Abstract
Despite successful treatment of the primary tumor, uveal melanoma has a propensity to metastasize to the liver. Prognosis is poor due to the very aggressive nature of these tumors. Because systemic therapies are relatively ineffective and patient survival correlates to disease control in the liver, locoregional therapies provide a means of prolonging survival. We review various techniques including chemoembolization, immunoembolization, radioembolization, arterial fotemustine infusion, and hepatic perfusion for the treatment of liver metastases from uveal melanoma.
Keywords: uveal melanoma, liver metastases, chemoembolization, immunoembolization, radioembolization
Objectives: Upon completion of this article, the reader will be able to identify the role of transarterial therapies, including the future directions of such therapies, in the treatment of uveal melanoma metastatic to the liver.
Accreditation: This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Uveal melanoma is the most common primary intraocular malignant tumor in adults. The incidence in the United States is 5.1 per million, or ∼3% of all melanomas. More than 95% of patients have detectable disease limited to the eye at the time of diagnosis. The median age at diagnosis is 62 years, and almost all patients are white, often with northern European features such as fair skin and light-colored eyes. This tumor is rarely hereditary, and the incidence is not increasing. No convincing link to ultraviolet exposure has been demonstrated, but there is a weak association with cutaneous melanoma.1,2,3
The uveal tract is composed of the iris, ciliary body, and choroid. Ninety percent of these tumors are located in the choroid, the thin vascular layer between the sclera and the retina. Diagnosis is generally made by ophthalmologic examination, with further evaluation by ocular ultrasound or fluorescein angiography. These tumors are often detected incidentally, but symptoms may include blurred vision, floaters, flashes, visual field defects, and shadows or misting. Pain is rare. The most common treatment is radiation in the form of plaque brachytherapy or external charged particle beam therapy. Enucleation is performed for large tumors, tumors with extraocular extension, or for patients with symptoms including significant pain.3
There is substantial interest in identifying patients at risk for metastases. Increased thickness and diameter of the primary tumor have been closely linked to the propensity for metastasis.4,5 Chromosomal abnormalities such as monosomy 3, in addition to amplification of the 8q chromosome, are associated with an increased risk for metastasis.6 Seventy percent of patients with monosomy 3 die within 4 years of initial diagnosis of the primary tumor due to metastases.7 Gene expression profiling using a 15-gene assay based on a sample of the primary eye tumor stratifies the half of patients in class 1 who have a 1% risk of developing metastases compared with those in class 2 who have an 80 to 90% 5-year mortality.8,9
The approximate 5-year survival from diagnosis of the primary tumor is 70%; the approximate 10-year survival is 60%. Up to half of all patients develop systemic metastases. These hematogenous micrometastases are considered to occur prior to diagnosis of the eye tumor. The liver is the predominant organ of involvement in >90% of patients with metastases. It tends to be the first manifestation of systemic disease, and in half of patients it remains the only site of metastases. Other sites of metastases include lungs, bones, brain, subcutaneous tissues, peritoneal cavity, and other visceral organs. Nonetheless, the clinical course of most patients with metastatic disease is generally determined by control of the tumors in the liver. This disease tends to be very aggressive, with patients typically progressing to liver failure; historically, survival following development of liver metastases ranges from 2 to 9 months.2,10
Unlike cutaneous melanoma, there is no effective systemic chemotherapy regimen for metastatic uveal melanoma.2,10 There is no proven adjuvant therapy for patients at high risk for developing metastases. Surgery and ablation techniques are rarely useful due to the multiplicity of tumors. In two studies, patients who underwent surgical resection for liver metastases <3.75 years11 or <5 years12 after diagnosis of the primary tumor experienced early disease recurrence. Because survival of most patients with metastatic uveal melanoma is based on the status of the disease in the liver, locoregional therapy is important for control of these metastases.
Chemoembolization
Chemoembolization combines the hepatic artery infusion of cytotoxic drugs with the blockage of the tumor blood supply. This treatment has shown effective results, increasing the overall survival of patients with metastatic liver disease (Table 1). In a study by Bedikian et al, systemic therapies, intra-arterial chemotherapy infusion, and chemoembolization were compared in 201 patients with liver metastases from uveal melanoma.13 For patients treated with chemoembolization, a 36% response rate was achieved, whereas systemic therapies yielded a response rate <1%. Although the difference in overall survival for the two patient populations was not statistically significant (median: 6.0 versus 5.0 months; p = 0.2), chemoembolization responders had a significantly longer overall survival than patients who did not respond to chemoembolization (median: 14.5 versus 5.0 months; p = 0.003) or patients who received intravenous systemic chemotherapy (median: 14.5 versus 5.0 months; p = 0.003). The authors concluded that compared with other therapies, chemoembolization demonstrated effective results and should be the treatment modality of choice for patients with liver metastases due to uveal melanoma.13
Table 1. Reported Studies Evaluating Chemoembolization of Uveal Melanoma Hepatic Metastases using Various Chemotherapeutic Agents.
| Study | No. of patients | Drug(s) | OS responders (mo) | OS nonresponders (mo) | Median OS (mo) |
|---|---|---|---|---|---|
| Mavligit et al15 | 30 | Cisplatin | 14 | 6 | 11 |
| Cantore et al22 | 8 | Carboplatin | – | – | 15 |
| Bedikian et al13 | 44 | Cisplatin | 14.5 | 5 | 6 |
| Sato et al16 | 14 | Cisplatin | – | – | 6.6 |
| Patel et al17 | 24 | BCNU (100 mg) | 21.9 | 3.3 | 5.2 |
| Huppert et al23 | 14 | Cisplatin/Carboplatin | 14.5 | 10 | 11.5 |
| Vogl et al19 | 12 | Mitomycin C | 21 | 16.5 | 21 |
| Dayani et al21 | 21 | Mitomycin C, cisplatin, doxorubicin | 12.7 | 3.7 | 7.6 (mean) |
| Gupta et al24 | 125 | Mostly cisplatin | 15.8 | 6.1 | 6.7 |
Abbreviation: OS, overall survival.
Median overall survival along with the comparison of survival between responders and nonresponders.
For >20 years, chemoembolization has been used for the treatment of liver metastases from uveal melanoma. However, there are no standard protocols and no comparative clinical trials demonstrating superior results of one particular chemotherapeutic drug over another. In fact, several different chemotherapeutic agents have been used for chemoembolization along with either transient or permanent embolic material (Table 1). In 1986, Carrasco et al reported the first results of chemoembolization using cisplatin and polyvinyl alcohol particles to control the growth of liver metastases from uveal melanoma.14 Significant regression of liver metastases, lasting 6 and 19 months, was demonstrated in two patients following chemoembolization. In 1988, Mavligit et al published their results using the same technique in a larger series (n = 30) of patients.15 The overall response rate was 46%; one patient (3%) had a complete response, and 13 patients (43%) demonstrated partial response following chemoembolization. The median overall survival was 11 months, with a 14-month (range: 9.0- to 54-month) median overall survival for responders compared with a 6-month (range: 2.0- to 19-month) median overall survival for patients who did not respond to treatment. Although these results were encouraging, the authors' institution failed to achieve similar results for chemoembolization using the same chemotherapeutic agent. The median overall survival for the authors' patient population (n = 14) was 6.6 months with a 0% response rate.16
In a separate study, the results of a phase 2 trial using 100 mg 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) with Gelfoam for 30 patients with liver metastases from uveal melanoma were published.17 BCNU was selected based on the high rate of hepatic extraction, solubility in Ethiodol, and efficacy in treating melanoma. The median overall survival in this study was 5.2 months (range: 0.1 to 27.6 months). The authors attributed this short overall survival to the inclusion of patients who did not complete at least one treatment to the affected hepatic lobes due to rapid progression of disease. If these patients were excluded, the median overall survival increased to 7.4 months (range: 1.6 to 27.6 months). Similar to the study by Mavligit et al, overall survival differed significantly based on response to treatment. Patients who achieved a complete or partial response had an overall survival of 21.9 months (range: 7.4 to 27.6 months), those with stable disease experienced an overall survival of 8.7 months (range: 2.9 to 14.4 months), and patients who developed progressive disease had an overall survival of only 3.3 months (range: 1.6 to 5.6 months). Survival benefit and response to chemoembolization were also related to the volume of hepatic metastases at the time of initial treatment. For patients with <20% (n = 6) tumor burden within the liver, the response rate (complete response plus partial response) was 33.3% with a median overall survival of 19 months (range: 3.8 to 27.6 months). For patients with a tumor burden >20% (n = 18), the response rate was 16.7%. For patients with a 20 to 50% tumor burden, the overall survival was 5.6 months (range: 0.1 to 14 months), and for those with >50% tumor burden (n = 11), the median overall survival was only 2.1 months (range: 0.6 to 7.5 months). Therefore, patients with limited tumor burden (<20%) had a significantly longer overall survival than those with larger tumor burdens at the time of initial presentation. Patients with limited tumor burden also had a better response to treatment than those with more disease burden. Of note, two patients in this study with >50% tumor burden had significant regression of hepatic metastases following chemoembolization. Patients with large tumor burdens who benefited from chemoembolization were those who presented with good performance status, normal bilirubin, and a patent hepatic vasculature. The authors therefore recommended that chemoembolization with BCNU be considered as a palliative treatment for bulky disease (>50% tumor burden) in patients with the characteristics just listed. Based on these results, the authors for several years have been treating patients with >50% tumor burden with chemoembolization using a higher dose (200 mg) of BCNU. The authors have noted encouraging results in this particular patient population with 22% surviving >1 year following bilobar treatment of all hepatic metastases (unpublished data).
Angiographic findings may also help predict overall survival and treatment response. Ethiodized oil is often combined with chemotherapeutic agents prior to chemoembolization (Figs. 1A and 1B) In a study by Monsky et al, the volume of ethiodized oil correlated with subsequent tumor necrosis, reduction in whole tumor volume, and patient survival.18 In 2007, Vogl et al evaluated the pattern of Lipiodol (Guerbet, Villepinte, France) uptake and response to treatment in 12 patients with liver metastases from uveal melanoma following chemoembolization with 10 mg/m2 mitomycin-C and resorbable microspheres.19 Patients who achieved a partial response (n = 3) or stable disease (n = 5) had more hypervascular tumors with significant Lipiodol uptake, whereas patients with relatively hypovascular tumors and minimal Lipiodol uptake were more likely to demonstrate progressive disease (n = 4). Furthermore, median overall survival for patients who demonstrated a partial response to treatment had a significantly longer overall survival than those who failed to respond to chemoembolization (median: 21 versus 16.5 months), respectively (p < 0.01).
Figure 1.
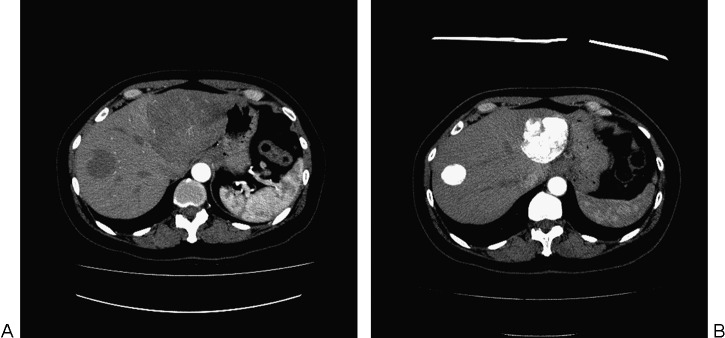
(A) Axial contrast-enhanced computed tomography (CT) of the liver prior to chemoembolization demonstrates two uveal melanoma hepatic metastases. The largest tumor replaces the lateral segment of the left lobe. A smaller tumor is present in the right lobe of the liver. (B) Axial contrast-enhanced CT of the liver 1 year following four chemoembolization treatments with BCNU/Lipiodol. Dense Lipiodol uptake is present within the tumors with a significant decrease in the size of the left lateral segment tumor.
In 2008, Sharma et al performed chemoembolization using 50 mg cisplatin, 50 mg doxorubicin, and 10 mg mitomycin C emulsified with ethiodized oil followed by either Gelfoam or polyvinyl alcohol particles to slow arterial flow.20 Of the 20 evaluable patients, 13 patients (65%) achieved stable disease and 7 patients (35%) developed progressive disease. The median overall survival was 271 days. Patients with tumors that had a nodular angiographic appearance had a longer overall survival than patients with an infiltrative pattern of disease (median: 750 versus 109 days; p = 0.0002). The authors hypothesized that the angiographic findings and subsequent survival benefit might correspond to the genetic profile of the metastatic tumor and that the infiltrative pattern would more likely be associated with aggressive tumor genetics.
In 2009, the same group further evaluated patients for angiographic patterns predictive of survival following chemoembolization.21 They divided patients into the same two angiographic patterns as their initial study: a nodular pattern with discrete well-defined tumors (n = 11) and a diffuse infiltrative (n = 9) pattern of metastases. Results showed a significantly longer overall survival time for patients with the nodular pattern (mean: 12.7 months) than those with the infiltrative pattern of disease (mean: 3.7 months). Furthermore, the 1-year survival rate for patients with the nodular pattern was 58%, whereas 0% of the patients with infiltrative disease survived 1 year. The authors once again concluded that the angiographic pattern of liver metastases was strongly predictive of overall survival following chemoembolization. Unlike their initial study, biopsies were performed in a subgroup of patients to determine histopathologic and genetic features of the liver metastases. The biopsy results were subsequently compared with the angiographic pattern of metastatic disease. Epithelioid cytology, indicative of a poor prognosis, was found in five patients with the infiltrative pattern and in only one patient with nodular metastases. Evaluation of genetic features (n = 9) also demonstrated a more aggressive tumor cell behavior in those with infiltrative tumor. The deletion of a metastatic suppressor gene on chromosome 8p was found in four patients with the infiltrative disease and in no patients with the nodular pattern of metastases. Although these findings did not reach statistical significance, the infiltrative angiographic pattern showed a trend toward more aggressive histopathologic and genetic features than the nodular pattern of metastatic disease, which correlated with survival benefit.
Several other reports in the literature have shown effective results for chemoembolization in the treatment of liver metastases from uveal melanoma. Table 1 summarizes these studies.13,15,16,17,19,21,22,23,24 Of note, when evaluated, the overall survival for chemoembolization responders was consistently longer than for those who failed chemoembolization.
Drug-Eluting Beads
Over the past several years, drug-eluting beads (DC/LC Beads, Biocompatibles, Surrey, UK) have been used to treat both primary and secondary tumors of the liver. Drug-eluting beads are produced from a polyvinyl alcohol hydrogel that has been modified with sulphonate groups for the controlled loading and delivery of chemotherapy agents. Therefore, embolization of tumors with drug-eluting beads allows for local delivery and sustained release of cytotoxic drugs directly into the tumor while concurrently rendering tumors ischemic.
Drug-eluting beads loaded with irinotecan or doxorubicin have both been used to treat liver metastases from uveal melanoma (Figs. 2A and 2B). In 2009, Fiorentini et al reported their phase 2 trial using irinotecan (100 mg) drug-eluting beads to treat 10 patients.25 Following chemoembolization, all patients achieved a partial response to treatment using modified Response Evaluation Criteria in Solid Tumors (RECIST). Three patients (30%) had a major response with a reduction in tumor size of 90%, three patients (30%) had a tumor reduction of 80%, and four patients (40%) had a tumor reduction between 60% and 70% following transarterial chemoembolization. Patients with limited tumor burden (≤25% liver volume) at the time of initial chemoembolization had the greatest response to treatment (90%); patients with larger tumor burden (≤75% liver volume) demonstrated less of a treatment response. The median overall survival time was 6.5 months (range: 4 to 9 months). Eight patients were alive at the time of their analysis. Two patients with a 60% and 75% tumor burden died at 6 and 4 months, respectively, due to tumor progression after chemoembolization. Treatment-related toxicity was short lived and included grade 2 abdominal pain following 12 chemoembolization procedures. Grade 3 toxicity occurred after three treatments and included one transient paralytic ileus and two cases of nonicteric hepatitis. More recently, Venturini et al found an 80% response rate following treatment of five patients with irinotecan (100 mg) drug-eluting beads (100 to 300 µm).26 One patient achieved a complete response, two patients had a partial response, one patient achieved stable disease, and another patient had progressive disease. Follow-up ranged from 8 to 13 months (mean: 10.6 months), and all patients were alive at last follow-up. Although both of these studies show encouraging results, our initial experience using irinotecan drug-eluting beads has not been as promising.
Figure 2.
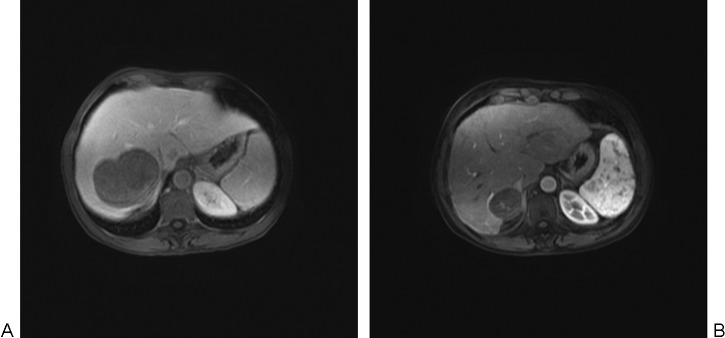
(A) Contrast-enhanced axial magnetic resonance (MR) image demonstrates one of the patient's multiple large metastases from uveal melanoma prior to treatment. (B) Contrast-enhanced MR image demonstrates significant shrinkage of this tumor 14 months after initial treatment with drug-eluting beads loaded with doxorubicin, followed by three treatments with BCNU chemoembolization.
A phase 2 multicenter clinical trial (n = 20 patients) for metastatic melanoma to the liver using doxorubicin drug-eluting beads was recently completed in the United States. Data are currently being analyzed at the time of this writing. At the authors' institution, doxorubicin drug-eluting beads are currently part of the armamentarium for treating patients with metastatic uveal melanoma, both as salvage therapy and in patients presenting with >50% tumor replacement.
Immunoembolization
Immunoembolization consists of infusion of an immunologic stimulant into the hepatic artery followed by embolization. The liver contains >70% of all tissue macrophages (Kupffer cells), many other antigen-presenting cells, and innate immune cell populations. Despite this abundance of immune cells, the liver tends to induce tolerance rather than immunity. This avoids unnecessary activation of the immune system due to continuous exposure to food-derived antigens and probiotics from the gastrointestinal tract, and it prevents damage to hepatocytes. The rationale for immunoembolization starts with destruction of tumor by embolization to control tumor progression locally and to provide tumor antigens to the local immune system. Concurrent use of biological response modifiers would induce an inflammatory response in the tumor and surrounding tissue that may improve the antigen presentation to the local immune system. Local stimulation of the immune system may result in the development of a systemic immune response against tumor cells that may suppress the growth of the untreated tumors; immunoembolization could thereby potentially create an in situ tumor vaccine.27
Although there are a few prior descriptions of hepatic arterial infusion of cytokines,28 immunoembolization was first described in the treatment of patients with hepatocellular carcinoma.29,30 This approach was pursued at the authors' institution for patients with metastatic uveal melanoma in attempts to control progression of extrahepatic metastases that commonly developed after liver metastases were controlled by chemoembolization.17
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a glycoprotein secreted by immune cells such as activated T cells that increases myeloid cell production, stimulates macrophages, increases cytotoxicity of monocytes toward tumor cell lines, and promotes maturation of dendritic cells. This was selected as the cytokine used for the immunoembolization of metastatic uveal melanoma, based on an elegant study that examined the vaccination properties of murine tumor cells transduced with 10 retroviruses encoding various potential immunomodulators.31 Irradiated B16 melanoma cells alone generated no antitumor immunity; however, new tumors were prevented and established tumors regressed in mice injected with irradiated B16 melanoma cells containing the transduced GM-CSF gene. The immunity was long lived and specific; antitumor activity was present several months after injection of B16 melanoma cells producing GM-CSF, and this did not produce immunity against several other murine cancers. The antitumor activity required both CD4+ (helper) and CD8+ (killer) T cells. The absence of either T-cell fraction abrogated the systemic immunity. This antitumor activity was attributed to the influence of GM-CSF on the maturation and/or function of dendritic cells.
It was therefore hypothesized that introduction of GM-CSF into tumors via intra-arterial Ethiodol emulsion would produce an environment allowing an immune response and antitumor activity similar to tumor cells with the transduced GM-CSF gene.27 GM-CSF retains its activity after separation from an emulsion with Ethiodol. Immunoembolization was shown to be safe in a study using a normal porcine model.32
A phase 1 trial was initiated to investigate the feasibility and safety of immunoembolization in the treatment of liver tumors.33 A total of 34 of the 39 patients in this study had metastatic uveal melanoma. All patients were deemed unresectable, with <50% tumor involvement. Patients underwent lobar hepatic artery embolization every 4 weeks using an escalating dose of GM-CSF (25 to 2000 µg) emulsified with Ethiodol, followed by Gelfoam slurry. Computed tomography (CT) of chest/abdomen/pelvis and magnetic resonance imaging of the liver were performed after every two treatments to assess results using RECIST, along with clinical assessment. Primary end points of the study were dose-limiting toxicity and the maximum tolerated dose.
Of the 34 patients with metastatic uveal melanoma, the median age was 56 years, and 35% were male. A total of 97% of patients had a very good clinical performance (Eastern Cooperative Oncology Group Performance Status: 0). A total of 29% had <20% tumor involvement, 29% had limited extrahepatic metastases, and 29% had an elevated lactase dehydrogenase (LDH). There was a median of six procedures per patient (range: 1 to 14). Two patients had a complete response, and eight patients had a partial response, for a 32% response rate (Figs. 3A and 3B, 4A and 4B). Another 32% had stable disease. The median overall survival was 14.4 months, with a survival rate at 1 and 2 years of 62% and 26%, respectively. The survival was 33.7 months for those experiencing a complete or partial response compared with 12.4 months for those with stable or progressive disease. The median progression-free survival in the liver was 4.8 months, compared with a 10.4-month systemic progression-free survival. Twenty-one percent of patients developed progression of extrahepatic metastases prior to growth of liver metastases, whereas 32% developed progression of liver metastases before extrahepatic progression. Eighteen percent developed simultaneous progression of liver and extrahepatic metastases, but 29% died of liver metastases without extrahepatic progression.
Figure 3.
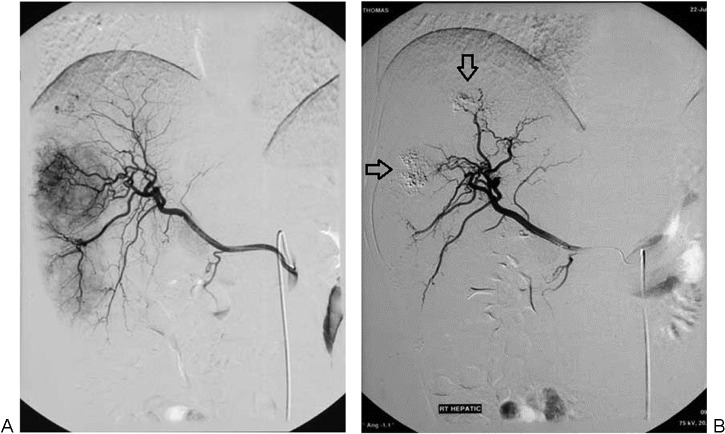
(A) Arteriogram prior to immunoembolization demonstrates several large hypervascular metastases from uveal melanoma. (B) Arteriogram 2 months after the initial immunoembolization procedure shows that these tumors are nearly avascular, with residual Ethiodol (arrows). This effect is largely due to the embolization component.
Figure 4.
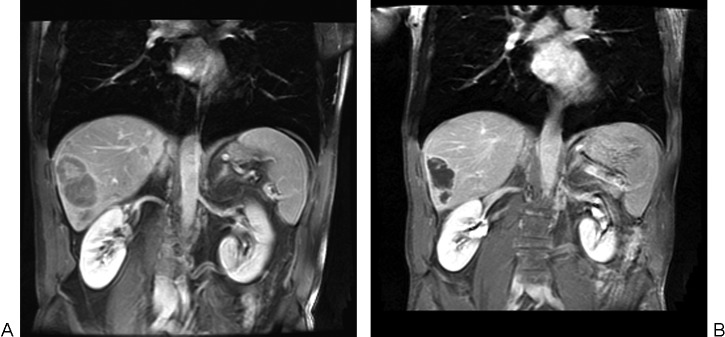
(A) Coronal contrast-enhanced magnetic resonance (MR) image demonstrates several hepatic metastases from uveal melanoma prior to treatment. (B) Coronal contrast-enhanced MR image shows tumor shrinkage and necrosis following one immunoembolization treatment to each lobe.
The authors compared patients undergoing high-dose immunoembolization (≥1500 µg) to those who received low-dose immunoembolization (≤1000 µg).34 In patients receiving high-dose immunoembolization, there was a longer systemic progression-free survival of 12.4 months compared with 5.6 months in patients receiving low-dose immunoembolization (p < 0.05), suggesting induction of a systemic immune response against the melanoma cells. Although there was a trend toward longer progression-free survival in the liver and overall survival, these results did not reach statistical significance. When patients undergoing high-dose immunoembolization were compared with similar patients (<50% tumor involvement) treated at the authors' institution with BCNU chemoembolization, the high-dose immunoembolization group demonstrated a longer median overall survival (20.4 versus 9.8 months; p = 0.005) and a longer median systemic progression-free survival (12.4 versus 4.8 months; p = 0.001), although there was no significant improvement in progression-free survival in the liver. There was no significant difference in overall or progression-free survival between patients who received BCNU chemoembolization and low-dose immunoembolization. It was proposed that stabilization of hepatic metastases was most likely achieved by the ischemic effects of embolization rather than by the administered medication. Tumor regression was sustained after discontinuation of immunoembolization in several patients, and a response took as long as 4 months to achieve. Repeated immune stimulation has been shown to be necessary to overcome tolerance and induce a vigorous response.35,36 Of note, six patients underwent resection of a remote extrahepatic metastasis, as deemed appropriate for their medical management. On pathologic examination, two showed signs of immune response, with CD4+ and CD8+ T cell and dendritic cell infiltration; one showed monocyte infiltration with tumor necrosis.
In 10 patients, the maximum tolerated dose was not demonstrated following administration of 2000 µg of GM-CSF. There were no treatment-related deaths or life-threatening adverse events. These procedures were tolerated quite well; most of the patients developed only mild postembolization symptoms for 1 or 2 days. Of 10 patients undergoing 55 procedures with the administration of 2000 µg of GM-CSF, there was only one grade 3 toxicity (asymptomatic elevation of liver function tests) and one grade 4 toxicity (respiratory suppression due to narcotic use that did not require intubation).
The favorable results of this study led to a randomized double-blind phase 2 clinical study of immunoembolization in patients with metastatic uveal melanoma.37 Fifty-two patients were randomized to undergo embolization with Ethiodol and Gelfoam (Pfizer, New York, NY) slurry, with or without 2000 µg GM-CSF. The primary end point was regression of liver metastases, and the secondary end points were time to progression and overall survival. Development of a local and systemic immune response was also assessed. Patients were stratified based on amount of liver involvement by tumor (<20% versus 20 to 50%) and HLA-A2 status. Patients with prior local liver therapies or extrahepatic metastases were excluded. Patients were otherwise treated in similar fashion to the phase 1 study, with lobar embolization every 4 weeks followed by imaging and clinical assessment every 8 weeks.
Both immunoembolization and bland embolization were well tolerated with acceptable toxicity profiles. Both approaches induced cytokine production, but it was more prominent in patients treated with immunoembolization. Survival in patients with 20 to 50% tumor involvement following immunoembolization was 18.2 months versus 16.0 months for those undergoing bland embolization (p = 0.047). There was a trend toward a longer systemic progression-free survival following immunoembolization. Unexpectedly, progression-free survival in the liver was shorter following immunoembolization in patients with <20% tumor involvement, suggesting a potential suppressive response to high-dose GM-CSF. There was a paradoxical negative correlation between posttreatment serum GM-CSF levels and systemic progression-free survival when the posttreatment serum GM-CSF level exceeded a certain threshold. A paradoxical suppressive effect from GM-CSF was previously described.38 Further analysis of data from this study is ongoing.
Radioactive Microspheres
Based on the excellent response rate of plaque radiotherapy for primary uveal melanoma, radioembolization using ytrrium-90 (90Y) has been used to treat patients with liver metastases from uveal melanoma.39,40,41 There are two types of 90Y microspheres commercially available: TheraSpheres and SIR-Spheres. TheraSpheres (MDS Nordion, Ottawa, Canada) are nonbiodegradable glass microspheres with a diameter of 20 to 30 µm. Each glass microsphere has a maximum activity of 2500 Bq at the time of calibration.42 A 3-GBq dose vial of TheraSpheres has 1.2 million glass microspheres. In contrast, SIR-Spheres (Sirtex, Sydney, Australia) are nonbiodegradable resin 90Y microspheres with a diameter of 20 to 40 µm. The activity per resin microsphere is ∼40 to 70 Bq; therefore a typical administration of SIR-Spheres involves 20 to 40 million microspheres, a much larger number of particles compared with a similar dose of TheraSpheres.43 Because of the larger number of microspheres delivered, SIR-Spheres also provide an embolic effect in addition to delivering radiation directly to the tumors. Therefore, SIR-Spheres are preferred in our institution for radioembolization of metastatic uveal melanoma because in most patients the tumors are hypervascular, numerous, and dispersed throughout both lobes of the liver.
In 2009, Kennedy et al reported their results of a retrospective study that included 11 patients from five centers around the world who underwent treatment for metastatic uveal melanoma using SIR-Spheres.39 The median activity delivered per treatment was 1.55 GBq. Toxicity was minimal with one grade 3 gastrointestinal toxicity of a gastric ulcer that healed within 6 weeks with supportive care. At 3-month follow-up, CT and positron emission tomography (PET) showed a 100% response rate, with one patient (9.1%) achieving a complete response. The 1-year survival for the 10 patients with available follow-up was 80%.
In 2011, the authors reported 32 patients treated with SIR-Spheres as salvage therapy after failure of initial therapies such as immunoembolization and chemoembolization.40 A dose reduction of 25% was applied to each patient due to multiple prior embolization procedures. The median dose delivered per patient was 1.08 GBq (29.28 mCi) (range: 0.63 to 1.86 GBq [16.77 to 50.10 mCi]). Patients were divided into three groups based on pretreatment tumor burden within the liver: <25% (n = 25), 25 to 50% (n = 5), and >50% (n = 2). Clinical follow-up lasted 1.0 to 29.0 months (median: 10 months). At the conclusion of the follow-up period, 10 patients were still alive 4.7 to 27.0 months (median: 9.4 months) following radioembolization. Twenty-two patients died 1.0 to 29.0 months (median: 5.8 months) postprocedure due to progressive disease (n = 13), extrahepatic disease (n = 4), or both (n = 5). Overall survival for the 32 patients ranged from 1.0 to 29.0 months (median: 10 months). Patients with <25% tumor burden had a significantly longer overall survival period than those with ≥25% tumor burden (10.5 versus 3.9 months; p = 0.0003). With respect to treatment response, one patient (3.1%) achieved a complete response, one patient (3.1%) had a partial response, 18 patients (56.3%) had stable disease, and 12 patients (37.5%) developed progressive disease (Figs. 5A and 5B). Patients with stable disease or better had significantly longer overall survival times (14.7 months) compared with patients with progressive disease (4.9 months) (p = 0.006). Median progression-free survival of hepatic metastasis was 4.7 months (range: 1.0 to 26.5 months). Patients with limited pretreatment tumor burden (<25%) had significantly longer progression-free survival of hepatic metastasis than did patients with ≥25% pretreatment tumor burden (6.4 versus 3.0 months; p = 0.03). In addition, patients who had a complete response, partial response, or stable disease had a significantly longer progression-free survival of hepatic metastasis compared with patients with progressive disease following radioembolization (7.9 versus 3.1 months; p < 0.0001). Systemic toxicity after treatment was mainly grade 1 to 2 fatigue (n = 9) and gastrointestinal symptoms such as indigestion (n = 2) and abdominal discomfort (n = 5), all of which were self-limited.
Figure 5.
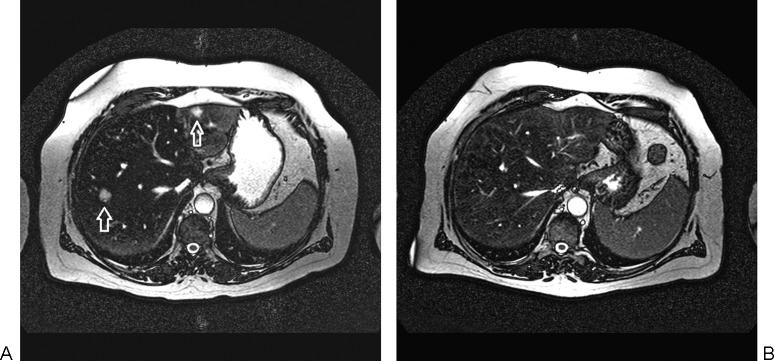
(A) Axial T2-weighted magnetic resonance (MR) image prior to radioembolization of the liver showing a metastasis from uveal melanoma in the left lateral segment and in the right lobe of the liver (arrows). (B) Axial T2-weighted MR image 6 months following radioembolization demonstrating complete tumor response.
In a similar study, Klingenstein et al published their results of 13 patients treated with radioembolization following failure of first-line therapy.41 In this study, eight patients (61.5%) achieved a partial response, two patients (15.4%) achieved stable disease, and three patients (23.1%) had progressive disease. The median overall survival was 7 months.
Fotemustine Infusion Therapy
Fotemustine is an alkylating agent with a short half-life and a high first-pass extraction resulting in hepatic concentration of 8 to 47 times compared with other tissues. This drug has been investigated in the treatment of metastatic uveal melanoma, based on its efficacy in the treatment of metastatic cutaneous melanoma.43,44 In a multi-institutional trial involving 101 patients,44 fotemustine was administered via a surgically implanted hepatic arterial port system as a 4-hour outpatient infusion weekly for 4 weeks followed by a 5-week treatment break. The therapy was repeated every 3 weeks until disease progression, unacceptable toxicity, or patient refusal. The liver was the only site of metastases in 88% of patients. Patients underwent a median of eight infusions. A total of 15% had a complete response, and 21% had a partial response. Forty-eight percent had stable disease for a median duration of 9.4 months. Overall survival was 15 months with 1- and 2-year survival rates of 67% and 29%, respectively. Additional trials are underway in Europe investigating use of this medication for both adjuvant therapy and in the treatment of liver metastases. This agent is not currently available for administration in the United States.
Hepatic Perfusion Therapy
Isolated hepatic perfusion is a surgical technique developed for the treatment of liver metastases in patients without significant extrahepatic disease.45 The vascular supply to the liver is isolated, and branches of the suprarenal inferior vena cava are also ligated. High-dose chemotherapy is infused through an arterial catheter in the liver, and hepatic venous blood draining into the inferior vena cava flows out through a cannula to allow reperfusion of the liver. Melphalan and warmed perfusate circulate in this system for an hour. Veno-veno bypass is simultaneously used to maintain perfusion through the rest of the body. This operation typically takes ∼8 hours, with a 7- to 10-day hospitalization afterward.2 Significant hemodynamic changes often occur during the procedure, and operative mortality is 4%.45 In a series of 29 patients with ocular melanoma treated by isolated hepatic perfusion using melphalan, there was a 62% response rate (10% complete response, 52% partial response), with an overall survival of 12 months and progression-free survival of 8 months. Two thirds of patients recurred initially in the liver. There were no operative mortalities in this small group.46
A phase 3 randomized trial comparing percutaneous hepatic perfusion using melphalan with best alternative care was completed in 2010.47 This technique uses a unique double-balloon inferior vena cava catheter (Delcath Systems, New York, NY) to allow high-dose chemosaturation of the liver using melphalan infused via the hepatic artery while limiting systemic distribution by extracting the hepatic venous blood for filtration before return to the body. This device allows use of the perfusion technique while avoiding the major abdominal operation. A total of 44 patients were randomized to percutaneous hepatic perfusion; 49 patients received best alternative care. Patients receiving best alternative care were allowed to cross over to hepatic perfusion after progression. The planned treatment regimen allowed four to six treatments at 4- to 5-week intervals. The median hepatic progression-free survival was 8 months for patients undergoing hepatic perfusion with an overall response rate of 34%, compared with hepatic progression-free survival of 1.6 months in those patients receiving best alternative care. The median overall survival for patients undergoing percutaneous hepatic perfusion was 9.8 months.48 These data are currently under review by the Food and Drug Administration as the application for device approval proceeds.
Summary
Various locoregional therapies have played a significant role in prolonging the lives of patients with metastatic uveal melanoma. Unfortunately, these are palliative without potential for cure.
In general, for patients with liver metastases with <50% tumor replacement, good performance status, preserved liver function, and deemed inappropriate for resection, the authors start treatment in our institution with either immunoembolization or SIR-Spheres. At our institution, a phase 2 clinical trial is underway to further evaluate the efficacy and safety of SIR-Spheres for both initial and salvage therapy. Our experience has shown that patients with tumors >5 cm diameter do not respond as well to immunoembolization, so we generally treat these patients initially with chemoembolization. Likewise, patients who present with >50% tumor replacement are treated with chemoembolization. We are currently investigating the use of an initial treatment with drug-eluting beads loaded with doxorubicin to each lobe, followed by BCNU chemoembolization. Chemoembolization is also used as salvage therapy following tumor progression after treatment with immunoembolization or SIR-Spheres.
Further research is necessary to help determine the efficacy of these various treatments. Patient accrual is often challenging in such a rare disease. To help better understand the value of these treatments, future studies should stratify patients based on tumor burden at the time of therapy initiation. Also, knowledge of the underlying tumor genetics would be quite helpful in evaluating the outcome following treatment. This may help physicians counsel patients and their families regarding response to locoregional therapies and survival benefit.
There are several clinical trials of potential systemic therapies such as ipilimumab, PD-1 and PDL-1 inhibitors, protein kinase C inhibitors, and MEK inhibitors. Clinical benefit to patients has not been established. Therefore, locoregional therapy will likely play a significant role in the treatment of patients with liver metastases from uveal melanoma for at least the next several years.
Disclosures
David J. Eschelman and Takami Sato have received an honorarium from Biocompatibles. David J. Eschelman is a paid consultant for Guerbet.
References
- 1.Singh A D, Turell M E, Topham A K. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology. 2011;118(9):1881–1885. doi: 10.1016/j.ophtha.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 2.Sato T. Locoregional management of hepatic metastasis from primary uveal melanoma. Semin Oncol. 2010;37(2):127–138. doi: 10.1053/j.seminoncol.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 3.Shields C L, Shields J A. Ocular melanoma: relatively rare but requiring respect. Clin Dermatol. 2009;27(1):122–133. doi: 10.1016/j.clindermatol.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Shields C L, Furuta M, Thangappan A. et al. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch Ophthalmol. 2009;127(8):989–998. doi: 10.1001/archophthalmol.2009.208. [DOI] [PubMed] [Google Scholar]
- 5.Damato B, Coupland S E. A reappraisal of the significance of largest basal diameter of posterior uveal melanoma. Eye (Lond) 2009;23(12):2152–2160; quiz 2161–2162. doi: 10.1038/eye.2009.235. [DOI] [PubMed] [Google Scholar]
- 6.van den Bosch T, van Beek J GM, Vaarwater J. et al. Higher percentage of FISH-determined monosomy 3 and 8q amplification in uveal melanoma cells relate to poor patient prognosis. Invest Ophthalmol Vis Sci. 2012;53(6):2668–2674. doi: 10.1167/iovs.11-8697. [DOI] [PubMed] [Google Scholar]
- 7.Prescher G, Bornfeld N, Hirche H, Horsthemke B, Jöckel K H, Becher R. Prognostic implications of monosomy 3 in uveal melanoma. Lancet. 1996;347(9010):1222–1225. doi: 10.1016/s0140-6736(96)90736-9. [DOI] [PubMed] [Google Scholar]
- 8.Onken M D, Worley L A, Char D H. et al. Collaborative Ocular Oncology Group report number 1: prospective validation of a multi-gene prognostic assay in uveal melanoma. Ophthalmology. 2012;119(8):1596–1603. doi: 10.1016/j.ophtha.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolata G A game changer in revealing a cancer's prognosis New York Times, July 9, 2012 [Google Scholar]
- 10.Spagnolo F, Caltabiano G, Queirolo P. Uveal melanoma. Cancer Treat Rev. 2012;38(5):549–553. doi: 10.1016/j.ctrv.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Hsueh E C, Essner R, Foshag L J, Ye X, Wang H J, Morton D L. Prolonged survival after complete resection of metastases from intraocular melanoma. Cancer. 2004;100(1):122–129. doi: 10.1002/cncr.11872. [DOI] [PubMed] [Google Scholar]
- 12.Aoyama T, Mastrangelo M J, Berd D. et al. Protracted survival after resection of metastatic uveal melanoma. Cancer. 2000;89(7):1561–1568. doi: 10.1002/1097-0142(20001001)89:7<1561::aid-cncr21>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 13.Bedikian A Y, Legha S S, Mavligit G. et al. Treatment of uveal melanoma metastatic to the liver: a review of the M. D. Anderson Cancer Center experience and prognostic factors. Cancer. 1995;76(9):1665–1670. doi: 10.1002/1097-0142(19951101)76:9<1665::aid-cncr2820760925>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 14.Carrasco C H, Wallace S, Charnsangavej C, Papadopoulos N EJ, Patt Y Z, Mavligit G M. Treatment of hepatic metastases in ocular melanoma. Embolization of the hepatic artery with polyvinyl sponge and cisplatin. JAMA. 1986;255(22):3152–3154. [PubMed] [Google Scholar]
- 15.Mavligit G M, Charnsangavej C, Carrasco C H, Patt Y Z, Benjamin R S, Wallace S. Regression of ocular melanoma metastatic to the liver after hepatic arterial chemoembolization with cisplatin and polyvinyl sponge. JAMA. 1988;260(7):974–976. [PubMed] [Google Scholar]
- 16.Sato T, Nathan F E, Berd D, Sullivan K, Mastrangelo M J. Lack of effect from chemoembolization for liver metastasis from uveal melanoma. Proc Am Soc Clin Oncol. 1995;14:415. [Google Scholar]
- 17.Patel K, Sullivan K, Berd D. et al. Chemoembolization of the hepatic artery with BCNU for metastatic uveal melanoma: results of a phase II study. Melanoma Res. 2005;15(4):297–304. doi: 10.1097/00008390-200508000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Monsky W L, Kim I, Loh S. et al. Semiautomated segmentation for volumetric analysis of intratumoral Ethiodol uptake and subsequent tumor necrosis after chemoembolization. AJR Am J Roentgenol. 2010;195(5):1220–1230. doi: 10.2214/AJR.09.3964. [DOI] [PubMed] [Google Scholar]
- 19.Vogl T, Eichler K, Zangos S. et al. Preliminary experience with transarterial chemoembolization (TACE) in liver metastases of uveal malignant melanoma: local tumor control and survival. J Cancer Res Clin Oncol. 2007;133(3):177–184. doi: 10.1007/s00432-006-0155-z. [DOI] [PubMed] [Google Scholar]
- 20.Sharma K V, Gould J E, Harbour J W. et al. Hepatic arterial chemoembolization for management of metastatic melanoma. AJR Am J Roentgenol. 2008;190(1):99–104. doi: 10.2214/AJR.07.2675. [DOI] [PubMed] [Google Scholar]
- 21.Dayani P N, Gould J E, Brown D B, Sharma K V, Linette G P, Harbour J W. Hepatic metastasis from uveal melanoma: angiographic pattern predictive of survival after hepatic arterial chemoembolization. Arch Ophthalmol. 2009;127(5):628–632. doi: 10.1001/archophthalmol.2009.45. [DOI] [PubMed] [Google Scholar]
- 22.Cantore M, Fiorentini G, Aitini E. et al. Intra-arterial hepatic carboplatin-based chemotherapy for ocular melanoma metastatic to the liver. Report of a phase II study. Tumori. 1994;80(1):37–39. doi: 10.1177/030089169408000107. [DOI] [PubMed] [Google Scholar]
- 23.Huppert P E, Fierlbeck G, Pereira P. et al. Transarterial chemoembolization of liver metastases in patients with uveal melanoma. Eur J Radiol. 2010;74(3):e38–e44. doi: 10.1016/j.ejrad.2009.03.064. [DOI] [PubMed] [Google Scholar]
- 24.Gupta S, Bedikian A Y, Ahrar J. et al. Hepatic artery chemoembolization in patients with ocular melanoma metastatic to the liver: response, survival, and prognostic factors. Am J Clin Oncol. 2010;33(5):474–480. doi: 10.1097/COC.0b013e3181b4b065. [DOI] [PubMed] [Google Scholar]
- 25.Fiorentini G, Aliberti C, Del Conte A. et al. Intra-arterial hepatic chemoembolization (TACE) of liver metastases from ocular melanoma with slow-release irinotecan-eluting beads. Early results of a phase II clinical study. In Vivo. 2009;23(1):131–137. [PubMed] [Google Scholar]
- 26.Venturini M, Pilla L, Agostini G. et al. Transarterial chemoembolization with drug-eluting beads preloaded with irinotecan as a first-line approach in uveal melanoma liver metastases: tumor response and predictive value of diffusion-weighted MR imaging in five patients. J Vasc Interv Radiol. 2012;23(7):937–941. doi: 10.1016/j.jvir.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan K L. New York, NY: Cambridge University Press; 2008. Immunoembolization for melanoma; pp. 311–315. [Google Scholar]
- 28.Yamamoto A, Sato T. Locoregional treatment of malignant hepatic tumors with biologic response modifiers. Surg Oncol Clin N Am. 2008;17(4):935–955, xii. doi: 10.1016/j.soc.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Kanai T Monden M Sakon M et al. New development of transarterial immunoembolization (TIE) for therapy of hepatocellular carcinoma with intrahepatic metastases Cancer Chemother Pharmacol 199433(Suppl):S48–S54. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida T, Sakon M, Umeshita K. et al. Appraisal of transarterial immunoembolization for hepatocellular carcinoma: a clinicopathologic study. J Clin Gastroenterol. 2001;32(1):59–65. doi: 10.1097/00004836-200101000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Dranoff G, Jaffee E, Lazenby A. et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993;90(8):3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sullivan K L, Aoyama T, Sato T, McCue P. Safety of immunoembolization of normal swine liver with human GM-CSF (granulocyte macrophage colony stimulating factor) and Ethiodol. J Vasc Interv Radiol. 2001;12:S132–S133. [Google Scholar]
- 33.Sato T, Eschelman D J, Gonsalves C F. et al. Immunoembolization of malignant liver tumors, including uveal melanoma, using granulocyte-macrophage colony-stimulating factor. J Clin Oncol. 2008;26(33):5436–5442. doi: 10.1200/JCO.2008.16.0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamamoto A, Chervoneva I, Sullivan K L. et al. High-dose immunoembolization: survival benefit in patients with hepatic metastases from uveal melanoma. Radiology. 2009;252(1):290–298. doi: 10.1148/radiol.2521081252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Speiser D E, Miranda R, Zakarian A. et al. Self antigens expressed by solid tumors do not efficiently stimulate naive or activated T cells: implications for immunotherapy. J Exp Med. 1997;186(5):645–653. doi: 10.1084/jem.186.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dow S W, Elmslie R E, Willson A P, Roche L, Gorman C, Potter T A. In vivo tumor transfection with superantigen plus cytokine genes induces tumor regression and prolongs survival in dogs with malignant melanoma. J Clin Invest. 1998;101(11):2406–2414. doi: 10.1172/JCI510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eschelman D J, Gonsalves C F, Terai M. et al. The results of a randomized phase II study using embolization with or without granulocyte-macrophage colony-stimulating factor (GM-CSF) in uveal melanoma patients with hepatic metastasis. J Clin Oncol. 2011;29:544s. [Google Scholar]
- 38.Parmiani G, Castelli C, Pilla L, Santinami M, Colombo M P, Rivoltini L. Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Ann Oncol. 2007;18(2):226–232. doi: 10.1093/annonc/mdl158. [DOI] [PubMed] [Google Scholar]
- 39.Kennedy A S, Nutting C, Jakobs T. et al. A first report of radioembolization for hepatic metastases from ocular melanoma. Cancer Invest. 2009;27(6):682–690. doi: 10.1080/07357900802620893. [DOI] [PubMed] [Google Scholar]
- 40.Gonsalves C F, Eschelman D J, Sullivan K L, Anne P R, Doyle L, Sato T. Radioembolization as salvage therapy for hepatic metastasis of uveal melanoma: a single-institution experience. AJR Am J Roentgenol. 2011;196(2):468–473. doi: 10.2214/AJR.10.4881. [DOI] [PubMed] [Google Scholar]
- 41.Klingenstein A Haug A R Zech C J Schaller U C Radioembolization as locoregional therapy of hepatic metastases in uveal melanoma patients Cardiovasc Intervent Radiol 2012; April 21 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 42.Van de Wiele C, Maes A, Brugman E. et al. SIRT of liver metastases: physiological and pathophysiological considerations. Eur J Nucl Med Mol Imaging. 2012;39(10):1646–1655. doi: 10.1007/s00259-012-2189-6. [DOI] [PubMed] [Google Scholar]
- 43.Leyvraz S, Spataro V, Bauer J. et al. Treatment of ocular melanoma metastatic to the liver by hepatic arterial chemotherapy. J Clin Oncol. 1997;15(7):2589–2595. doi: 10.1200/JCO.1997.15.7.2589. [DOI] [PubMed] [Google Scholar]
- 44.Peters S, Voelter V, Zografos L. et al. Intra-arterial hepatic fotemustine for the treatment of liver metastases from uveal melanoma: experience in 101 patients. Ann Oncol. 2006;17(4):578–583. doi: 10.1093/annonc/mdl009. [DOI] [PubMed] [Google Scholar]
- 45.Alexander H R Jr, Butler C C. Development of isolated hepatic perfusion via the operative and percutaneous techniques for patients with isolated and unresectable liver metastases. Cancer J. 2010;16(2):132–141. doi: 10.1097/PPO.0b013e3181db9c0a. [DOI] [PubMed] [Google Scholar]
- 46.Alexander H R Jr, Libutti S K, Pingpank J F. et al. Hyperthermic isolated hepatic perfusion using melphalan for patients with ocular melanoma metastatic to liver. Clin Cancer Res. 2003;9(17):6343–6349. [PubMed] [Google Scholar]
- 47.Pingpank J F, Hughes M S, Faries M B. et al. A phase III random assignment trial comparing percutaneous hepatic perfusion with melphalan (PHP-mel) to standard of care for patients with hepatic metastases from metastatic ocular or cutaneous melanoma. J Clin Oncol. 2010;28:18s. [Google Scholar]
- 48.Zager J, Nutting C. Chemosaturation therapy with percutaneous hepatic perfusions of melphalan versus standard of care in patients with hepatic metastases from melanoma: A randomized multicenter phase 3 study. J Vasc Interv Radiol. 2012;23:S3. [Google Scholar]