the physiological normal state. Therefore, the occurrence of side effects is even less likely in patients with febrile seizures than in healthy controls.
In addition to 5% carbon dioxide, carbogen gas contains 95% oxygen. The placebo contains 100% oxygen. Oxygen might in principle have a toxic effect due to the formation of reactive oxygen species (ROS). It should be noted, however, that the application in the study is very brief and not hyperbaric. In patients with febrile seizures, oxygen saturation (SaO2) may drop below 90% during a seizure because of irregular breathing. The additional administration of oxygen would improve the SaO2 and would be in line with the guidelines [9].
Randomization and treatment allocation
After inclusion into the study, patients will receive a running patient number. For each patient we reserve a package containing two blinded cans (“A” and “B”). Block randomization will be done by issuing to the parents the first blinded “A” can (randomly containing either verum or placebo) out of consecutively numbered packages. After the first seizure recurrence the parents are issued the “B” can of their package, which contains verum if “A” was placebo and vice versa. After a second seizure recurrence the parents will receive further open-label cans that all contain verum. The study duration will be 24 months for each patient. Patients who have at least one seizure recurrence during this time will be included into the (modified) intention-to-treat analysis.
Study procedures
At study entry all patients receive a thorough physical examination. Neurological assessment includes vigilance, muscle strength and tone, coordination, sensibility, walking performance und cranial nerve status. Parents are instructed to call the study team as soon as the seizure had occurred. Hence a study physician “on call” can be reached round the clock 24/7 by mobile phone, who will then explain and ask a set of standardized questions about the seizure. Parents are asked about the circumstances and the details of the seizure recurrence including the date and time, duration, focality, body temperature at the time of the seizure, cause of the febrile infection and maximum temperature during the entire febrile period. Questions regarding the endpoint comprise: (i) cessation of the seizure during the 3 minute application of the study medication, or (ii) the necessity to use diazepam thereafter. Further questions are related to side effects, ratings on the practicability, quality of life, anxiousness, contentedness and mobility with regard to the use of the can. Parents are further asked to present to the study center as soon as possible to exchange the study medication. If the family cannot come to the study center, a study physician visits the family at home. If parents do not contact the study center for 6 months, the study team will phone the family to make sure that no seizure recurrence had occurred in the meantime and to inquire about potential problems.
The study will be performed in accordance with the Good Clinical Practice (GCP) guidelines, the Declaration of Helsinki, the German Medical Drug Law and Data Protection Laws in their current or effective versions. An extensive GCP monitoring will be conducted. Additionally, we put strategies in place to maximize data quality, such as intensive training of the study team, nursing personal and parents. Adverse event management will be done according to standard regulations.
Safety
The overall risk of the study is considered to be minimal. In the verum group, administration of carbogen will only reconstitute the normal physiological state. According to the laws of respiratory physiology it is impossible to increase the arterial pCO2 to toxic levels with 3 liter carbogen. Hence, the study-related pCO2-elevation is only short and normalizes within 30 seconds after termination of the gas application. The short-term oxygen application does not bear any risks and even improves the oxygenation of the patient during the seizure. Finally, we investigated in an adult volunteer what would happen if the gas flow ceased while the parents would continue to hold the mask in front of the child’s face. The experiment was continued for 6 minutes. After 3 minutes a small re-breathing effect was observed with elevation of the inspiratory pCO2 by 1-2 mmHg, but no elevation of endtidal pCO2. The oxygen saturation was always >97%. Since the breathing mask is only loosely fitting, ambient air would have enough access to prevent suffocation.
Definition, documentation and reporting of adverse and serious adverse events follow established conventions and regulatory requirements. The entire trial will be terminated prematurely in the event that more than 30% of the patients terminate their participation prematurely for the following individual reasons: (i) retraction of informed consent, (ii) subsequent occurrence one or more exclusion criteria, (iii) occurrence of a suspected unexpected serious adverse reaction (SUSAR), (iv) non-authorized use of the study medication as well as non-compliance of the parents or custodians of the study participant. Furthermore, the study will be terminated in the event that the risk-benefit-ratio changes towards a higher risk. Sealed unblinding envelopes are available at the study centre. Unblinding for any reason inevitably results in the exclusion of the patient. Parents are thoroughly trained by the study physician about what to do during a seizure or an emergency situation and when to call an ambulance to present to the Accident and Emergency Department of the Children’s University Hospital of the Charité, Berlin. The study team can be reached by mobile phone around the clock.
Statistical analyses
Statistical planning is based on the modified intention-to-treat principle, i.e. only patients suffering from a febrile seizure recurrence during the study period of 24 months will be assigned to the intention-to-treat population. Analyses will be performed using SPSS, R, and SAS in their newest versions.
The aim of the study is the proof of the superiority of the experimental intervention (carbogen) versus control (oxygen) to suppress a seizure recurrence within 3 minutes. Success rates under intervention and control being pI and pK, the statistical null hypothesis is pI = pK, and the alternative hypothesis pI ≠ pK. The analyses will be carried out according to Bauer & Köhne with α = 0.025, cα= 0.00380 and α0 = 0.5 (in each case one-sided) [29]. The main variable “diazepam application “no” ( = success) versus “yes” (= failure)” will be analyzed as a binary endpoint by means of the Fisher´s exact test. The secondary endpoints safety and practicability will be analyzed by relative frequency and two-sided 95% confidence interval. The other secondary endpoints quality of life, anxiousness, mobility and contentedness will be analyzed in a descriptive manner according to their scaling; p-values will be reported but are not to be interpreted in a confirmatory way. The dependency of the repeated measures (“crossover” and “open label” phase) will be adjusted for by means of generalized estimating equation for a logistic regression model.
One-sided significance level will be 0.025. With an expected difference of 75% versus 25% the power for the two-sided Fisher´s exact test will be 86% when including 2 x 26 patients. The effective power will probably be even higher, as the sample size can only be increased but not decreased. After 2 x 10 patients with at least one seizure recurrence have been included, all data of those patients will be unblinded and will be analyzed with respect to safety. Furthermore, an interim analysis will be performed to adjust the sample size. A medical expert will decide whether the safety data allow the continuation of the study. If the study is continued, the new sample sized will be defined by means of the Bauer & Köhne method [29].
Ethics
Following the legal requirements, no patient will be deprived of standard care due to study participation [34]. The study medication is applied during a time window of 3 minutes, at which the parents are advised, according to the guidelines, to wait before application of rectal diazepam (standard care). If the seizures persists even after application of diazepam parents are advised to call the emergency number, as it is the case in standard care. The study has been reviewed and approved by the Ethical Review Board of the State of Berlin.
The scheme for recruitment, randomization, and crossover of the CARDIF trial is depicted in the Flow diagram at Figure 4.
Discussion
The CARDIF study will provide important data on a treatment alternative for patients with febrile seizures recurrences. This closes the gap between promising preclinical observations and so far missing evidence-based data on the efficacy and safety of such an approach. Possibly, the use of benzodiazepines might become dispensable or at least second choice behind the application of carbogen, which has fewer side effects. To our knowledge, CARDIF is presently the only registered clinical trial that investigates a new treatment to suppress an acute recurrence of a febrile seizure. Since this study is a non-commercial investigator-initiated trial mainly based on own preclinical and clinical data, it is a nice example for an academic translational approach. Some challenges and limitations of the CARDIF study may deserve a closer discussion.
Because febrile seizures only occur in children, the study hypothesis can only be tested in minors. All legal and ethical criteria specific for the conduct of clinical trials in minors (e.g. to be of benefit for the entire group of affected patients and to expose the study participants to the lowest possible risk and burden) had to be fulfilled by the study design to obtain regulatory approval.
Before initiating the CARDIF trial we attempted in another study to treat patients with a febrile seizure using a re-breathing device in a controlled in-patient setting. As febrile seizures are short unpredicted events and mainly occur at home, we were unable to treat more than 2 patients over a period of 12 months. As re-breathing from a plastic bag without safety monitoring is potentially dangerous, such approach must not be transferred into a domestic environment.
With this in mind we looked for an alternative and identified the low-pressure cans, which could be used to apply the desired amount of carbogen at home. The limited volume of gas prevents overdosage and the oxygen offers the added benefit to prevent hypoxia during the seizure. The main challenge resulting from this change of strategy was to find a company, which would be willing and certified to produces such cans with mask containing the gas in “medical quality” according to the requirements of the German Medical Drug Law.
A possible difficulty of the study results from the domestic use of the study medication. Hence, the primary outcome will not be assessed by the investigators but by medical lay persons (here the parents). Thus, the investigators have to rely on the compliance and reliability of these individuals with regard to correct application of the gas as well as the reporting of the outcome. Taking this into account, parents will receive intensive hands-on training and supervision, and will be contacted or visited regularly.
Only approximately 25-30% of children with a life history of one febrile seizure have further ones. Accordingly, thrice as many patients have to be recruited to the study than needed for the analysis. This includes the informed consent procedure, randomization, distribution of the study medication, and training. This will lead predictably to a high percentage of non-treated patients and non-used study medication.
In conclusion, the CARDIF study represents a prime example of an intramural translational approach of an academic institution: researchers from one research consortium working hand-in-hand with clinicians to translate their findings from basic science leading to novel therapies. This is made possible by the NeuroCure consortium at the Charité that offers an interdisciplinary forum, training for physician-scientists, protected research time for clinicians, as well as intramural funding for investigator initiated trials in order to overcome the common “translational roadblocks” [35,36].
Abbreviations
CARDIF: CARbon DIoxide against Febrile seizures; CO2: Carbon dioxide; O2: Oxygen; pCO2: Partial pressure of CO2.
Competing interests
The authors do not report any conflict of interest.
Authors’ contributions
Design of the study, draft of the study protocol (SO, DS, MS), organization of all regulatory affairs (SO), draft of the manuscript (SO, MS), biometrical and biostatistical planning (PM), performing the respiratory investigations with the carbogen cans (MS, SWC, TW), acquisition of funding (DS, MS), critically revising the manuscript for intellectual content (SO, TW, SWC, CW, PM, DS, MS). All authors have given final approval of the version to be published.
Contributor Information
Stephanie Ohlraun, Email: stephanie.ohlraun@charite.de.
Tobias Wollersheim, Email: tobias.wollersheim@charite.de.
Claudia Weiß, Email: claudia.weiss@charite.de.
Peter Martus, Email: peter.martus@med.uni-tuebingen.de.
Steffen Weber-Carstens, Email: steffen.weber-carstens@charite.de.
Dietmar Schmitz, Email: dietmar.schmitz@charite.de.
Markus Schuelke, Email: markus.schuelke@charite.de.
Acknowledgements
The CARDIF trial is funded by the DFG (Deutsche Forschungsgemeinschaft) via the NeuroCure Cluster of Excellence (German Research Foundation, DFG, Exc 257) and the Collaborative Research Center SFB 665 TP C4. We are indebted to the MTI Industrie-Gase AG for excellent logistic support, cooperation and the manufacture of the investigational product.
References
- Guidelines for epidemiologic studies on epilepsy. Commission on epidemiology and prognosis, international league against epilepsy. Epilepsia. 1993;34:592–596. doi: 10.1111/j.1528-1157.1993.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Nelson KB, Ellenberg JH. Predictors of epilepsy in children who have experienced febrile seizures. N Engl J Med. 1976;295:1029–1033. doi: 10.1056/NEJM197611042951901. [DOI] [PubMed] [Google Scholar]
- Verity CM, Golding J. Risk of epilepsy after febrile convulsions: a national cohort study. BMJ. 1991;303:1373–1376. doi: 10.1136/bmj.303.6814.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser WA. The prevalence and incidence of convulsive disorders in childern. Epilepsia. 1994;35(Suppl):S1–S6. doi: 10.1111/j.1528-1157.1994.tb05932.x. [DOI] [PubMed] [Google Scholar]
- Shinnar S, Glauser TA. Febrile seizures. J Child Neurol. 2002;17(1 suppl):S44–S52. doi: 10.1177/08830738020170010601. [DOI] [PubMed] [Google Scholar]
- Waruiru C, Appleton R. Febrile seizures: an update. Arch Dis Child. 2004;89:751–756. doi: 10.1136/adc.2003.028449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard M, Christensen J. Register-based studies on febrile seizures in Denmark. Brain Dev. 2009;31:372–377. doi: 10.1016/j.braindev.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Hesdorffer DC, Benn EKT, Bagiella E, Nordli D, Pellock J, Hinton V, Shinnar S. Team for the FS. Distribution of febrile seizure duration and associations with development. Ann Neurol. 2011;70:93–100. doi: 10.1002/ana.22368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capovilla G, Mastrangelo M, Romeo A, Vigevano F. Recommendations for the management of “febrile seizures” Ad hoc task force of LICE guidelines commission. Epilepsia. 2009;50:2–6. doi: 10.1111/j.1528-1167.2008.01963.x. [DOI] [PubMed] [Google Scholar]
- Duffner PK, Baumann RJ, Berman P, Green JL, Schneider S, Hodgson ES, Glade GB, Harbaugh N Jr, McInerny TK, Miller MR, Moyer VA, Sevilly XD, Simpson L, Takata GS. for the steering committee on quality improvement and management, subcommittee on febrile seizures. Febrile seizures: clinical practice guideline for the long-term management of the child with simple febrile seizures. Pediatrics. 2008;121:1281–1286. doi: 10.1542/peds.2008-0939. [DOI] [PubMed] [Google Scholar]
- Knudsen FU. Febrile seizures: treatment and prognosis. Epilepsia. 2000;41:2–9. doi: 10.1111/j.1528-1157.2000.tb01497.x. [DOI] [PubMed] [Google Scholar]
- Annegers JF, Hauser WA, Shirts SB, Kurland LT. Factors prognostic of unprovoked seizures after febrile convulsions. N Engl J Med. 1987;316:493–498. doi: 10.1056/NEJM198702263160901. [DOI] [PubMed] [Google Scholar]
- Berg AT. Febrile seizures and epilepsy: the contributions of epidemiology. Paediatr Perinat Epidemiol. 1992;6:145–152. doi: 10.1111/j.1365-3016.1992.tb00756.x. [DOI] [PubMed] [Google Scholar]
- Hesdorffer DC, Shinnar S, Lewis DV, Moshé SL, Nordli DR, Pellock JM, MacFall J, Shinnar RC, Masur D, Frank LM, Epstein LG, Litherland C, Seinfeld S, Bello JA, Chan S, Bagiella E, Sun S. Team for the F study. Design and phenomenology of the FEBSTAT study. Epilepsia. 2012;53:1471–1480. doi: 10.1111/j.1528-1167.2012.03567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnar S, Bello JA, Chan S, Hesdorffer DC, Lewis DV, MacFall J, Pellock JM, Nordli DR, Frank LM, Moshe SL, Gomes W, Shinnar RC, Sun S. MRI abnormalities following febrile status epilepticus in children the FEBSTAT study. Neurology. 2012;79:871–877. doi: 10.1212/WNL.0b013e318266fcc5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen FU. Rectal administration of diazepam in solution in the acute treatment of convulsions in infants and children. Arch Dis Child. 1979;54:855–857. doi: 10.1136/adc.54.11.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppu K, Santavuori P. Diazepam rectal solution for home treatment of acute seizures in children. Acta Paediatr Scand. 1981;70:369–372. doi: 10.1111/j.1651-2227.1981.tb16565.x. [DOI] [PubMed] [Google Scholar]
- Norris E, Marzouk O, Nunn A, McIntyre J, Choonara I. Respiratory depression in children receiving diazepam for acute seizures: a prospective study. Dev Med Child Neurol. 1999;41:340–343. doi: 10.1017/S0012162299000742. [DOI] [PubMed] [Google Scholar]
- McIntyre J, Robertson S, Norris E, Appleton R, Whitehouse WP, Phillips B, Martland T, Berry K, Collier J, Smith S, Choonara I. Safety and efficacy of buccal midazolam versus rectal diazepam for emergency treatment of seizures in children: a randomised controlled trial. The Lancet. 2005;16(366):205–210. doi: 10.1016/S0140-6736(05)66909-7. [DOI] [PubMed] [Google Scholar]
- Appleton R, Sweeney A, Choonara I, Robson J, Molyneux E. Lorazepam versus diazepam in the acute treatment of epileptic seizures and status epilepticus. Dev Med Child Neurol. 1995;37:682–688. doi: 10.1111/j.1469-8749.1995.tb15014.x. [DOI] [PubMed] [Google Scholar]
- Schuchmann S, Schmitz D, Rivera C, Vanhatalo S, Salmen B, Mackie K, Sipilä ST, Voipio J, Kaila K. Experimental febrile seizures are precipitated by a hyperthermia-induced respiratory alkalosis. Nat Med. 2006;12:817–823. doi: 10.1038/nm1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuchmann S, Hauck S, Henning S, Grüters-Kieslich A, Vanhatalo S, Schmitz D, Kaila K. Respiratory alkalosis in children with febrile seizures. Epilepsia. 2011;52:1949–1955. doi: 10.1111/j.1528-1167.2011.03259.x. [DOI] [PubMed] [Google Scholar]
- Schuchmann S, Schmitz D, Grüters-Kieslich A, Vanhatolo S, Kaila K. Suppression of complex febrile seizures by elevating respiratory CO2 using a rebreathing technique – two case reports. Epilepsia. 2009;50(Suppl):245. [Google Scholar]
- Tolner EA, Hochman DW, Hassinen P, Otáhal J, Gaily E, Haglund MM, Kubová H, Schuchmann S, Vanhatalo S, Kaila K. Five percent CO2 is a potent, fast-acting inhalation anticonvulsant. Epilepsia. 2011;52:104–114. doi: 10.1111/j.1528-1167.2010.02731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuchmann S, Vanhatalo S, Kaila K. Neurobiological and physiological mechanisms of fever-related epileptiform syndromes. Brain Dev. 2009;31:378–382. doi: 10.1016/j.braindev.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel group randomized trials. Bmc Med Res Methodol. 2001;1:2. doi: 10.1186/1471-2288-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Trials. 2010;11:32. doi: 10.1186/1745-6215-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman JM, Browne ST, Papp LA, Martinez J, Welkowitz L, Coplan JD, Goetz RR, Kent J, Klein DF. Effect of antipanic treatment on response to carbon dioxide. Biol Psychiatry. 1997;42:982–991. doi: 10.1016/S0006-3223(97)00160-1. [DOI] [PubMed] [Google Scholar]
- Bauer P, Köhne K. Evaluation of experiments with adaptive interim analyses. Biometrics. 1994;50:1029–1041. doi: 10.2307/2533441. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency. Guideline on the evaluation of medicinal products indicated for treatment of bacterial infections. Comm Med Prod Hum Use. 2010;2:1–26. CPMP/EWP/558/95 rev. [Google Scholar]
- Kergoat H, Tinjust D. Neuroretinal function during systemic hyperoxia and hypercapnia in humans. Optom Vis Sci Off Publ Am Acad Optom. 2004;81:214–220. doi: 10.1097/00006324-200403000-00014. [DOI] [PubMed] [Google Scholar]
- Ashkanian M, Gjedde A, Mouridsen K, Vafaee M, Hansen KV, Østergaard L, Andersen G. Carbogen inhalation increases oxygen transport to hypoperfused brain tissue in patients with occlusive carotid artery disease: increased oxygen transport to hypoperfused brain. Brain Res. 2009;1304:90–95. doi: 10.1016/j.brainres.2009.09.076. [DOI] [PubMed] [Google Scholar]
- Voipio J, Tallgren P, Heinonen E, Vanhatalo S, Kaila K. Millivolt-scale DC shifts in the human scalp EEG: evidence for a nonneuronal generator. J Neurophysiol. 2003;89:2208–2214. doi: 10.1152/jn.00915.2002. [DOI] [PubMed] [Google Scholar]
- Directive 2001/20/EC of the European parliament and of the council on the approximation of the laws, regulations and administrative provisions of the member states relating to the implementation of good clinical practice in the conduct of clinical trials on medical products for human use. 2001. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2001:121:0034:0044:en:PDF. [PubMed]
- Vesterinen HV, Egan K, Deister A, Schlattmann P, Macleod MR, Dirnagl U. Systematic survey of the design, statistical analysis, and reporting of studies published in the 2008 volume of the journal of cerebral blood flow and metabolism. J Cereb Blood Flow Metab. 2011;31:1064–1072. doi: 10.1038/jcbfm.2010.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod MR, Fisher M, O’Collins V, Sena ES, Dirnagl U, Bath PMW, Buchan A, van der Worp HB, Traystman RJ, Minematsu K, Donnan GA, Howells DW. Reprint. Good laboratory practice: preventing introduction of bias at the bench. J Cereb Blood Flow Metab. 2008;29:221–223. doi: 10.1161/STROKEAHA.108.525386. [DOI] [PMC free article] [PubMed] [Google Scholar]