Abstract
Purpose
Treatment intensity for elderly patients with end-stage renal disease has escalated beyond population growth. Ageism seems to have given way to a powerful imperative to treat patients irrespective of age, prognosis, or functional status. Hemodialysis (HD) is a prime example of this trend. Recent articles have questioned this practice. This paper aims to identify existing pre-synthesized evidence on HD in the very elderly and frame it from the perspective of a clinician who needs to involve their patient in a treatment decision.
Patients and methods
A comprehensive search of several databases from January 2002 to August 2012 was conducted for systematic reviews of clinical and economic outcomes of HD in the elderly. We also contacted experts to identify additional references. We applied the rigorous framework of decisional factors of the Grading of Recommendation, Assessment, Development and Evaluation (GRADE) to evaluate the quality of evidence and strength of recommendations.
Results
We found nine eligible systematic reviews. The quality of the evidence to support the current recommendation of HD initiation for most very elderly patients is very low. There is significant uncertainty in the balance of benefits and risks, patient preference, and whether default HD in this patient population is a wise use of resources.
Conclusion
Following the GRADE framework, recommendation for HD in this population would be weak. This means it should not be considered standard of care and should only be started based on the well-informed patient’s values and preferences. More studies are needed to delineate the true treatment effect and to guide future practice and policy.
Keywords: cost, quality of life, renal replacement therapy, resource utilization survival, symptom burden
Introduction
Over the past two decades treatment intensity for elderly patients with terminal conditions has escalated beyond population growth.1–3 Ageism, characteristic of the early days of Hemodialysis (HD),4 seems to have given way to a powerful imperative to treat patients irrespective of age, prognosis, or functional status.5 End-stage renal disease (ESRD) is a prime example of this trend with a 57% age-adjusted increase in HD for octo- and nonagenarians between 1996 and 2003,1 partly due to a push for earlier initiation of HD, especially in patients over 75.6 This has happened despite an Institute of Medicine report in 1991 calling attention to an increasing number of dialysis patients with poor quality of life (QoL) and limited survival possibilities7, and subsequent treatment guidelines in 2000 emphasizing shared decision making and outlining when HD treatment may be considered of minimal benefit.8 Patients report not being given enough information to make informed decisions and to be given a choice of dialysis or nothing.5,9
Although the benefits of HD in aggregate are undeniable, benefits to certain high-risk subgroups are uncertain,10,11 and the quality of evidence available to guide practice and policy is questionable, particularly in the very elderly. Meanwhile, the evidence of significant harm for certain subpopulations is mounting.11–13 Moreover, more than half of ESRD patients on dialysis experience significant symptoms, such as pain, fatigue, pruritus, nausea, and constipation.14 Data are quite limited on symptom prevalence in ESRD patients managed conservatively without dialysis.14
To develop evidence-based recommendations regarding HD in the very elderly (≥75 years old), several factors should be considered including the quality of evidence supporting benefit and harm, patients’ values, preferences, and clinical and social context, as well as resource availability. In this paper, we incorporate these decisional factors following the rigorous framework of the Grading of Recommendation, Assessment, Development and Evaluation, (GRADE) framework.15 To our knowledge, this is the first description of applying this framework, which is increasingly becoming the state-of-the-art guideline development process, to this important clinical question.
Methods
This study follows an umbrella systematic review design16 and aims to identify existing pre-synthesized evidence in published systematic reviews, as well as to frame them from the perspective of a clinician who needs to make a single treatment decision.
Data sources and search strategies
A comprehensive search of several databases from January 2002 to August 2012, in any language, was conducted. The databases included Ovid Medline In-Process and Other Non-Indexed Citations, Ovid MEDLINE, Ovid EMBASE, Ovid Cochrane Database of Systematic Reviews, and Scopus. The search strategy was designed and conducted by an experienced librarian with input from the study’s principal investigator. Controlled vocabulary, supplemented with keywords, was used to search for systematic reviews of outcomes and economics of HD in the elderly. Reference lists were hand searched and expert colleagues were approached for relevant articles. We then applied A Measurement Tool to Assess Systematic Reviews (AMSTAR) grading system17 to the systematic reviews to assess their quality (Supplementary material).
Rating the quality of evidence and strength of recommendations
We applied the GRADE framework to the available research evidence. The quality of evidence depends on the elements of risk of bias, imprecision, indirectness, reporting and publication bias and inconsistency. The strength of recommendations depends on the quality of evidence, patients’ values and preferences, balance between harms and benefits and resource utilization. The recommendations can be strong (apply to most patients in most settings) or weak (conditional, can vary based on patients context, resources and preferences).15
Results
The search strategy yielded 92 articles, and reference review and expert contact yielded five additional articles. Lastly, we included nine systematic reviews that provided data on the outcomes of interest and are included in this review (Figure 1).9,14,18–24
Figure 1.
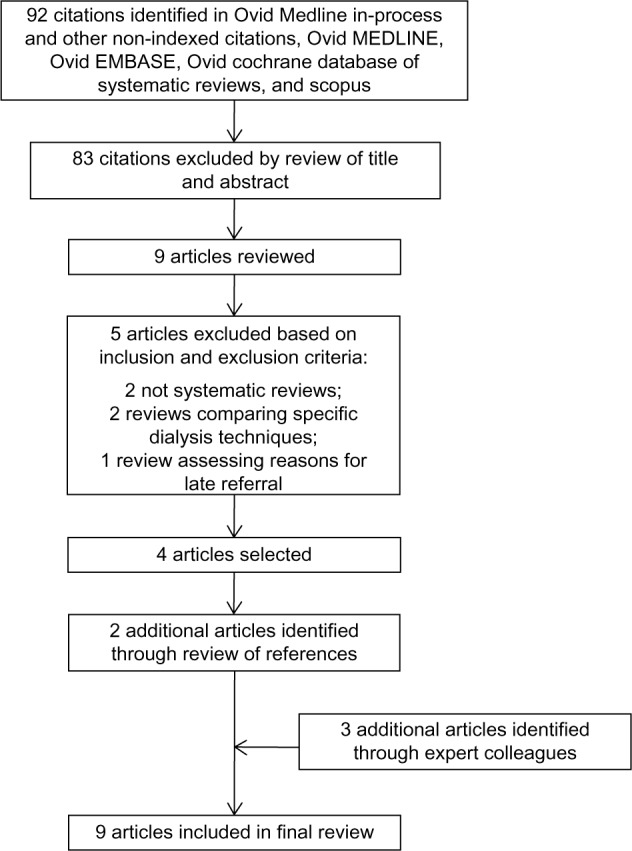
Search strategy results and identification of publications included in review.
The methodological quality of these systematic reviews was moderate to high, satisfying most of the AMSTAR quality indicators (Table 1). The included systematic reviews were particularly deficient in the areas of restricting their search to the English language and their inability to evaluate for publication bias (Figure 1 and Table 1).
Table 1.
Quality of systematic review (AMSTAR quality indicators)
| Article | Primary goal | included studies | Quality of systematic review (AMSTAR quality indicators)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Was a priori design provided? | Was there duplicate study selection and data extraction? | Was a comprehensive literature search performed? | Was the status of publication (ie, grey literature) used as an inclusion criterion? | Was a list of studies (included and excluded) provided? | Were the characteristics of the included studies provided? | Was the scientific quality of the included studies assessed and documented? | Was the scientific quality of the included studies used appropriately in formulating conclusions? | Were the methods used to combine the findings of studies appropriate? | Was the likelihood of publication bias assessed? | Was the conflict of interest included? | |||
| AMSTAR | |||||||||||||
| Winkelmayer22 | To evaluate the cost-effectiveness of renal replacement therapy | 13 observational and economic modeling studies | Yes | Not mentioned | No, search restricted to English | Yes | Yes included No excluded | Yes | No | Yes | Yes | No | No |
| Mowatt24 | To assess the effectiveness and cost effectiveness of home HD compared with HD carried out in a hospital or satellite unit | 27 reviews, comparative observational studies and randomized crossover trial | Yes | Yes | Non-English studies identified but not evaluated | No | Yes | Yes | Yes | Yes | Yes | No | Funding source listed no conflict of interest statement |
| Murtagh14 | To identify the prevalence of symptoms in patients with ESRD on HD, non-dialytic management and discontinuing dialysis | 60 prospective and retrospective cross sectional data one of which collected longitudinal data | Yes | Study selection No data extraction Yes on a random sample | No, search restricted to English | No | Yes included No excluded | Yes | Yes | Yes | Yes | No | No |
| Lazarides21 | To compare outcomes of various angioaccess procedures in elderly patients | 13 cohort observational studies | Yes | No | No, search restricted to English | No | Yes | Yes | No | Yes | Yes | Yes | No |
| Schmitt19 | To determine the incidence of non-recovery of kidney function after acute kidney injury | 17 retrospective cohort and randomized controlled trials | Yes | Yes | No, search restricted to English | Yes | Yes included No excluded | Yes | Yes | Yes | Yes | No | Yes |
| Morton9 | To synthesize and analyze the views of patients and caregivers on decision making and choice for treatments in patients with chronic kidney disease | 18 qualitative studies | Yes | Yes | No, search restricted to English | No | Yes included No excluded | Yes | Yes | Yes | Yes | No | Yes |
| Johnson20 | To formulate and express a prognostic assessment for a patient with acute renal failure requiring RRT in the Intensive care unit | 41 cohort, and RCT studies | Yes | No | No, search restricted to English | Yes | Yes included No excluded | Yes but just number of patients, setting and outcomes, not study type | No | No | Yes | No | Yes |
| Menzin23 | To identify interventions in chronic kidney disease that provide reasonable value and potential to lower cost and improve quality | 84 observational and economic modeling studies | Yes | Yes | No, search restricted to English | Yes | No | Some, description, time horizon perspective but not study type | No | Yes | Yes | No | Yes |
| O’Connor18 | To summarize evidence on non-dialytic management of ESRD regarding prognosis and QoL | 13 cohort, cross sectional and observational studies | Yes | Yes | Yes | No | Yes included No excluded | Yes | Yes | Yes | N/A | No | Yes |
Notes: Ninety-two citations identified in Ovid Medline In-Process and Other Non-Indexed Citations, Ovid MEDLINE, Ovid EMBASE, Ovid Cochrane Database of Systematic Reviews, and Scopus. Eighty-three citations excluded by review of title and abstract. Nine articles reviewed. Five articles excluded based on inclusion and exclusion criteria. Two nonsystematic reviews. Two reviews comparing specific dialysis techniques. One review assessing reasons for late referral. Four articles selected. Two additional articles identified through review of references. Three additional articles identified through expert colleagues. Nine articles included in final review.
Abbreviations: AMSTAR, a measurement tool to assess systematic reviews; ESRD, end-stage renal disease; HD, hemodialysis; QoL, quality of life; RRT, renal replacement therapy; RCT, randomized controlled trial.
Quality of evidence for benefit
Based on the United States Renal Data System (USRDS), the expected remaining lifetime for patients 75–79 years old with ESRD undergoing HD is 2.8 years, 2.3 years for 80- to 84-year-old patients, and 1.9 years for the age group ≥ 85 years old.25 No comparable national registry or large cohort data is available for patients who opt for conservative treatment. Smaller cohort studies were recently summarized in a systematic review of conservatively managed ESRD patients that demonstrated a median survival of at least 6 months with a range of 6.3–23.4 months.18 Five prognostic studies compared patients on HD with supportive care. All of them reported statistically significant survival benefits, but the groups were poorly balanced on either age,26–28 other prognostic factors,27,29 or both,27,30 and there was significant heterogeneity in their population and methods. One of the studies looked specifically at the survival of a small subgroup of patients for whom palliative care had been recommended and showed no significant survival benefit.27 Another study looked at median survival from the first known date of glomerular filtration rate < 15 and found minimal survival benefit with HD.28 The studies were all cohort studies; three of which were prospective26,27,29 and two were retrospective.28,30 Three of the studies specifically evaluated the older patients (over age 70 or 75),26,28,30 and even in those studies the groups were poorly balanced in terms of age.
In addition, elderly patients who suffer acute kidney injury are less likely to recover kidney function and become independent from dialysis therapy19 and are more likely to suffer fistula failure than younger patients.21
Thus, the overall quality of evidence supporting a modest survival benefit with HD in the elderly is considered to be very low (due to the methodological limitations of the studies and heterogeneity).
Quality of evidence for harm
In recent studies there is mounting evidence of a risk of harm from HD in the very elderly.11 A large retrospective registry cohort study of 3702 nursing-home patients was conducted using functional status from the Minimal Data Set Activities of Daily Living (MDS-ADL) score from 3 months prior, to until 12 months after the onset of HD. There was no comparison group of conservatively treated patients. The study demonstrated a rapid decline in functional status and high mortality. Only 13% maintained functional status at 12 months, and the 1-year mortality rate was 58%.12 A small (n = 97) single-center retrospective analysis of prospectively collected data on living status showed that the proportion of independently living elderly patients rapidly declined from 78% at baseline to 23% at 1 year, 11% at 2 years, and 4% at the end of follow-up.13 Similarly, there was no comparison group in this study.
Qualitative studies report significant patient suffering.31 Symptom burden for patients with ESRD is consistently reported as very high for patients on HD,14 with limited comparative data for patients on supportive care only.14 Contrary to common belief, HD does not always improve symptom control, and symptom burden is higher at 6 months than at HD initiation.32 Withdrawal rates in the oldest USRDS population range from 25%–34%.33 Palliative and hospice care is underutilized even for those patients who decide to discontinue HD.34 Symptoms are often undertreated35 despite available and effective treatments.36,37
Once on HD, aggressive end-of-life care is pervasive and more aggressive than for patients with other chronic life-limiting illnesses as demonstrated by a retrospective cohort study of over 99,000 USRDS decedents.38 Patients on HD spend significantly more days in the hospital and in the HD unit and are much less likely to die at home than patients receiving supportive care (odds ratio for home death on supportive care is 4.15; 95% CI 1.67–10.25).26 Patients may spend the majority of any extra days survived in the hospital or HD unit.26 These data are based on a prospective cohort study of 202 patients over 70 years of age. Two other prospective studies of 321 and 71 patients, respectively, have shown similar findings for site of death.27,39
In summary, the quality of evidence suggesting harms associated with HD in the elderly is also low as it is mostly based on observational studies lacking comparison groups, except for the risk for institutional death which is based on prospective cohort data.
Uncertainty or variability in values and preferences
In a situation in which the balance of benefits and risks are uncertain, eliciting the values and preferences of patients and empowering them and their surrogates to make decisions consistent with their goals of care becomes even more important. Defining the benefit of treatment should be in the patients’ purview.40
Qualitative studies and surveys suggest that most patients on HD do not perceive that they have been given a choice of treatment41,42 and many regret having started HD.32,42 Receipt of intensive end-of-life care in HD patients also seems to be driven more by practice related factors than patient characteristics.38 Six prominent patient goals of care have been identified in a structured literature review43 and validated in subsequent studies.44 Patients can have multiple goals of care. While 84% of hospitalized patients endorsed living longer as one of their goals, it was the single most important goal for less than 10% of patients.44 A systematic review and synthesis of qualitative studies on the views of chronic kidney disease patients regarding treatment decision making also reached the conclusion that patients were more concerned about the impact on QoL than longevity.9 Despite this, none of the studies comparing survival between groups looked at QoL data or loss of functional status.26–30 Only two studies directly comparing QoL were identified by the systematic review on conservative management of ESRD.18 Yong et al reported better QoL in the conservatively managed group despite the fact that the patients were older and had higher comorbidity.45 De Biase et al compared two groups of patients who were recommended for palliative care and found that those who opted for HD had a similar QoL compared to those who opted for supportive care.46
There is significant variability in elderly patient’s values and preferences when facing decisions regarding treatment options for ESRD. The available evidence suggests a failure to honor those differences by failing to involve elderly patients in shared decision making before starting HD.
Uncertainty about whether the intervention represents a wise use of resources
There is increasing concern about the cost of care for the growing elderly population in the context of a looming bankruptcy of Medicare.47 Currently, Medicare spends over a quarter of its budget on the 5%–6% of its beneficiaries that die each year.48 High costs associated with the last months of life and terminal hospitalizations/ICU stays have been cited as an area of potential savings with minimal harm or even benefit.49 In addition, there is concern about the increasing medicalization of death and “bad hospital deaths” with significant patient suffering and financial and emotional burdens on loved ones left behind.50–52 The HD benefit is a significant portion of the Medicare budget consuming approximately 6% of the entire budget for a disease with a prevalence of 1,780 per million, representing 1% of the total Medicare population.33
The cost effectiveness of HD has been cited as a benchmark for societal willingness to pay for medical treatment.22 A meta-analytic review of the cost effectiveness of HD found the estimate to remain within a narrow range of $55,000 to $80,000 per life-year saved.22 However, the costs and cost effectiveness ratios in this analysis may have been underestimated since informal caregiver time was not included in most of the studies. Another weakness identified in all studies was the assumption of no life expectancy for patients who were not dialyzed, which does not hold, especially in the setting of early initiation of HD. The true cost based on USRDS data for the oldest HD patients, with congestive heart failure and diabetes mellitus as comorbidities, is at or above $100,000 in 2006 US dollars.25,53 Given the questionable survival benefit of HD outlined above, the dollars per quality-adjusted life-year saved are likely much higher. A recent empirical estimate to update the HD cost effectiveness standard arrived at an average of $129,000 per Quality Added Life Years (QALY) with a wide distribution.54 The patients in the highest quintile (mostly older and sicker) had costs of about $250,000/QALY. Another study showed a cost effectiveness ratio of $250,000 for early initiation of glomerular filtration rate (GFR) < 15 compared to current practice.55 Peritoneal dialysis is more cost effective than HD24,56 and is currently underutilized in the US.57
There is significant uncertainty in the cost effectiveness estimates for the oldest HD patients secondary to the uncertainty about the true treatment benefit. Hemodialysis in the frail elderly and others with multiple comorbidities is very costly and, depending on the societal benchmark for willingness to pay, may not constitute a wise use of resources.
Research gaps
This analysis of the existing evidence suggests that there is sufficient equipoise regarding the benefits of HD for the oldest patients to warrant a randomized controlled trial of HD vs best supportive care. Better evidence is needed to enable sound policy decisions that preserve access to HD yet minimize the risk of overutilization and possible harm to patients who are likely to benefit only marginally or suffer harms from this expensive treatment.
Ideally, the recruitment for the study would utilize well-developed patient decision aids to convey the available evidence on patient survival as well as the uncertainty of the estimate. Peer educators would also be valuable to provide the experiential aspect of dialysis care.58 Patients should be risk stratified using one of the available tools for ESRD patients.59 In the analysis, correcting for the GFR at the start of HD should also be used to correct for lead time bias with the current early initiation of HD.
The study would need to consider all patient important outcomes including survival from diagnosis, frequency of hospitalizations and number of hospital and ICU days, QoL and symptom burden (measured with validated tools such as the Kidney Disease Quality Of Life 3660 or Short Form1261), and finally the proportion of hospital vs home deaths. Calculating the cost of care for both treatment arms would also be important for comparative effectiveness purposes.
We realize that there might be significant barriers to recruitment into such a study given the powerful technical and moral imperative to treat5, as well as the risk of being accused of advocating death panels or of being ageist.62,63 Patients may also be resistant to randomization based on their goals of care. If this proves to be the case, we suggest the creation of a large-scale cohort of elderly patients with ESRD who opt for conservative management to create a valid comparison group to the USRDS database on HD patients.
Conclusion
We have demonstrated that the quality of the evidence to support the current practice of HD initiation for most very elderly patients is very low. Survival benefit is questionable and modest at best, and there are significant concerns for harm such as decline in functional status, high treatment and symptom burden, poor QoL, aggressive end-of-life care, and institutionalized death. Moreover, this is a costly treatment. More studies are needed to delineate the true treatment effect and to guide future practice and policy.
Following the GRADE framework, recommendation for HD in this population would be weak, which means it should not be considered default treatment in the majority of cases and should only be offered based on the well-informed patient’s values and preferences.
The recent push for early HD initiation in this age group is not justified. The suggestion of a risk of harm, coupled with a failure of early initiation to demonstrate improved survival,64,65 would support holding off from HD as long as clinically possible. A significant number of patients are likely to die of other causes before they reach the point of inevitable HD.66
Patients’ goals of care should be the guiding light in all treatment decisions and physicians should not feel obliged to dialyze everyone. In fact the Hippocratic maxim “first do no harm” should be weighed against the moral imperative to treat.
Supplementary material
Search strategies by database.
Ovid
Database(s): Embase 1988 to 2012 Week 33, Ovid MEDLINE(R) In-Process and Other Non-Indexed Citations and Ovid MEDLINE(R) 1946 to Present, EBM Reviews – Cochrane Database of Systematic Reviews 2005 to July 2012.
Search strategy:
| # | Searches | Results |
|---|---|---|
| 1 | exp Renal Dialysis/ | 180343 |
| 2 | exp hemodialysis/ | 137673 |
| 3 | (((renal or kidney* or blood) adj5 (dialyses or dialysis)) or hemodialysis or haemodialysis or hemodialyses or haemodialyses or “extracorporeal dialysis” or “extracorporeal dialyses” or “extracorporeal blood cleansing” or hemodialyse or hemorenodialysis or hemorenodialyses or hemotrialysate).mp. [mp = ti, ab, sh, hw, tn, ot, dm, mf, dv, kw, nm, ps, rs, ui, tx, ct] | 176110 |
| 4 | (((renal or kidney* or blood or Peritoneal) adj5 (dialyses or dialysis)) or hemodialysis or haemodialysis or hemodialyses or haemodialyses or “extracorporeal dialysis” or “extracorporeal dialyses” or “extracorporeal blood cleansing” or hemodialyse or hemorenodialysis or hemorenodialyses or hemotrialysate or Hemodiafiltration).mp. [mp = ti, ab, sh, hw, tn, ot, dm, mf, dv, kw, nm, ps, rs, ui, tx, ct] | 206776 |
| 5 | or/1–4 | 220497 |
| 6 | limit 5 to yr = “2002 – Current” | 112269 |
| 7 | limit 6 to (“all aged (65 and over)” or “aged (80 and over)”) [Limit not valid in Embase,CDSR; records were retained] | 85643 |
| 8 | limit 7 to aged “65+ years” [Limit not valid in Ovid MEDLINE(R),Ovid MEDLINE(R) In-Process,CDSR; records were retained] | 31235 |
| 9 | (elderly or octagenarian* or nonagenarian* or “very old” or “75 year*” or “80 year*” or “90 year*” or “100 year*” or ((“75” or “80” or “90” or “100”) adj (age or aged))).mp. [mp = ti, ab, sh, hw, tn, ot, dm, mf, dv, kw, nm, ps, rs, ui, tx, ct] | 445517 |
| 10 | 6 and 9 | 2886 |
| 11 | 8 or 10 | 32086 |
| 12 | systematic review/ | 52193 |
| 13 | (systematic* adj3 review*).mp. [mp = ti, ab, sh, hw, tn, ot, dm, mf, dv, kw, nm, ps, rs, ui, tx, ct] | 127590 |
| 14 | 11 and (12 or 13) | 231 |
| 15 | from 14 keep 1–69 | 69 |
| 16 | from 10 keep 2833–2885 | 53 |
| 17 | 15 or 16 | 122 |
| 18 | remove duplicates from 17 | 107 |
| 19 | exp treatment outcome/or outcome*.mp. or economic*.mp. or exp Economics/or exp “Costs and Cost Analysis” or cost.mp. or costs.mp. or benefit*.mp. or harm*.mp. or preference*.mp. or exp Patient Preference/or “quality of life”.mp. or exp “Quality of Life”/or survival.mp. or exp Survival/or exp survival rate/or “functional status”.mp. or morbidity.mp. or mortality.mp. or exp Morbidity/or exp Mortality/or satisfaction.mp. or exp Patient Satisfaction/[mp = ti, ab, sh, hw, tn, ot, dm, mf, dv, kw, nm, ps, rs, ui, tx, ct] | 6917810 |
| 20 | 18 and 19 | 92 |
| 21 | limit 20 to (editorial or erratum or letter or note or addresses or autobiography or bibliography or biography or dictionary or directory or interactive tutorial or lectures or legislation or news or newspaper article or patient education handout or periodical index or portraits or published erratum or video-audio media or webcasts) [Limit not valid in Embase,Ovid MEDLINE(R),Ovid MEDLINE(R) In-Process,CDSR; records were retained] | 54 |
| 22 | from 21 keep 1–2 | 2 |
| 23 | 20 not 22 | 90 |
Scopus
TITLE-ABS-KEY((renal W/5 dialyses) or (kidney w/5 dialyses) or (blood w/5 dialyses) or (peritoneal w/5 dialyses) or (renal W/5 dialysis) or (kidney w/5 dialysis) or (blood w/5 dialysis) or (peritoneal w/5 dialysis) or hemodialysis or haemodialysis or hemodialyses or haemodialyses or “extracorporeal dialysis” or “extracorporeal dialyses” or “extracorporeal blood cleansing” or hemodialyse or hemorenodialysis or hemorenodialyses or hemotrialysate or Hemodiafiltration) AND PUBYEAR > 2001
TITLE-ABS-KEY(elderly or octagenarian* or nonagenarian* or “very old” or “75 year*” or “80 year*” or “90 year*” or “100 year*” or (75 w/1 age) or (80 w/1 age) or (90 w/1 age) or (100 w/1 age) or (75 w/1 aged) or (80 w/1 aged) or (90 w/1 aged) or (100 w/1 aged))
TITLE-ABS-KEY(outcome* or economic* or cost or costs or benefit* or harm* or preference* or “quality of life” or survival or “functional status” or morbidity or mortality or satisfaction)
and 2 and 3
PMID(0*) OR PMID(1*) OR PMID(2*) OR PMID(3*) OR PMID(4*) OR PMID(5*) OR PMID(6*) OR PMID(7*) OR PMID(8*) OR PMID(9*)
and not 6
DOCTYPE(le) OR DOCTYPE(ed) OR DOCTYPE(bk) OR DOCTYPE(er) OR DOCTYPE(no) OR DOCTYPE(sh)
and not 7
TITLE-ABS-KEY(systematic* w/3 review*)
and 9
Acknowledgments
This publication was supported by Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
We thank Gladys Hebl from Mayo Clinic Grant and Publication Support Services for her help in preparing this manuscript for publication.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kurella M, Covinsky KE, Collins AJ, Chertow GM. Octogenarians and nonagenarians starting dialysis in the United States. Ann Intern Med. 2007;146(3):177–183. doi: 10.7326/0003-4819-146-3-200702060-00006. [DOI] [PubMed] [Google Scholar]
- 2.Sharma G, Freeman J, Zhang D, Goodwin JS. Trends in end-of-life ICU use among older adults with advanced lung cancer. Chest. 2008;133(1):72–78. doi: 10.1378/chest.07-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Earle CC, Landrum MB, Souza JM, Neville BA, Weeks JC, Ayanian JZ. Aggressiveness of cancer care near the end of life: is it a quality-of-care issue? J Clin Oncol. 2008;26(23):3860–3866. doi: 10.1200/JCO.2007.15.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kjellstrand C. All elderly patients should be offered dialysis. Geriatr Nephrol Urol. 1997;6:129–136. [Google Scholar]
- 5.Kaufman SR, Shim JK, Russ AJ. Revisiting the biomedicalization of aging: clinical trends and ethical challenges. Gerontologist. 2004;44(6):731–738. doi: 10.1093/geront/44.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Hare AM, Choi AI, Boscardin WJ, et al. Trends in timing of initiation of chronic dialysis in the United States. Arch Intern Med. 2011;171(18):1663–1669. doi: 10.1001/archinternmed.2011.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Institute of Medicine, Division of Health Care Services, Committee for the Study of the Medicare End-Stage Renal Disease Program . Kidney Failure and the Federal Government. Washington, DC: National Academy Press; 1991. [Google Scholar]
- 8.Renal Physicians Association . Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis. Clinical Practice Guideline. Rockville, MD: Renal Physicians Association; 2010. [Google Scholar]
- 9.Morton RL, Tong A, Howard K, Snelling P, Webster AC. The views of patients and carers in treatment decision making for chronic kidney disease: systematic review and thematic synthesis of qualitative studies. BMJ. 2010;340:c112. doi: 10.1136/bmj.c112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thorsteinsdottir B, Swetz KM, Feely MA, Mueller PS, Williams AW. Are there alternatives to hemodialysis for the elderly patient with end-stage renal failure? Mayo Clin Proc. 2012;87(6):514–516. doi: 10.1016/j.mayocp.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosansky SJ. The sad truth about early initiation of dialysis in elderly patients. JAMA. 2012;307(18):1919–1920. doi: 10.1001/jama.2012.3522. [DOI] [PubMed] [Google Scholar]
- 12.Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361(16):1539–1547. doi: 10.1056/NEJMoa0904655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jassal SV, Chiu E, Hladunewich M. Loss of independence in patients starting dialysis at 80 years of age or older. N Engl J Med. 2009;361(16):1612–1613. doi: 10.1056/NEJMc0905289. [DOI] [PubMed] [Google Scholar]
- 14.Murtagh FEM, Addington-Hall J, Higginson IJ. The prevalence of symptoms in end-stage renal disease: a systematic review. Adv Chronic Kidney Dis. 2007;14(1):82–99. doi: 10.1053/j.ackd.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomson D, Russell K, Becker L, Klassen T, Hartling L. The evolution of a new publication type: Steps and challenges of producing overviews of reviews. Res Synth Method. 2010;1(3–4):198–211. doi: 10.1002/jrsm.30. [DOI] [PubMed] [Google Scholar]
- 17.Shea BJ, Hamel C, Wells GA, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. 2009;62(10):1013–1020. doi: 10.1016/j.jclinepi.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 18.O’Connor NR, Kumar P. Conservative management of end-stage renal disease without dialysis: a systematic review. J Palliat Med. 2012;15(2):228–235. doi: 10.1089/jpm.2011.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmitt R, Coca S, Kanbay M, Tinetti ME, Cantley LG, Parikh CR. Recovery of kidney function after acute kidney injury in the elderly: a systematic review and meta-analysis. Am J Kidney Dis. 2008;52(2):262–271. doi: 10.1053/j.ajkd.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Johnson RF, Gustin J. Acute renal failure requiring renal replacement therapy in the intensive care unit: impact on prognostic assessment for shared decision making. J Palliat Med. 2011;14(7):883–889. doi: 10.1089/jpm.2010.0452. [DOI] [PubMed] [Google Scholar]
- 21.Lazarides MK, Georgiadis GS, Antoniou GA, Staramos DN. A meta-analysis of dialysis access outcome in elderly patients. J Vasc Surg. 2007;45(2):420–426. doi: 10.1016/j.jvs.2006.10.035. [DOI] [PubMed] [Google Scholar]
- 22.Winkelmayer WC, Weinstein MC, Mittleman MA, Glynn RJ, Pliskin JS. Health economic evaluations: the special case of end-stage renal disease treatment. Med Decis Making. 2002;22(5):417–430. doi: 10.1177/027298902236927. [DOI] [PubMed] [Google Scholar]
- 23.Menzin J, Lines LM, Weiner DE, et al. A review of the costs and cost effectiveness of interventions in chronic kidney disease: implications for policy. Pharmacoeconomics. 2011;29(10):839–861. doi: 10.2165/11588390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 24.Mowatt G, Vale L, Perez J, et al. Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of home versus hospital or satellite unit haemodialysis for people with end-stage renal failure. Health Technol Assess. 2003;7(2):1–174. doi: 10.3310/hta7020. [DOI] [PubMed] [Google Scholar]
- 25.US Renal Data System . USRDS 2008 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2008. [Google Scholar]
- 26.Carson RC, Juszczak M, Davenport A, Burns A. Is maximum conservative management an equivalent treatment option to dialysis for elderly patients with significant comorbid disease? Clin J Am Soc Nephrol. 2009;4(10):1611–1619. doi: 10.2215/CJN.00510109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith C, Da Silva-Gane M, Chandna S, Warwicker P, Greenwood R, Farrington K. Choosing not to dialyse: evaluation of planned non-dialytic management in a cohort of patients with end-stage renal failure. Nephron Clin Pract. 2003;95(2):c40–c46. doi: 10.1159/000073708. [DOI] [PubMed] [Google Scholar]
- 28.Murtagh FE, Marsh JE, Donohoe P, Ekbal NJ, Sheerin NS, Harris FE. Dialysis or not? A comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephrol Dial Transplant. 2007;22(7):1955–1962. doi: 10.1093/ndt/gfm153. [DOI] [PubMed] [Google Scholar]
- 29.Joly D, Anglicheau D, Alberti C, et al. Octogenarians reaching end-stage renal disease: cohort study of decision-making and clinical outcomes. J Am Soc Nephrol. 2003;14(4):1012–1021. doi: 10.1097/01.asn.0000054493.04151.80. [DOI] [PubMed] [Google Scholar]
- 30.Chandna SM, Da Silva-Gane M, Marshall C, Warwicker P, Greenwood RN, Farrington K. Survival of elderly patients with stage 5 CKD: comparison of conservative management and renal replacement therapy. Nephrol Dial Transplant. 2011;26(5):1608–1614. doi: 10.1093/ndt/gfq630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russ AJ, Shim JK, Kaufman SR. “Is there life on dialysis?”: time and aging in a clinically sustained existence. Med Anthropol. 2005;24(4):297–324. doi: 10.1080/01459740500330639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stringer S, Baharani J. Why did I start dialysis? A qualitative study on views and expectations from an elderly cohort of patients with end-stage renal failure starting haemodialysis in the United Kingdom. Int Urol Nephrol. 2012;44(1):295–300. doi: 10.1007/s11255-011-0045-4. [DOI] [PubMed] [Google Scholar]
- 33.US Renal Data System . USRDS 2011 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011. [Google Scholar]
- 34.Murray AM, Arko C, Chen SC, Gilbertson DT, Moss AH. Use of hospice in the United States dialysis population. Clin J Am Soc Nephrol. 2006;1(6):1248–1255. doi: 10.2215/CJN.00970306. [DOI] [PubMed] [Google Scholar]
- 35.Claxton RN, Blackhall L, Weisbord SD, Holley JL. Undertreatment of symptoms in patients on maintenance hemodialysis. J Pain Symptom Manage. 2010;39(2):211–218. doi: 10.1016/j.jpainsymman.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 36.Cohen LM, Moss AH, Weisbord SD, Germain MJ. Renal palliative care. J Palliat Med. 2006;9(4):977–992. doi: 10.1089/jpm.2006.9.977. [DOI] [PubMed] [Google Scholar]
- 37.Barakzoy AS, Moss AH. Efficacy of the world health organization analgesic ladder to treat pain in end-stage renal disease. J Am Soc Nephrol. 2006;17(11):3198–3203. doi: 10.1681/ASN.2006050477. [DOI] [PubMed] [Google Scholar]
- 38.Wong SP, Kreuter W, O’Hare AM. Treatment intensity at the end of life in older adults receiving long-term dialysis. Arch Intern Med. 2012;172(8:):661–663. doi: 10.1001/archinternmed.2012.268. discussion 663–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong CF, McCarthy M, Howse ML, Williams PS. Factors affecting survival in advanced chronic kidney disease patients who choose not to receive dialysis. Ren Fail. 2007;29(6):653–659. doi: 10.1080/08860220701459634. [DOI] [PubMed] [Google Scholar]
- 40.Pellegrino ED. Decisions to withdraw life-sustaining treatment: a moral algorithm. JAMA. 2000;283(8):1065–1067. doi: 10.1001/jama.283.8.1065. [DOI] [PubMed] [Google Scholar]
- 41.Russ AJ, Kaufman SR. Discernment rather than decision-making among elderly dialysis patients. Semin Dial. 2012;25(1):31–32. doi: 10.1111/j.1525-139X.2011.01047.x. [DOI] [PubMed] [Google Scholar]
- 42.Davison SN. End-of-life care preferences and needs: perceptions of patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5(2):195–204. doi: 10.2215/CJN.05960809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaldjian LC, Curtis AE, Shinkunas LA, Cannon KT. Goals of care toward the end of life: a structured literature review. Am J Hosp Palliat Care. 2008;25(6):501–511. doi: 10.1177/1049909108328256. [DOI] [PubMed] [Google Scholar]
- 44.Kaldjian LC, Erekson ZD, Haberle TH, et al. Code status discussions and goals of care among hospitalised adults. J Med Ethics. 2009;35(6):338–342. doi: 10.1136/jme.2008.027854. [DOI] [PubMed] [Google Scholar]
- 45.Yong DS, Kwok AO, Wong DM, Suen MH, Chen WT, Tse DM. Symptom burden and quality of life in end-stage renal disease: a study of 179 patients on dialysis and palliative care. Palliat Med. 2009;23(2):111–119. doi: 10.1177/0269216308101099. [DOI] [PubMed] [Google Scholar]
- 46.De Biase V, Tobaldini O, Boaretti C, et al. Prolonged conservative treatment for frail elderly patients with end-stage renal disease: the Verona experience. Nephrol Dial Transplant. 2008;23(4):1313–1317. doi: 10.1093/ndt/gfm772. [DOI] [PubMed] [Google Scholar]
- 47.Roy A.Trustees: Medicare Will Go Broke in 2016, If You Exclude Obamacare’s Double-Counting. Forbes [serial on the Internet] April232012Available from: http://www.forbes.com/sites/aroy/2012/04/23/trustees-medicare-will-go-broke-in-2016-if-you-exclude-obamacares-double-counting/Accessed May 8, 2013
- 48.Riley GF, Lubitz JD. Long-term trends in Medicare payments in the last year of life. Health Serv Res. 2010;45(2):565–576. doi: 10.1111/j.1475-6773.2010.01082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zilberberg MD, Shorr AF. Economics at the end of life: hospital and ICU perspectives. Semin Respir Crit Care Med. 2012;33(4):362–369. doi: 10.1055/s-0032-1322399. [DOI] [PubMed] [Google Scholar]
- 50.Institute of Medicine, Committee on Care at the End of Life . Approaching Death: Improving Care at the End of Life. Washington, DC: The National Academies Press; 1997. [PubMed] [Google Scholar]
- 51.Himmelstein DU, Thorne D, Warren E, Woolhandler S. Medical bankruptcy in the United States, 2007: results of a national study. Am J Med. 2009;122(8):741–746. doi: 10.1016/j.amjmed.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 52.Wendler D, Rid A. Systematic review: the effect on surrogates of making treatment decisions for others. Ann Intern Med. 2011;154(5):336–346. doi: 10.7326/0003-4819-154-5-201103010-00008. [DOI] [PubMed] [Google Scholar]
- 53.Knauf F, Aronson PS. ESRD as a window into America’s cost crisis in health care. J Am Soc Nephrol. 2009;20(10):2093–2097. doi: 10.1681/ASN.2009070715. [DOI] [PubMed] [Google Scholar]
- 54.Lee CP, Chertow GM, Zenios SA. An empiric estimate of the value of life: updating the renal dialysis cost-effectiveness standard. Value Health. 2009;12(1):80–87. doi: 10.1111/j.1524-4733.2008.00401.x. [DOI] [PubMed] [Google Scholar]
- 55.Lee CP, Chertow GM, Zenios SA. A simulation model to estimate the cost and effectiveness of alternative dialysis initiation strategies. Med Decis Making. 2006;26(5):535–549. doi: 10.1177/0272989X06290488. [DOI] [PubMed] [Google Scholar]
- 56.Teerawattananon Y, Mugford M, Tangcharoensathien V. Economic evaluation of palliative management versus peritoneal dialysis and hemodialysis for end-stage renal disease: evidence for coverage decisions in Thailand. Value Health. 2007;10(1):61–72. doi: 10.1111/j.1524-4733.2006.00145.x. [DOI] [PubMed] [Google Scholar]
- 57.Mitka M. Developed countries lag in use of cheaper and easier peritoneal dialysis. JAMA. 2012;307(22):2360–2361. doi: 10.1001/jama.2012.5761. [DOI] [PubMed] [Google Scholar]
- 58.Perry E, Swartz J, Brown S, Smith D, Kelly G, Swartz R. Peer mentoring: a culturally sensitive approach to end-of-life planning for long-term dialysis patients. Am J Kidney Dis. 2005;46(1):111–119. doi: 10.1053/j.ajkd.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 59.Couchoud C. Dialysis: Can we predict death in patients on dialysis? Nat Rev Nephrol. 2010;6(7):388–389. doi: 10.1038/nrneph.2010.42. [DOI] [PubMed] [Google Scholar]
- 60.Hays RD, Kallich JD, Mapes DL, Coons SJ, Carter WB. Development of the kidney disease quality of life (KDQOL) instrument. Qual Life Res. 1994;3(5):329–338. doi: 10.1007/BF00451725. [DOI] [PubMed] [Google Scholar]
- 61.Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 62.Vierra M. Death Panels. Ann Intern Med. 2012;156(5):394–395. doi: 10.7326/0003-4819-156-5-201203060-00016. [DOI] [PubMed] [Google Scholar]
- 63.Kettl P. A piece of my mind. One vote for death panels. JAMA. 2010;303(13):1234–1235. doi: 10.1001/jama.2010.376. [DOI] [PubMed] [Google Scholar]
- 64.Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363(7):609–619. doi: 10.1056/NEJMoa1000552. [DOI] [PubMed] [Google Scholar]
- 65.Winkelmayer WC, Liu J, Chertow GM, Tamura MK. Predialysis nephrology care of older patients approaching end-stage renal disease. Arch Intern Med. 2011;171(15):1371–1378. doi: 10.1001/archinternmed.2011.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164(6):659–663. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
| # | Searches | Results |
|---|---|---|
| 1 | exp Renal Dialysis/ | 180343 |
| 2 | exp hemodialysis/ | 137673 |
| 3 | (((renal or kidney* or blood) adj5 (dialyses or dialysis)) or hemodialysis or haemodialysis or hemodialyses or haemodialyses or “extracorporeal dialysis” or “extracorporeal dialyses” or “extracorporeal blood cleansing” or hemodialyse or hemorenodialysis or hemorenodialyses or hemotrialysate).mp. [mp = ti, ab, sh, hw, tn, ot, dm, mf, dv, kw, nm, ps, rs, ui, tx, ct] | 176110 |
| 4 | (((renal or kidney* or blood or Peritoneal) adj5 (dialyses or dialysis)) or hemodialysis or haemodialysis or hemodialyses or haemodialyses or “extracorporeal dialysis” or “extracorporeal dialyses” or “extracorporeal blood cleansing” or hemodialyse or hemorenodialysis or hemorenodialyses or hemotrialysate or Hemodiafiltration).mp. [mp = ti, ab, sh, hw, tn, ot, dm, mf, dv, kw, nm, ps, rs, ui, tx, ct] | 206776 |
| 5 | or/1–4 | 220497 |
| 6 | limit 5 to yr = “2002 – Current” | 112269 |
| 7 | limit 6 to (“all aged (65 and over)” or “aged (80 and over)”) [Limit not valid in Embase,CDSR; records were retained] | 85643 |
| 8 | limit 7 to aged “65+ years” [Limit not valid in Ovid MEDLINE(R),Ovid MEDLINE(R) In-Process,CDSR; records were retained] | 31235 |
| 9 | (elderly or octagenarian* or nonagenarian* or “very old” or “75 year*” or “80 year*” or “90 year*” or “100 year*” or ((“75” or “80” or “90” or “100”) adj (age or aged))).mp. [mp = ti, ab, sh, hw, tn, ot, dm, mf, dv, kw, nm, ps, rs, ui, tx, ct] | 445517 |
| 10 | 6 and 9 | 2886 |
| 11 | 8 or 10 | 32086 |
| 12 | systematic review/ | 52193 |
| 13 | (systematic* adj3 review*).mp. [mp = ti, ab, sh, hw, tn, ot, dm, mf, dv, kw, nm, ps, rs, ui, tx, ct] | 127590 |
| 14 | 11 and (12 or 13) | 231 |
| 15 | from 14 keep 1–69 | 69 |
| 16 | from 10 keep 2833–2885 | 53 |
| 17 | 15 or 16 | 122 |
| 18 | remove duplicates from 17 | 107 |
| 19 | exp treatment outcome/or outcome*.mp. or economic*.mp. or exp Economics/or exp “Costs and Cost Analysis” or cost.mp. or costs.mp. or benefit*.mp. or harm*.mp. or preference*.mp. or exp Patient Preference/or “quality of life”.mp. or exp “Quality of Life”/or survival.mp. or exp Survival/or exp survival rate/or “functional status”.mp. or morbidity.mp. or mortality.mp. or exp Morbidity/or exp Mortality/or satisfaction.mp. or exp Patient Satisfaction/[mp = ti, ab, sh, hw, tn, ot, dm, mf, dv, kw, nm, ps, rs, ui, tx, ct] | 6917810 |
| 20 | 18 and 19 | 92 |
| 21 | limit 20 to (editorial or erratum or letter or note or addresses or autobiography or bibliography or biography or dictionary or directory or interactive tutorial or lectures or legislation or news or newspaper article or patient education handout or periodical index or portraits or published erratum or video-audio media or webcasts) [Limit not valid in Embase,Ovid MEDLINE(R),Ovid MEDLINE(R) In-Process,CDSR; records were retained] | 54 |
| 22 | from 21 keep 1–2 | 2 |
| 23 | 20 not 22 | 90 |


