Abstract
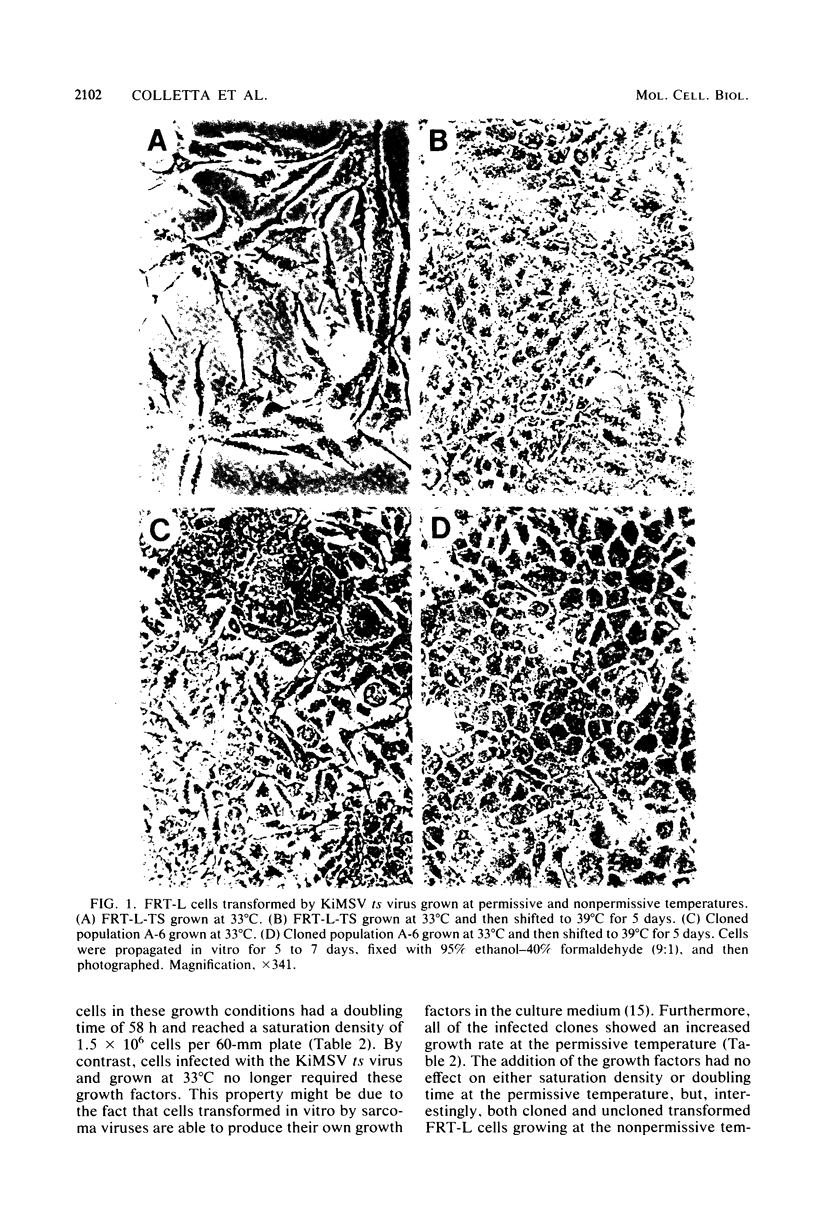
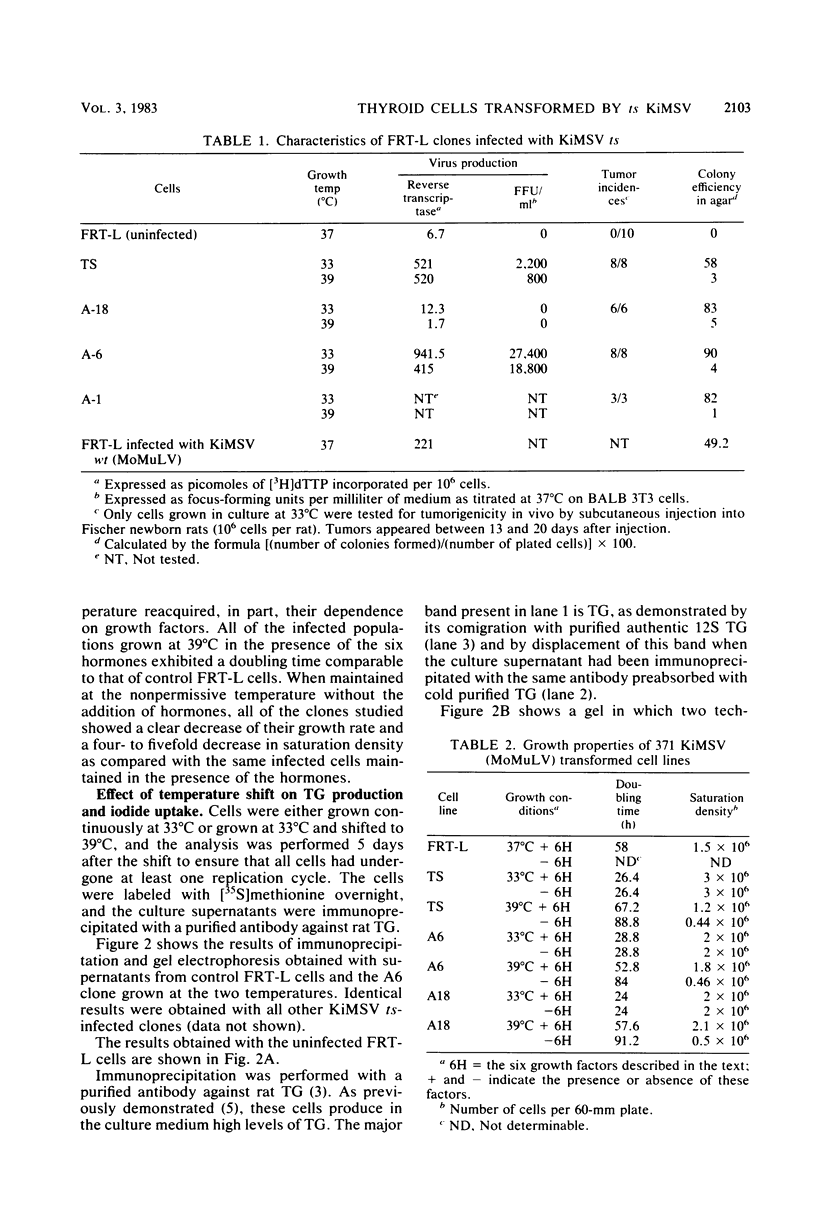
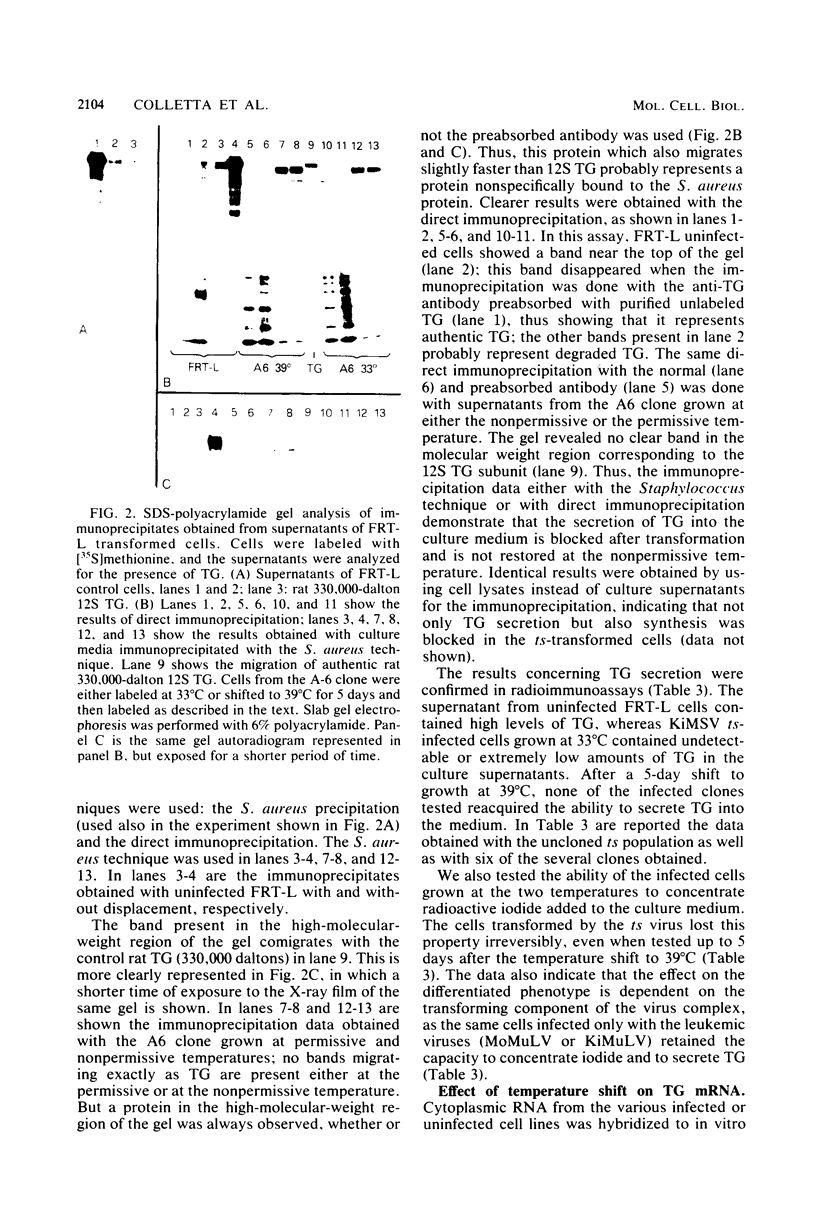
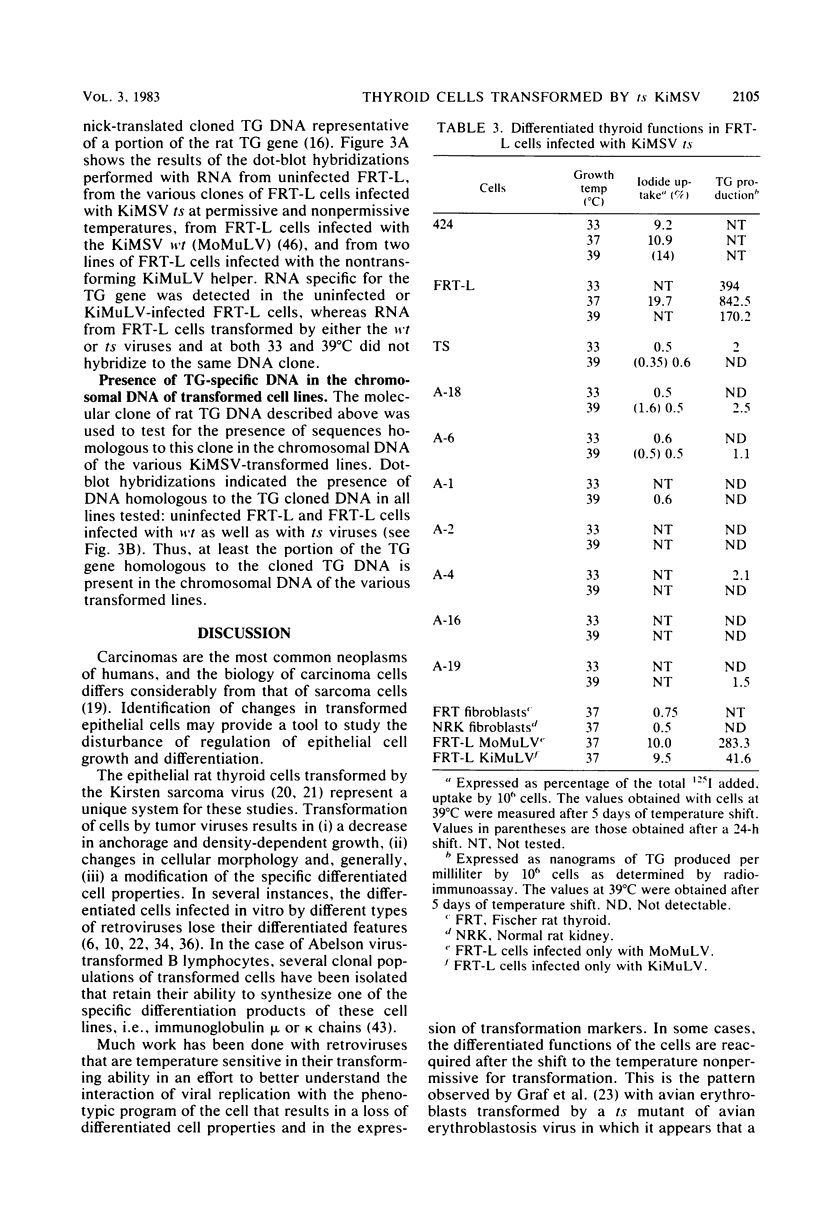
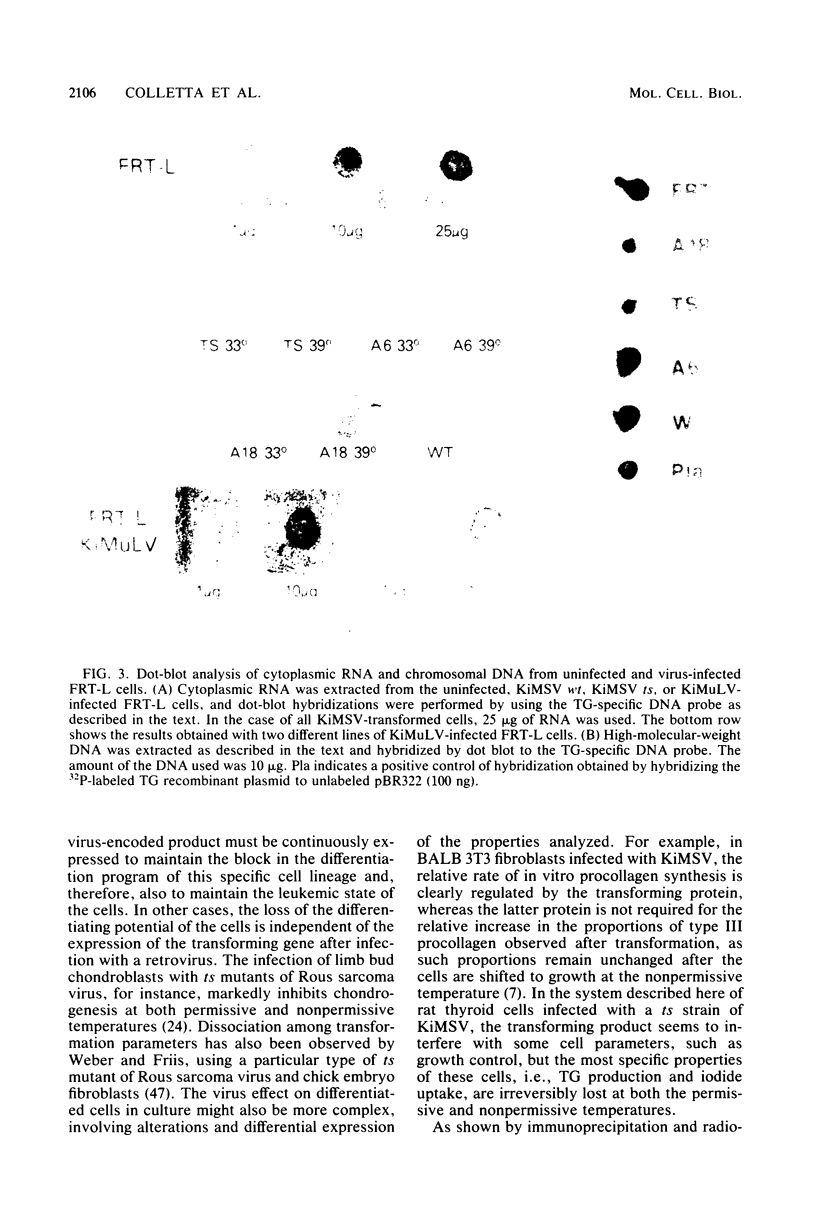
Differentiated rat thyroid epithelial cells, infected in vitro with a temperature-sensitive mutant of the Kirsten murine sarcoma virus, expressed at the permissive temperature (33 degrees C) some phenotypic properties typical of transformed cells, including morphological features, colony formation in agar, and induction of tumors in newborn animals. Specific functional markers of these differentiated cells, i.e., synthesis/secretion of thyroglobulin, synthesis of thyroglobulin mRNA and iodide uptake, were blocked during growth at 33 degrees C. Normal morphology, failure to grow in agar, and the requirement of hormones for optimal growth were all restored after shifting to the temperature nonpermissive for transformation (39 degrees C), though the typical differentiated functions remained blocked. Infection with a leukemia helper virus clone (Moloney or Kirsten murine leukemia virus) did not lead to the loss of the differentiated phenotype of rat epithelial thyroid cells, thus demonstrating that the loss of the differentiated phenotype is caused by the sarcoma virus component. These results indicate that the expression of some of the phenotypic properties of transformed differentiated rat thyroid epithelial cells is under the direct control of the p21 thermosensitive activity, whereas the block in the expression of two typical differentiation markers of thyroid epithelial cells is irreversible and probably controlled by different mechanisms.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. L., Sobel M. E., Howard B. H., Olden K., Yamada K. M., de Crombrugghe B., Pastan I. Levels of translatable mRNAs for cell surface protein, collagen precursors, and two membrane proteins are altered in Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3399–3403. doi: 10.1073/pnas.74.8.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvino C. G., Tassi V., Paterson B. M., Di Lauro R. In vitro synthesis of 300 00 Mr rat thyroglobulin subunit. FEBS Lett. 1982 Jan 25;137(2):307–313. doi: 10.1016/0014-5793(82)80373-6. [DOI] [PubMed] [Google Scholar]
- Ambesi-Impiombato F. S., Parks L. A., Coon H. G. Culture of hormone-dependent functional epithelial cells from rat thyroids. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3455–3459. doi: 10.1073/pnas.77.6.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbogast B. W., Yoshimura M., Kefalides N. A., Holtzer H., Kaji A. Failure of cultured chick embryo fibroblasts to incorporate collagen into their extracellular matrix when transformed by Rous sarcoma virus. An effect of transformation but not of virus production. J Biol Chem. 1977 Dec 25;252(24):8863–8868. [PubMed] [Google Scholar]
- Bateman J. F., Peterkofsky B. Mechanisms of Kirsten murine sarcoma virus transformation-induced changes in the collagen phenotype and synthetic rate of BALB 3T3 cells. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6028–6032. doi: 10.1073/pnas.78.10.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beug H., von Kirchbach A., Döderlein G., Conscience J. F., Graf T. Chicken hematopoietic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell. 1979 Oct;18(2):375–390. doi: 10.1016/0092-8674(79)90057-6. [DOI] [PubMed] [Google Scholar]
- Bilello J. A., Colletta G., Warnecke G., Koch G., Frisby D., Pragnell I. B., Ostertag W. Analysis of the expression of spleen focus-forming virus (SFFV)-related RNA and gp55, a Friend and Rauscher virus-specific protein. Virology. 1980 Dec;107(2):331–344. doi: 10.1016/0042-6822(80)90301-3. [DOI] [PubMed] [Google Scholar]
- Boettiger D., Roby K., Brumbaugh J., Biehl J., Holtzer H. Transformation of chicken embryo retinal melanoblasts by a temperature-sensitive mutant of Rous sarcoma virus. Cell. 1977 Aug;11(4):881–890. doi: 10.1016/0092-8674(77)90299-9. [DOI] [PubMed] [Google Scholar]
- Calothy G., Poirier F., Dambrine G., Pessac B. A transformation defective mutant of Rous sarcoma virus inducing chick embryo neuroretinal cell proliferation. Virology. 1978 Aug;89(1):75–84. doi: 10.1016/0042-6822(78)90041-7. [DOI] [PubMed] [Google Scholar]
- Chou J. Y. Human placental cells transformed by tsA mutants of simian virus 40: a model system for the study of placental functions. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1409–1413. doi: 10.1073/pnas.75.3.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y., Schlegel-Haueter S. E. Study of liver differentiation in vitro. J Cell Biol. 1981 May;89(2):216–222. doi: 10.1083/jcb.89.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colletta G., Di Fiore P. P., Ferrentino M., Pietropaolo C., Turco M. C., Vecchio G. Enhancement of viral gene expression in Friend erythroleukemic cells by 12-O tetradecanoylphorbol-13-acetate. Cancer Res. 1980 Sep;40(9):3369–3373. [PubMed] [Google Scholar]
- Di Lauro R., Obici S., Acquaviva A. M., Alvino C. G. Construction of recombinant plasmids containing rat thyroglobulin mRNA sequences. Gene. 1982 Jul-Aug;19(1):117–125. doi: 10.1016/0378-1119(82)90196-2. [DOI] [PubMed] [Google Scholar]
- Easton T. G., Reich E. Muscle differentiation in cell culture. Effects of nucleoside inhibitors and Rous sarcoma virus. J Biol Chem. 1972 Oct 25;247(20):6420–6431. [PubMed] [Google Scholar]
- Ellis R. W., Defeo D., Shih T. Y., Gonda M. A., Young H. A., Tsuchida N., Lowy D. R., Scolnick E. M. The p21 src genes of Harvey and Kirsten sarcoma viruses originate from divergent members of a family of normal vertebrate genes. Nature. 1981 Aug 6;292(5823):506–511. doi: 10.1038/292506a0. [DOI] [PubMed] [Google Scholar]
- Fusco A., Pinto A., Ambesi-Impiombato F. S., Vecchio G., Tsuchida N. Transformation of rat thyroid epithelial cells by Kirsten murine sarcoma virus. Int J Cancer. 1981 Nov 15;28(5):655–662. doi: 10.1002/ijc.2910280519. [DOI] [PubMed] [Google Scholar]
- Fusco A., Pinto A., Tramontano D., Tajana G., Vecchio G., Tsuchida N. Block in the expression of differentiation markers of rat thyroid epithelial cells by transformation with Kirsten murine sarcoma virus. Cancer Res. 1982 Feb;42(2):618–626. [PubMed] [Google Scholar]
- Gazzolo L., Moscovici C., Moscovici M. G., Samarut J. Response of hemopoietic cells to avian acute leukemia viruses: effects on the differentiation of the target cells. Cell. 1979 Mar;16(3):627–638. doi: 10.1016/0092-8674(79)90036-9. [DOI] [PubMed] [Google Scholar]
- Graf T., Ade N., Beug H. Temperature-sensitive mutant of avian erythroblastosis virus suggests a block of differentiation as mechanism of leukaemogenesis. Nature. 1978 Oct 12;275(5680):496–501. doi: 10.1038/275496a0. [DOI] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Gross J. L., Rifkin D. B. The effect of avian retroviruses on limb bud chondrogenesis in vitro. Cell. 1979 Nov;18(3):707–718. doi: 10.1016/0092-8674(79)90125-9. [DOI] [PubMed] [Google Scholar]
- Holtzer H., Biehl J., Yeoh G., Meganathan R., Kaji A. Effect of oncogenic virus on muscle differentiation. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4051–4055. doi: 10.1073/pnas.72.10.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikekubo K., Pervos R., Schneider A. B. Clearance of normal and tumor-related thyroglobulin from the circulation of rats: role of the terminal sialic acid residues. Metabolism. 1980 Jul;29(7):673–681. doi: 10.1016/0026-0495(80)90113-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levinson W., Bhatnagar R. S., Liu T. Z. Loss of ability to synthesize collagen in fibroblasts transformed by rous sarcoma virus. J Natl Cancer Inst. 1975 Oct;55(4):807–810. doi: 10.1093/jnci/55.4.807. [DOI] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama M., Yoshimura M., Muto M., Chi J., Roth S., Kaji A. Transformation of chicken chondrocytes by Rous sarcoma virus. Cancer Res. 1977 Mar;37(3):712–717. [PubMed] [Google Scholar]
- Olden K., Yamada K. M. Mechanism of the decrease in the major cell surface protein of chick embryo fibroblasts after transformation. Cell. 1977 Aug;11(4):957–969. doi: 10.1016/0092-8674(77)90307-5. [DOI] [PubMed] [Google Scholar]
- Ozanne B., Fulton R. J., Kaplan P. L. Kirsten murine sarcoma virus transformed cell lines and a spontaneously transformed rat cell-line produce transforming factors. J Cell Physiol. 1980 Oct;105(1):163–180. doi: 10.1002/jcp.1041050118. [DOI] [PubMed] [Google Scholar]
- Pacifici M., Boettiger D., Roby K., Holtzer H. Transformation of chondroblasts by Rous sarcoma virus and synthesis of the sulfated proteoglycan matrix. Cell. 1977 Aug;11(4):891–899. doi: 10.1016/0092-8674(77)90300-2. [DOI] [PubMed] [Google Scholar]
- Paraskeva C., Gallimore P. H. Tumorigenicity and in vitro characteristics of rat liver epithelial cells and their adenovirus-transformed derivatives. Int J Cancer. 1980 May 15;25(5):631–639. doi: 10.1002/ijc.2910250513. [DOI] [PubMed] [Google Scholar]
- Parker I., Fitschen W. Procollagen mRNA metabolism during the fibroblast cell cycle and its synthesis in transformed cells. Nucleic Acids Res. 1980 Jun 25;8(12):2823–2833. doi: 10.1093/nar/8.12.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp U. R., Todaro G. J. Generation of oncogenic mouse type C viruses: in vitro selection of carcinoma-inducing variants. Proc Natl Acad Sci U S A. 1980 Jan;77(1):624–628. doi: 10.1073/pnas.77.1.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy-Burman P., Klement V. Derivation of mouse sarcoma virus (Kirsten) by acquisition of genes from heterologous host. J Gen Virol. 1975 Aug;28(2):193–198. doi: 10.1099/0022-1317-28-2-193. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Weeks M. O., Young H. A., Scolnick E. M. p21 of Kirsten murine sarcoma virus is thermolabile in a viral mutant temperature sensitive for the maintenance of transformation. J Virol. 1979 Aug;31(2):546–546. doi: 10.1128/jvi.31.2.546-546.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siden E. J., Baltimore D., Clark D., Rosenberg N. E. Immunoglobulin synthesis by lymphoid cells transformed in vitro by Abelson murine leukemia virus. Cell. 1979 Feb;16(2):389–396. doi: 10.1016/0092-8674(79)90014-x. [DOI] [PubMed] [Google Scholar]
- Varshavsky A. Phorbol ester dramatically increases incidence of methotrexate-resistant mouse cells: possible mechanisms and relevance to tumor promotion. Cell. 1981 Aug;25(2):561–572. doi: 10.1016/0092-8674(81)90074-x. [DOI] [PubMed] [Google Scholar]
- Weber M. J., Friis R. R. Dissociation of transformation parameters using temperature-conditional mutants of Rous sarcoma virus. Cell. 1979 Jan;16(1):25–32. doi: 10.1016/0092-8674(79)90184-3. [DOI] [PubMed] [Google Scholar]
- Yavin E., Yavin Z., Schneider M. D., Kohn L. D. Monoclonal antibodies to the thyrotropin receptor: implications for receptor structure and the action of autoantibodies in Graves disease. Proc Natl Acad Sci U S A. 1981 May;78(5):3180–3184. doi: 10.1073/pnas.78.5.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Larco J. E., Todaro G. J. Growth factors from murine sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4001–4005. doi: 10.1073/pnas.75.8.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]