Abstract
Hepatic metastases, which are frequently seen in patients with neuroendocrine tumors (NETs), have a major adverse impact on the patient's quality of life and survival. Surgery is the treatment of choice for hepatic metastases but is possible in only a small percentage of patients. Systemic chemotherapy yields disappointing results. Somatostatin analogs are effective in controlling symptoms in many of these patients; however, the disease can become refractory to treatment. Transcatheter intra-arterial liver-directed therapies, such as hepatic artery embolization, chemoembolization, and radioembolization are frequently used in patients with NETs metastatic to the liver, especially in patients with refractory, unresectable, or recurrent disease. These treatments are effective in palliating the hormonal symptoms as well as achieving objective tumor responses. This review focuses on the technique, safety, and clinical efficacy of hepatic artery embolization, chemoembolization, and radioembolization in patients with metastatic NETs.
Keywords: neuroendocrine, metastases, chemoembolization, radioembolization, interventional radiology
Objectives: Upon completion of this article, the reader will be able to discuss the role of transarterial therapy in the treatment of neuroendocrine liver metastatic disease.
Accreditation: This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Neuroendocrine tumors (NETs) include a variety of tumors that have the capacity to synthesize and secrete hormonally active polypeptide products.1 NETs are slow-growing tumors with a predisposition to metastasize to the liver.2 At presentation, 46 to 93% of patients with NETs have hepatic metastases.3 Liver metastases can severely impair a patient's quality of life and are associated with a poor prognosis, with 5-year survival rates of 0 to 40%.1,4
Treatment options for patients with liver metastases are limited. Patients with neuroendocrine hepatic metastases, especially those experiencing debilitating hormonal symptoms, are treated initially with somatostatin analog therapy.5 However, the disease can become increasingly refractory to this treatment. Surgery is the only therapeutic option that offers the possibility of cure for liver metastases. However, only a small percentage of patients (10 to 15% according to various studies) are suitable candidates for curative resection.2,5,6 Systemic chemotherapies yield response rates varying between 0% and 38% for patients with metastatic carcinoid tumors.7,8 Islet cell neoplasms are generally more responsive to systemic chemotherapy, with reported response rates between 30% and 70% with streptozocin-based chemotherapies.4,5,6,7,8
Transcatheter intra-arterial tumor therapies involve the intravascular delivery of therapeutic agents via selective catheter placement with imaging guidance.9 Chemotherapeutic agents, embolic particles, and radioactive materials can be injected via feeding vessels into tumor(s) in an attempt to achieve tumor cell death. Transcatheter intra-arterial tumor therapies are widely used to treat patients with NETs metastatic to the liver. They are effective in controlling hormonal symptoms and in achieving a durable tumor response.10,11,12,13 This article reviews the current role of intra-arterial treatment options in patients with liver metastases attributable to NETs.
Indications for Liver-Directed Intra-Arterial Therapies
The presence of hepatic metastatic disease in patients with NETs is associated with poor prognosis, with an established 5-year survival rate of 20 to 25%.1,4 An aggressive multidisciplinary treatment approach that includes surgery and locoregional therapies improves survival and quality of life in these patients.14
Intra-arterial liver-directed therapies are generally used in NET patients with liver-dominant metastatic disease who have symptoms related to hormonal excess or tumor bulk or to those who present with rapid progression of liver disease, especially in patients with refractory, unresectable, or recurrent disease.10,11,12,13 Liver-directed treatment can occasionally also be used as an adjuvant therapy to reduce tumor load before hepatic resection, hepatic transplantation, or tumor ablation.11
Hepatic Arterial Embolization and Chemoembolization: Technique and Outcomes
NETs that have metastasized to the liver are generally hypervascular and receive between 80% and 90% of their blood supply from the hepatic artery. This feature, combined with the fact that normal liver receives 80% of its blood supply from the portal vein, provides a good rationale for using either hepatic arterial embolization or chemoembolization to treat these metastases. Hepatic artery embolization involves administering embolic agent into the hepatic artery that is supplying the tumors with the goal of inducing tumor ischemia. Hepatic artery chemoembolization combines intra-arterial delivery of chemotherapeutic agents with particulate embolization and combines local cytotoxic effect of chemotherapeutic agents with selective ischemia induced by embolization. Slowing of blood flow by embolization also prolongs drug retention in a tumor.
Patient Evaluation/Work-Up
Preprocedure evaluation should include a tissue diagnosis, cross-sectional imaging, history and physical examination, and laboratory evaluation. Laboratory studies include complete blood count, coagulation profile, renal and liver function tests, and relevant tumor markers. Contrast-enhanced computed tomography or magnetic resonance imaging should be performed, ideally within 1 month prior to treatment, to assess volume and distribution of liver disease, presence and extent of extrahepatic disease, and portal vein patency.
Technical Aspects
Initially, diagnostic superior mesenteric and celiac angiography is performed to evaluate the hepatic vasculature (including anatomical variants), vessels supplying the liver tumor(s), and tumor vascularity, and to ensure patency of the portal vein. After diagnostic angiography, embolization or chemoembolization is then performed using a microcatheter placed in a selective location to minimize nontarget embolization. For hepatic artery embolization, the embolic agent of choice is infused through the microcatheter until the selected vessel demonstrates complete or near-complete stasis of flow (Fig. 1). Commonly used embolic agents for hepatic artery embolization include Gelfoam sponge (Gelfoam, Upjohn, Kalamazoo, MI), polyvinyl alcohol particles (Contour; Boston Scientific, Natick, MA, USA), and tris-acryl gelatin microspheres (Embospheres, Biosphere Medical, Rockland, MA). For chemoembolization, the combination of an embolic agent and the chemotherapeutic drug is administered in a fashion similar to that used for hepatic artery embolization (Fig. 2).
Figure 1.
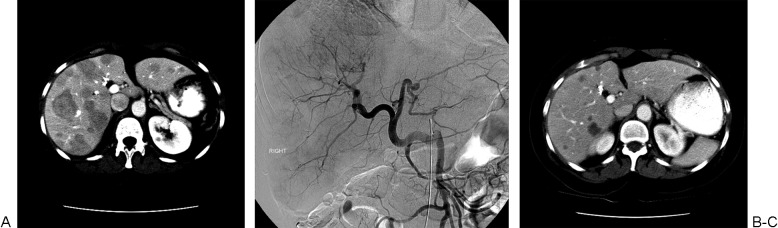
A 60-year-old man with metastatic carcinoid treated with lobar embolization. (A) Computed tomography (CT) scan shows multiple liver lesions involving both lobes of the liver. (B) Superior mesenteric angiogram shows replaced common hepatic artery with multiple hypervascular liver tumors. The patient was treated with right and left hepatic artery bland embolization in two treatment sessions, separated by a 4-week interval. (C) Follow-up CT scan shows decrease in number and size of liver tumors.
Figure 2.
A 45-year-old man with metastatic insulinoma presenting with persistent hypoglycemia. (A) Computed tomography (CT) scan shows a large lesion in segment 7. There were three other liver lesions in the right liver (not shown). (B) Selective right hepatic arteriogram shows multiple hypervascular liver lesions. Chemoembolization was performed using 5-fluoruracil, doxorubicin, and streptozocin mixed with 300 to 500 polyvinyl alcohol particles. (C) Postembolization angiogram shows stasis in right hepatic artery. (D) Posttreatment follow-up CT scan shows tumor necrosis and lack of vascularity within the tumor.
Embolization technique and extent depend on the number, size, and distribution of liver lesions. Patients with unilobar disease can be treated with lobar embolization or selective catheterization and embolization of individual branches supplying the tumor. In patients with bilobar liver involvement, embolization of the entire liver during a single treatment session is not recommended because of risk of liver failure.12,13 The lobe with the largest tumor burden is typically treated first. In the case of extensive bilobar disease with >75% liver involvement, embolization procedures can be staged, and different liver segments may be embolized at each treatment session.10,11,12,13 The timing of subsequent embolizations is determined primarily by a patient's tumor burden and his or her ability to tolerate the procedure.12,13 Depending on the extent of liver involvement, the entire liver can usually be treated in two to four sessions. Although there is no absolute contraindication to embolization or chemoembolization, complete portal vein occlusion, poor performance status, and hepatic insufficiency are considered relative contraindications to these treatments.15,16 Embolization should not be performed in patients with bilirubin levels >2 to 3 mg/dL. Patients who have undergone previous biliary-enteric anastomoses are at very high risk for developing a liver abscess after chemoembolization, even when standard prophylactic antibiotic therapy is used.17 A few recent reports suggest that use of aggressive antibiotic prophylaxis with bowel preparation may protect against hepatic abscesses after chemoembolization in such patients.17 Geschwind et al described no abscesses in a group of four patients who received intravenous (IV) tazobactam/piperacillin along with bowel preparation before treatment.18 Similarly, Patel and others used long-term antibiotic prophylaxis and bowel preparation; they reported a trend toward a lower rate of abscess formation among patients who received more aggressive antibiotic prophylaxis compared with those who received standard prophylaxis.19 At our institution, patients with biliary-enteric anastomoses undergoing embolization or chemoembolization are treated with the following regimen19: oral levofloxacin 500 mg daily and metronidazole 500 mg twice daily 2 days before chemoembolization and continued for 2 weeks after discharge, along with bowel preparation with neomycin 1 g plus erythromycin base 1 g orally at 1 pm, 2 pm, and 11 pm the day before chemoembolization. In a more recent study, Khan et al showed that prophylactic treatment with moxifloxacin alone was successful in preventing liver abscess in 10 patients treated with 25 embolization or chemoembolization procedures, avoiding the need for bowel preparation.20
Some reports have suggested that embolization and chemoembolization should not be performed in patients with a tumor burden involving >50% of the liver because of the high risk of inducing liver failure. Carrasco and associates reported a high mortality rate for patients with >50% liver involvement by metastatic disease.21,22 In another report by Kress and colleagues,23 most of the patients with a tumor burden >75% died 30 days to 6 months after undergoing chemoembolization as a result of liver failure or tumor progression. However, results of a study from MD Anderson Cancer Center suggest that, although response rates and survival durations are lower in patients with >75% liver involvement than in those with lesser liver involvement, many of these patients can be safely treated and can even benefit from this treatment if only small portions of the liver are embolized during each session.12,15 In a 2008 study of patients with hepatic NET metastases and liver involvement >75% who were treated at the author institution with selective segmental sequential embolization or chemoembolization, radiologic response or disease stabilization was seen in 82% of patients; symptomatic response was seen in 65% of patients; and the median progression-free and overall survival durations were 9.2 months and 17.9 months, respectively.15 However, this 2008 study also indicated that embolization or chemoembolization can be associated with a high risk for procedure-related mortality in patients with massive liver tumor burden if they have additional risk factors such as carcinoid heart disease, sepsis, rapidly worsening performance status, or anasarca.15
The therapeutic benefit of chemoembolization over bland embolization for this patient population remains unclear. A review of various published studies suggests that no difference in response rates exists between these treatment methods.12,13,14,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50 Several different chemotherapeutic agents have been used in various studies, and there is no consensus on which chemotherapeutic agent to use. Most commonly used drugs for chemoembolization include doxorubicin and streptozocin, alone or in combination with cisplatin, 5-fluorouracil, and mitomycin-C. Results of the author experience at MD Anderson reveal that although chemoembolization did not demonstrate a therapeutic advantage over bland embolization in patients with carcinoid tumors, patients with islet cell carcinoma treated with chemoembolization had a tendency toward a more prolonged survival (31.5 months versus 18.2 months) and a better radiologic response rate (50% versus 25%) than did those treated with embolization alone; however, these differences did not reach statistical significance.12 This was not an unexpected result because carcinoid tumors are known to be more resistant to systemic chemotherapy than are islet cell carcinomas. In another retrospective multi-institutional review comparing chemoembolization and bland embolization, patients treated with chemoembolization demonstrated a trend toward improved time to progression, symptom control, and overall survival.41 Again, statistical significance was not achieved because of the small cohort, warranting the necessity for further prospective randomized trials. At MD Anderson, patients with metastatic carcinoid tumors are treated with bland embolization; those with metastatic islet cell tumors are treated with chemoembolization. There is marked variability in the chemoembolization protocol based on institutional and operator preferences. Some mix the chemotherapy with particulate agents, some infuse the chemotherapy initially followed by embolization with particles, some emulsify the chemotherapy with lipiodol, and others use the sandwich technique that involves particle embolization before and after chemotherapy infusion. Literature review shows there is no difference in the outcomes between the various techniques, and there are no data supporting superiority of any one chemoembolization regimen over the others.
Outcomes
Many nonrandomized retrospective reports have shown that embolization and chemoembolization can reduce hormone levels, palliate symptoms, and reduce tumor burden in patients with NET hepatic metastases.12,13,14,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50 A review of the literature shows that embolization and chemoembolization can result in imaging response in 25 to 95% of patients and symptomatic response in 50 to 100% of patients with NET liver metastasis (Table 1). Reported 5-year survival rates vary between 13.7% and 83%.12,13,14,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50 This wide range in response rates and survival durations is a reflection of the marked heterogeneity in the reported studies in terms of patient demographics; extent of liver involvement by metastatic disease; presence or absence of extrahepatic disease; tumor histology and degree of differentiation; treatment regimens used (bland embolization versus chemoembolization, number of embolization sessions, choice and/or dose of the chemotherapeutic drug and embolic agent); the time when embolization was performed (i.e., early or late in the clinical course); concurrent use of octreotide and systemic chemotherapy; frequency and timing of follow-up imaging; criteria used to evaluate response; and methods used to report survival.
Table 1. Literature findings on Hepatic Arterial Embolization and Chemoembolization for Treatment of Metastatic Neuroendocrine Tumors.
| Study | Tumor type | N | EHD (%) | Treatment | Imaging response (%) | Symptom response (%) |
Tumor marker response (%) |
Median TTP/PFS (mo) |
Median survivala (mo) | 5-y survivala (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CR plus PR | SD | PD | ||||||||||
| Ajani et al24 | Islet cell | 22 | NR | TAE | 60 | NR | NR | NR | 33.7 | NR | ||
| Hanssen et al25 | Carcinoid | 36 | NR | TAE | 60 | 20 | 20 | 100 | NR | NR | NR | 75 |
| Rusznieweski et al40 | Carcinoid | 18 | NR | TACE (dox) | 33 | 73 | 57 | NR | NR | NR | ||
| Islet cell | 5 | NR | TACE (dox) | 60 | NR | NR | NR | NR | ||||
| Therasse et al43 | Carcinoid | 23 | NR | TACEb | 33 | 50 | 11 | 100 | 91 | NR | 24 | NR |
| Perry et al38 | NET | 30 | NR | TACE (dox) | 95 | 90 | 69 | NR | 24 | NR | ||
| Wangberg45 | Carcinoid | 40 | NR | TAE | 42.5 | 37.5 | NR | 100 | NR | NR | 63 | |
| Eriksson et al31 | Carcinoid | 29 | NR | TAE | 52 | 31 | NR | 38 | NR | 80 | 60 | |
| Islet cell | 12 | NR | 50 | 8 | NR | 42 | NR | 20 | NR | |||
| Diamandidouet al28 | NET | 20 | NR | TACE (cis) | 33.3 | 66.6 | 67 | 73 | NR | NR | NR | |
| Brown et al27 | NET | 35 | NR | TAE | 96 | NR | NR | NR | 54 | |||
| Kim et al47 | Carcinoid | 16 | NR | TACE (cis/dox) | 25 | NR | 75 | NR | NR | |||
| Islet cell | 14 | NR | TACE (STZ/5-FU) |
50 | NR | 90 | NR | NR | ||||
| Chamberlain et al14 | NET | 33 | 45 | TAE | 96 | NR | NR | NR | 51 | |||
| Yao et al46 | NET | 20 | NR | TACE (dox/cis/mit-C) | 25 | 10 | 50 | NR | NR | 32 | 40 | |
| Roche et al39 | NET | 14 | 7.1 | TACE (dox) | 43 | 90 | NR | NR | NR | 83c | ||
| Loewe et al34 | Carcinoid | 23 | ≥48 | TAE | 73 | 22 | NR | 61.5 | 69 | 65.4 | ||
| Kress et al23 | NET | 26 | TACE (dox) | 7.7 | 53.8 | 19.2 | NR | NR | NR | NR | 48c | |
| Gupta et al12 | Carcinoid | 69 | 70 | TAE or TACEb | 66.7 | 24.6 | 8.7 | NR | NR | 22.7 | 33.8 | 26.6 |
| Islet cell | 54 | 57 | 35.2 | 61.1 | 3.7 | NR | NR | 16.1 | 23.2 | 13.7 | ||
| Osborne et al37 | NET | 59 | TAE | 91 | NR | NR | 24d | NR | ||||
| Strosberg et al42 | NET | 84 | 26 | TAE | 48 | 52 | 80 | NR | 36 | NR | NR | |
| Varker et al44 | Carcinoid | 122 | NR | 1st TACE (cis/dox/mit-C) | 82 | 92 | NR | 11.8 | 33.3 | NR | ||
| Carcinoid | 27 | NR | Repeat TACE (cis/dox/mit-C) | 61 | 77 | NR | NR | 28.1 | NR | |||
| Bloomston et al26 | Carcinoid | 122 | 28 | TACEb | 82 | 12 | 6 | 92 | 80 | NR | 33.3 | NR |
| Ruutiainen et al41 | NET | 67 | NR | TAE | 50 | 38 | 13 | 93 | NR | 6 | 39 | 33 |
| NR | TACE (cis/dox/mit-C) | 66 | 22 | 12 | 92 | NR | 12 | 44 | 50 | |||
| Ho et al33 | Carcinoid | 31 | NR | TAE or TACE (cis/dox/mit-C) | 23 | 32 | 23 | 78 | NR | 22.7d | 41.8d | 32 |
| Islet cell | 15 | NR | 18 | 45 | 9 | 75 | NR | 16.1d | 43.7d | 35 | ||
| de Baere et al48 | NET | 8 | 60 | TACE (DEB/dox) | 80 | 15 | 1 | NR | NR | 15 | NR | NR |
| Dong and Carr50 | NET | 123 | NR | TACEb | 62 | 24 | 14 | NR | NR | NR | 65 | 36 |
Abbreviations: chemo, chemotherapy; cis, cisplatin; CR, complete response; dox, doxorubicin; EHD, extrahepatic disease; 5-FU, 5-fluorouracil; IV, intravenous; mit-C, mitomycin-C; NET, neuroendocrine tumor; NR, not recorded; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease; STZ, streptozocin; TACE, transcatheter arterial chemoembolization; TAE, transcatheter arterial embolization; TTP, time to progression; vinb, vinblastine; DEB, drug-eluting beads.
From the date of TAE/TACE.
Multiple chemotherapeutic regimens.
From diagnosis.
Mean.
In keeping with the results reported in the medical and surgical oncology literature, patients with carcinoid tumors treated with embolization or chemoembolization experience better outcomes than do those with islet cell tumors treated in a similar fashion (Table 1). Eriksson et al reported the median survival duration in patients with carcinoid tumors at 80 months, but only 20 months in patients with islet cell tumors.31 Moertel and colleagues reported a longer median survival (27 months versus 9 months) in patients with carcinoid tumors treated with liver embolization or surgical ligation of the hepatic artery than in those with islet cell tumors.36 A study from MD Anderson showed that patients with carcinoid tumors had significantly higher radiologic response rates (66.7% versus 35.2%; p = 0.0001) and longer median progression-free survival (22.7 months versus 16.1 months; p = 0.046) and overall survival durations (33.8 months versus 23.2 months; p = 0.012) than did patients with islet cell tumors.12
Although many studies have reported on the use of embolization or chemoembolization in patients with metastatic NETs, several unresolved issues remain. There is, however, a lack of consensus among interventional radiologists regarding the ideal chemoembolic regimen, procedure end points, the degree of vascular stasis to be achieved, and the ideal time interval between treatment sessions. In addition, because of the lack of randomized controlled trials, unequivocal survival benefit has not yet been established. In a 2011 study with long-term follow-up (mean follow-up:3.2 years; range: 2 weeks to 18.3 years), Dong and Carr found that the overall 3-, 5-, and 10-year patient survivals were 59%, 36%, and 20%, respectively, with a mean patient survival of 3.3 years.50 These authors concluded that chemoembolization for unresectable NETs metastatic to the liver is associated with prolonged survival. The time at which embolization treatment should be performed, whether early or late in the course of disease, also remains unclear. Although some investigators advocate early embolization, others suggest late embolization can be equally effective. Hanssen and colleagues, who observed a higher response rate in patients with carcinoid tumors treated at the time of diagnosis with embolization followed by interferon therapy than the response in patients who received interferon only (86% versus 42%), concluded that embolization should be performed early in the course of disease.25 However, Erickson and associates performed embolization or chemoembolization at a median of 37 months after diagnosis and observed a median survival of 80 months and a 5-year survival rate of 60%, indicating that relatively late embolization also is effective.31 These findings complement the author's experience at MD Anderson; the duration of liver disease before embolization did not influence response rates or survival durations after embolization or chemoembolization.12
In recent years, drug-eluting beads have been used to administer chemoembolization as a means to deliver a more accurate dose of anticancer drugs to liver tumors over a prolonged period. Drug-eluting beads are biocompatible, hydrophilic, nonresorbable hydrogel microspheres that are available in various sizes. These beads can be loaded with chemotherapeutic medications, which then release in a controlled and sustained fashion. However, data on the use of chemoembolization using drug-eluting beads in patients with NETs are limited. De Baere et al used doxorubicin-eluting beads (500 to 700 μm) in 20 patients with progressive liver metastases resulting from NETs.48 Partial response (PR) and stable disease (SD) were noted in 16 (80%) and 3 (15%) patients, respectively. At 15-month follow-up, nine patients (45%) maintained SD. These authors concluded that chemoembolization with drug-eluting beads is safe and effective. In another study, Gaur and associates performed 38 drug-eluting bead (100- to 300-μm or 300- to 500-μm beads preloaded with 50 to 100 mg doxorubicin) chemoembolization procedures in 18 patients with NETs metastatic to the liver.49 At short-term follow-up (<3 months), 22 of 38 procedures (58%) produced an radiologic response, with the remainder having SD. At intermediate-term follow-up (mean: 445 days; range: 163 to 1247 days), 17 of 26 procedures (65%) produced a radiologic response. However, at present, there is no evidence in the published literature to suggest that drug-eluting beads offer any therapeutic advantage over bland embolization or conventional chemoembolization in patients with metastatic NETs.
Complications
Postembolization syndrome, characterized by right-upper-quadrant pain, fever, nausea, leukocytosis, and elevated liver function test findings, is seen most patients after embolization or chemoembolization; this syndrome is generally self-limited and improves within 3 to 5 days, but occasionally it is severe and requires prolonged hospitalization.12,15 Complications such as carcinoid crisis, acute liver failure, hepatic encephalopathy, emphysematous cholecystitis, gastric ulcer and hemorrhage, abscess formation, and tumor lysis syndrome are uncommon.12,14,15 Hepatic artery embolization in patients with functional carcinoid metastases can occasionally precipitate massive acute release of serotonin and other vasoactive peptides resulting in a carcinoid crisis. Carcinoid crisis is a potentially life-threatening event characterized by hypotension or hypertension, arrhythmias, bronchospasm, facial flushing, edema, and metabolic acidosis. Carcinoid crisis is treated by IV administration of 200 μg IV octreotide, repeated as needed until the symptoms are controlled. Octreotide may also be useful in preventing a carcinoid crisis, and its prophylactic administration is recommended prior to any hepatic artery intervention in patients with metastatic carcinoid tumors.
Yttrium-90 Microsphere Radioembolization
Intra-arterial radioembolization with yttrium-90 (90Y) microspheres is increasingly being used in patients with unresectable primary and metastatic liver tumors.48,49,50 90Y is a β emitter with a mean soft tissue penetration of 2.5 mm. The two 90Y microsphere products approved by the Food and Drug Administration in clinical use are the glass-based TheraSphere (Nordion Inc., Ottawa, Ontario, Canada) and the resin-based SIR-Spheres (Sirtex Medical Ltd., Sydney, New South Wales, Australia). Particle sizes vary between 20 µm and 40 µm. Hepatic arterial administration of the 90Y microspheres results in preferential deposition of the microspheres into the tumoral and peritumoral vasculature, allowing the delivery of high doses of radiation to the tumor with relative sparing of the normal liver parenchyma.51,52
Technique
Radioembolization is generally performed in two steps. Diagnostic angiography is performed initially to evaluate the hepatic arterial anatomy and to determine the pulmonary shunt fraction via technetium-99 macroaggregated albumin infusion.51,52,53 Excessive pulmonary parenchymal deposition of the 90Y microspheres via pulmonary shunting could result in radiation pneumonitis, which can be avoided by keeping the lung exposure <30 Gy. To avoid nontarget delivery of radioactive microspheres to organs such as the stomach, duodenum, and pancreas, it is essential to perform an angiogram with selective embolization of all extrahepatic arteries before treatment with 90Y microspheres (Fig. 3).
Figure 3.
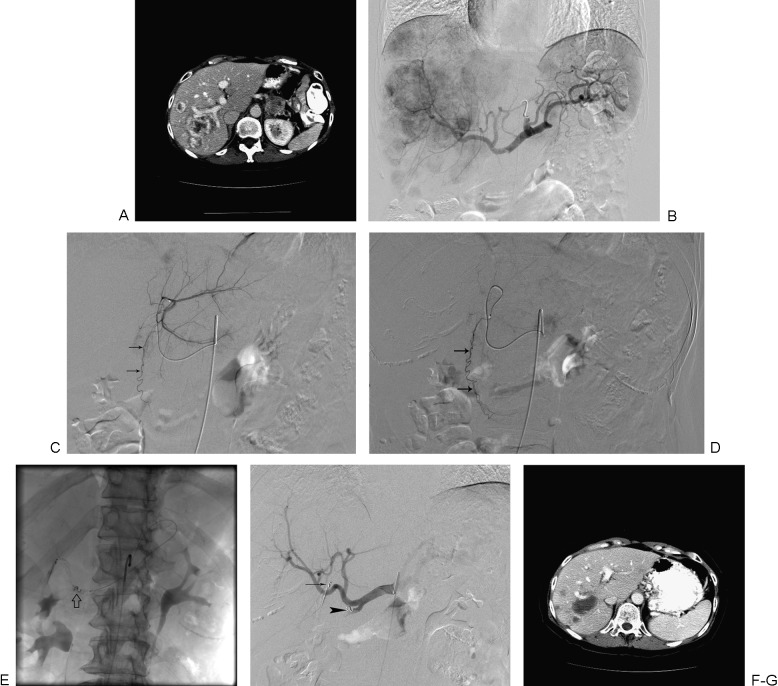
A 62-year-old female patient with metastatic islet cell carcinoma treated with radioembolization. (A) Computed tomography (CT) scan shows multiple right liver lesions. Imaging showed left liver lesions as well (not shown). (B) Celiac arteriogram shows multiple hypervascular liver lesions. (C) Selective left hepatic arteriogram shows an aberrant umbilical artery (arrows) arising from the left hepatic artery. (D) This branch (arrows) was selectively catheterized and embolized with coils. (E) Right gastric artery was also embolized with coils (arrow) through a microcatheter placed in the left gastric artery. (F) Celiac arteriogram shows successful coil embolization of the umbilical (arrow) and right gastric (arrowhead) arteries. The patient was treated with whole-liver infusion of 90Y microspheres. (G) Follow-up imaging shows necrosis of liver lesions.
In 2012, Mahvash and colleagues reported an alternative method to prevent nontarget gastrointestinal deposition of 90Y microspheres; the technique involves the use of a balloon catheter to temporarily occlude the common hepatic artery to reverse hepatoenteric flow for lobar administration of microspheres.54 However, the technique should not be used in patients with a significant tortuosity of the common hepatic artery or in patients with anatomical variants, such as the left hepatic artery arising from the gastrohepatic trunk or a replaced right hepatic artery. At MD Anderson, whenever feasible, the balloon occlusion technique is used for 90Y radioembolization. The second step involves the delivery of the radioactive microspheres. Sequential unilobar or whole-liver treatments can be performed depending on the extent of liver involvement, liver function, variant hepatic arterial anatomy, and institutional and operator preferences. For sequential unilobar infusions, a 4- to 6-week interval between infusions is recommended to allow for resolution of any treatment-related toxicities. For whole-liver infusion, the catheter can be positioned in the proper hepatic artery for infusion of 90Y microspheres. Alternatively, the whole liver can be treated in one session by selective catheterization and infusion in one, followed by the other hepatic artery. Device-specific dosimetry calculations have been discussed in detail in many previous reports and are not included in this article.51,52,53 Periprocedural management in patients undergoing radioembolization involves the use of a proton pump inhibitor or histamine-2 blocker to prevent gastrointestinal injury, antiemetics for posttreatment nausea, and a short-acting somatostatin analog in patients with functional carcinoid tumors. Although prophylactic antibiotics are not routinely used, aggressive antibiotic prophylaxis is recommended in patients with biliary-enteric anastomoses. Although portal vein thrombosis is considered a relative contraindication for embolization or chemoembolization, it is not necessarily a contraindication for radioembolization. TheraSpheres, because of their lower embolic potential, are the preferred microspheres by some authors for this patient population.51,52,53
Outcomes
Several studies have reported the results of radioembolization in patients with neuroendocrine liver metastases (Table 2).52,55,56,57,58,59,60,61 In a study involving 148 patients with NET liver metastases treated with 185 radioembolization procedures using resin 90Y microspheres, Kennedy et al reported a complete response in 2.7% of patients, PR in 60.5%, SD in 22.7%, and progressive disease in 4.9%.55 The median survival was 70 months. In another prospective study that involved nine patients with unresectable liver metastases from NETs treated with 90Y radioembolization, PR was seen in six patients (66%), and survival rates were 100% and 57% for 1 and 3 years, respectively.59 No major complications occurred. In a 2012 single-center study involving 40 patients with metastatic NETS treated with 90Y radioembolization, Memon and colleagues reported that the 1-, 2-, and 3-year overall survival rates were 72.5%, 62.5%, and 45%, respectively; overall median survival was 34.4 months.60 In a separate study involving radioembolization in 48 patients with unresectable metastatic NETs, Saxena and associates reported a complete or partial response in 54% of patients; the median survival was 35 months.61 These authors also evaluated the prognostic variables that influenced survival in patients with unresectable metastatic NETs and noted that that low hepatic tumor burden (p = 0.022), female gender (p = 0.022), well-differentiated tumor (p = 0.001), and absence of extrahepatic metastasis (p < 0.001) were associated with an improved survival.61 In a retrospective analysis involving eight patients with metastatic NETs, Murthy et al showed that 90Y radioembolization can be safely performed even after previous embolization or chemoembolization, with a median survival time of 14 months after radioembolization.52 These results suggest that 90Y radioembolization represents a viable alternative therapy for patients with hepatic NETs, especially those patients in whom traditional therapies have failed. Further investigation, long-term follow-up, and prospective clinical trials are warranted to determine the exact role of this treatment method in the management of NET hepatic metastases.
Table 2. Literature findings on Radioembolization for Treatment of Metastatic Neuroendocrine Tumors.
| Study | Line | N | Tumor type | EHD (%) | Treatment agent | Imaging/tumor marker response (%) | Symptom response (%) | Median TTP | Median survival | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CR plus PR | SD | PD | |||||||||
| Kennedy et al55 | First to salvage | 148 | 82% carcinoid; 18% other NETs | NR | SIR-Spheres | 63.2 | 22.7 | 4.9 | NR | NR | 70 mo |
| King et al57 | First or second line or more | 34 | 68% carcinoid; 32% other NETs | 59 | SIR-Spheres plus 5-FU infusion | 50 | 14.7 | 32.4 | 55 | NR | 24.2 mo |
| Murthy et al52 | Salvagea | 8 | 75% islet cell; 25% carcinoid | 62.5 | SIR-Spheres | 12 | 50 | 37.5 | NR | NR | 14 mo |
| Rheeet al58 | NR | 27 | 67% carcinoid; 33% islet cell | NR | SIR-Spheres or Therasphere | 93.8b | NR | NR | 18 mo | ||
| Kalinowski et al59 | NR | 9 | NR | NR | SIR-Spheres | 66.7 | 33.3 | NR | 11.1 mo | 3 y: 57% | |
| Saxena et al61 | First to salvage | 48 | 71% carcinoid; 29% other NETs | 48 | SIR-Spheres | 54 | 23 | 23 | NR | NR | 35 mo |
| Memon et al60 | First to salvage | 40 | 82% carcinoid; 18% other NETs | 35 | Therasphere | 64 | NR | NR | 84 | NR | 34.4 mo |
Abbreviations: CR, complete response; EHD, extrahepatic disease; 5-FU, 5-fluorouracil; NETs, neuroendocrine tumors; NR, not recorded; PD, progressive disease; PR, partial response; SD, stable disease; TTP, time to progression.
All failed previous transcatheter arterial embolization/transcatheter arterial chemoembolization.
PR plus SD.
Complications
The acute and subacute toxicities associated with radioembolization are less severe and better tolerated than those toxicities associated with conventional hepatic embolization procedures. This better toxicity profile, combined with the lower incidence of postembolization syndrome associated with radioembolization, allows for treatment in an outpatient setting.51,52,53 Complications including radiation gastritis, radiation pneumonitis, duodenal ulcers, and liver dysfunction have been reported. Radiation-induced liver disease is rare; the estimated incidence for all tumor types is <1%.62
Hepatic Arterial Infusion of Radiolabeled Somatostatin Analogs
Radiolabeled somatostatin analog imaging has been used to diagnose, localize, and monitor NET treatment response. Octreotide, octreotate, and lanreotide peptides have been used, and all have been modified with the addition of the metal chelator tetraazacyclododecanetetraacetic acid (DOTA).16 More recently, intra-arterial delivery of these somatostatin analogs labeled with therapeutic radioactive agents has been used to treat liver metastases from NETs. The commonly used radionuclides include 90Y, indium-111 (111In), and lutetium-177. A diagnostic 111In-pentetreotide scan is performed to assess receptor positivity and to determine whether a patient may benefit from treatment with radiolabeled somatostatin analogs; almost 90% of all carcinoid tumors are receptor positive. However, this percentage can drop to 50% in NETs of pancreatic origin, especially insulinomas.16,62 Patients for whom receptor activity at known tumor sites is more intense than activity in a healthy liver are likely to benefit from this treatment,63,64 and intra-arterial administration of these radiolabeled somatostatin analogs has been shown to result in modest treatment responses.63,64 McStay et al treated 23 patients with NET hepatic metastases with hepatic arterial infusion of 90Y-DOTA-lanreotide and observed a response rate of 16% and 1-year survival rate of 63%.65 Limouris and associates, in their series of 17 patients treated with hepatic arterial infusion of 111In-DTPA-D-Phe1-octreotide, observed a complete or partial response in 53% of patients and a survival of 32 months in 70.5% of patients.66
Hepatic Arterial Infusion of Chemotherapy
Hepatic arterial infusion of chemotherapeutic agents permits delivery of relatively higher doses of chemotherapy than systemic administration. There is very limited literature on the use of hepatic arterial infusion chemotherapy in patients with metastatic NETs. Takeuchi and colleagues achieved a PR that was maintained for 40 months in a patient with metastatic carcinoid tumor treated with 40 cycles of intra-arterial methotrexate and fluorouracil (5-FU).67 Christante et al treated 77 patients with NET hepatic metastases with four cycles of hepatic arterial infusion of 5-FU.68 The last two infusion cycles were combined with chemoembolization using cisplatin, doxorubicin, and mitomycin-C mixed with lipiodol. The authors reported a response rate of 80%, a median progression-free survival duration of 19 months, and a 5-year survival rate of 27%.68 At MD Anderson, hepatic arterial infusion chemotherapy is generally reserved for patients in whom embolization or radioembolization is not considered safe because of extensive tumor burden, hepatic insufficiency, or complete portal vein occlusion.
Conclusions
Transcatheter intra-arterial tumor therapies such as hepatic artery embolization, chemoembolization, and radioembolization reduce hormone levels, provide symptom relief, and reduce tumor burden in many patients with unresectable and symptomatic NET hepatic metastases. In selected patients, the use of liver-directed therapies can result in cytoreduction, allowing for potential resection or ablation. In the absence of large-scale controlled trials, it is not possible to draw any conclusion regarding the superiority of one intra-arterial therapy over the other. Treatment selection in individual patient should be based on the disease burden, presence and extent of extrahepatic disease, presence and severity of symptoms, performance status, anatomical factors, and quality-of-life considerations. Prospective clinical trials comparing the relative safety and efficacy of different treatment options are warranted.
References
- 1.Modlin I M, Lye K D, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97(4):934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 2.Neary P C, Redmond P H, Houghton T, Watson G R, Bouchier-Hayes D. Carcinoid disease: review of the literature. Dis Colon Rectum. 1997;40(3):349–362. doi: 10.1007/BF02050428. [DOI] [PubMed] [Google Scholar]
- 3.Chamberlain R S, Canes D, Brown K T. et al. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg. 2000;190(4):432–445. doi: 10.1016/s1072-7515(00)00222-2. [DOI] [PubMed] [Google Scholar]
- 4.Modlin I M, Sandor A. An analysis of 8305 cases of carcinoid tumors. Cancer. 1997;79(4):813–829. doi: 10.1002/(sici)1097-0142(19970215)79:4<813::aid-cncr19>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Modlin I M, Latich I, Kidd M, Zikusoka M, Eick G. Therapeutic options for gastrointestinal carcinoids. Clin Gastroenterol Hepatol. 2006;4(5):526–547. doi: 10.1016/j.cgh.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Loftus J P, van Heerden J A. Surgical management of gastrointestinal carcinoid tumors. Adv Surg. 1995;28:317–336. [PubMed] [Google Scholar]
- 7.O'Toole D, Hentic O, Corcos O, Ruszniewski P. Chemotherapy for gastro-enteropancreatic endocrine tumours. Neuroendocrinology. 2004;80 01:79–84. doi: 10.1159/000080747. [DOI] [PubMed] [Google Scholar]
- 8.Engstrom P F, Lavin P T, Moertel C G, Folsch E, Douglass H O Jr. Streptozocin plus fluorouracil versus doxorubicin therapy for metastatic carcinoid tumor. J Clin Oncol. 1984;2(11):1255–1259. doi: 10.1200/JCO.1984.2.11.1255. [DOI] [PubMed] [Google Scholar]
- 9.Brown D B Gould J E Gervais D A et al. Society of Interventional Radiology Technology Assessment Committee and the International Working Group on Image-Guided Tumor Ablation Transcatheter therapy for hepatic malignancy: standardization of terminology and reporting criteria J Vasc Interv Radiol 200920(7, Suppl):S425–S434. [DOI] [PubMed] [Google Scholar]
- 10.Madoff D C, Gupta S, Ahrar K, Murthy R, Yao J C. Update on the management of neuroendocrine hepatic metastases. J Vasc Interv Radiol. 2006;17(8):1235–1249; quiz 1250. doi: 10.1097/01.RVI.0000232177.57950.71. [DOI] [PubMed] [Google Scholar]
- 11.Liu D M, Kennedy A, Turner D. et al. Minimally invasive techniques in management of hepatic neuroendocrine metastatic disease. Am J Clin Oncol. 2009;32(2):200–215. doi: 10.1097/COC.0b013e318172b3b6. [DOI] [PubMed] [Google Scholar]
- 12.Gupta S, Johnson M M, Murthy R. et al. Hepatic arterial embolization and chemoembolization for the treatment of patients with metastatic neuroendocrine tumors: variables affecting response rates and survival. Cancer. 2005;104(8):1590–1602. doi: 10.1002/cncr.21389. [DOI] [PubMed] [Google Scholar]
- 13.Gupta S, Yao J C, Ahrar K. et al. Hepatic artery embolization and chemoembolization for treatment of patients with metastatic carcinoid tumors: the M.D. Anderson experience. Cancer J. 2003;9(4):261–267. doi: 10.1097/00130404-200307000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Chamberlain R S, Canes D, Brown K T. et al. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg. 2000;190(4):432–445. doi: 10.1016/s1072-7515(00)00222-2. [DOI] [PubMed] [Google Scholar]
- 15.Kamat P P, Gupta S, Ensor J E. et al. Hepatic arterial embolization and chemoembolization in the management of patients with large-volume liver metastases. Cardiovasc Intervent Radiol. 2008;31(2):299–307. doi: 10.1007/s00270-007-9186-3. [DOI] [PubMed] [Google Scholar]
- 16.Steward M J, Warbey V S, Malhotra A, Caplin M E, Buscombe J R, Yu D. Neuroendocrine tumors: role of interventional radiology in therapy. Radiographics. 2008;28(4):1131–1145. doi: 10.1148/rg.284075170. [DOI] [PubMed] [Google Scholar]
- 17.Kim W, Clark T W, Baum R A, Soulen M C. Risk factors for liver abscess formation after hepatic chemoembolization. J Vasc Interv Radiol. 2001;12(8):965–968. doi: 10.1016/s1051-0443(07)61577-2. [DOI] [PubMed] [Google Scholar]
- 18.Geschwind J F, Kaushik S, Ramsey D E, Choti M A, Fishman E K, Kobeiter H. Influence of a new prophylactic antibiotic therapy on the incidence of liver abscesses after chemoembolization treatment of liver tumors. J Vasc Interv Radiol. 2002;13(11):1163–1166. doi: 10.1016/s1051-0443(07)61959-9. [DOI] [PubMed] [Google Scholar]
- 19.Patel S, Tuite C M, Mondschein J I, Soulen M C. Effectiveness of an aggressive antibiotic regimen for chemoembolization in patients with previous biliary intervention. J Vasc Interv Radiol. 2006;17(12):1931–1934. doi: 10.1097/01.RVI.0000244854.79604.C1. [DOI] [PubMed] [Google Scholar]
- 20.Khan W, Sullivan K L, McCann J W. et al. Moxifloxacin prophylaxis for chemoembolization or embolization in patients with previous biliary interventions: a pilot study. AJR Am J Roentgenol. 2011;197(2):W343–W345. doi: 10.2214/AJR.10.6019. [DOI] [PubMed] [Google Scholar]
- 21.Carrasco C H, Chuang V P, Wallace S. Apudomas metastatic to the liver: treatment by hepatic artery embolization. Radiology. 1983;149(1):79–83. doi: 10.1148/radiology.149.1.6611956. [DOI] [PubMed] [Google Scholar]
- 22.Carrasco C H, Charnsangavej C, Ajani J, Samaan N A, Richli W, Wallace S. The carcinoid syndrome: palliation by hepatic artery embolization. AJR Am J Roentgenol. 1986;147(1):149–154. doi: 10.2214/ajr.147.1.149. [DOI] [PubMed] [Google Scholar]
- 23.Kress O, Wagner H J, Wied M, Klose K J, Arnold R, Alfke H. Transarterial chemoembolization of advanced liver metastases of neuroendocrine tumors—a retrospective single-center analysis. Digestion. 2003;68(2–3):94–101. doi: 10.1159/000074522. [DOI] [PubMed] [Google Scholar]
- 24.Ajani J A, Carrasco C H, Charnsangavej C, Samaan N A, Levin B, Wallace S. Islet cell tumors metastatic to the liver: effective palliation by sequential hepatic artery embolization. Ann Intern Med. 1988;108(3):340–344. doi: 10.7326/0003-4819-108-3-340. [DOI] [PubMed] [Google Scholar]
- 25.Hanssen L E, Schrumpf E, Jacobsen M B. et al. Extended experience with recombinant alpha-2b interferon with or without hepatic artery embolization in the treatment of midgut carcinoid tumours. A preliminary report. Acta Oncol. 1991;30(4):523–527. doi: 10.3109/02841869109092412. [DOI] [PubMed] [Google Scholar]
- 26.Bloomston M, Al-Saif O, Klemanski D. et al. Hepatic artery chemoembolization in 122 patients with metastatic carcinoid tumor: lessons learned. J Gastrointest Surg. 2007;11(3):264–271. doi: 10.1007/s11605-007-0089-z. [DOI] [PubMed] [Google Scholar]
- 27.Brown K T, Koh B Y, Brody L A. et al. Particle embolization of hepatic neuroendocrine metastases for control of pain and hormonal symptoms. J Vasc Interv Radiol. 1999;10(4):397–403. doi: 10.1016/s1051-0443(99)70055-2. [DOI] [PubMed] [Google Scholar]
- 28.Diamandidou E, Ajani J A, Yang D J. et al. Two-phase study of hepatic artery vascular occlusion with microencapsulated cisplatin in patients with liver metastases from neuroendocrine tumors. AJR Am J Roentgenol. 1998;170(2):339–344. doi: 10.2214/ajr.170.2.9456942. [DOI] [PubMed] [Google Scholar]
- 29.Dominguez S, Denys A, Madeira I. et al. Hepatic arterial chemoembolization with streptozotocin in patients with metastatic digestive endocrine tumours. Eur J Gastroenterol Hepatol. 2000;12(2):151–157. doi: 10.1097/00042737-200012020-00004. [DOI] [PubMed] [Google Scholar]
- 30.Drougas J G, Anthony L B, Blair T K. et al. Hepatic artery chemoembolization for management of patients with advanced metastatic carcinoid tumors. Am J Surg. 1998;175(5):408–412. doi: 10.1016/s0002-9610(98)00042-7. [DOI] [PubMed] [Google Scholar]
- 31.Eriksson B K, Larsson E G, Skogseid B M, Löfberg A M, Lörelius L E, Oberg K E. Liver embolizations of patients with malignant neuroendocrine gastrointestinal tumors. Cancer. 1998;83(11):2293–2301. [PubMed] [Google Scholar]
- 32.Hajarizadeh H, Ivancev K, Mueller C R, Fletcher W S, Woltering E A. Effective palliative treatment of metastatic carcinoid tumors with intra-arterial chemotherapy/chemoembolization combined with octreotide acetate. Am J Surg. 1992;163(5):479–483. doi: 10.1016/0002-9610(92)90392-5. [DOI] [PubMed] [Google Scholar]
- 33.Ho A S, Picus J, Darcy M D. et al. Long-term outcome after chemoembolization and embolization of hepatic metastatic lesions from neuroendocrine tumors. AJR Am J Roentgenol. 2007;188(5):1201–1207. doi: 10.2214/AJR.06.0933. [DOI] [PubMed] [Google Scholar]
- 34.Loewe C, Schindl M, Cejna M, Niederle B, Lammer J, Thurnher S. Permanent transarterial embolization of neuroendocrine metastases of the liver using cyanoacrylate and lipiodol: assessment of mid- and long-term results. AJR Am J Roentgenol. 2003;180(5):1379–1384. doi: 10.2214/ajr.180.5.1801379. [DOI] [PubMed] [Google Scholar]
- 35.Mavligit G M, Pollock R E, Evans H L, Wallace S. Durable hepatic tumor regression after arterial chemoembolization-infusion in patients with islet cell carcinoma of the pancreas metastatic to the liver. Cancer. 1993;72(2):375–380. doi: 10.1002/1097-0142(19930715)72:2<375::aid-cncr2820720211>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 36.Moertel C G, Johnson C M, McKusick M A. et al. The management of patients with advanced carcinoid tumors and islet cell carcinomas. Ann Intern Med. 1994;120(4):302–309. doi: 10.7326/0003-4819-120-4-199402150-00008. [DOI] [PubMed] [Google Scholar]
- 37.Osborne D A, Zervos E E, Strosberg J. et al. Improved outcome with cytoreduction versus embolization for symptomatic hepatic metastases of carcinoid and neuroendocrine tumors. Ann Surg Oncol. 2006;13(4):572–581. doi: 10.1245/ASO.2006.03.071. [DOI] [PubMed] [Google Scholar]
- 38.Perry L J, Stuart K, Stokes K R, Clouse M E. Hepatic arterial chemoembolization for metastatic neuroendocrine tumors. Surgery. 1994;116(6):1111–1116; discussion 1116–1117. [PubMed] [Google Scholar]
- 39.Roche A, Girish B V, de Baère T. et al. Trans-catheter arterial chemoembolization as first-line treatment for hepatic metastases from endocrine tumors. Eur Radiol. 2003;13(1):136–140. doi: 10.1007/s00330-002-1558-0. [DOI] [PubMed] [Google Scholar]
- 40.Ruszniewski P, Rougier P, Roche A. et al. Hepatic arterial chemoembolization in patients with liver metastases of endocrine tumors. A prospective phase II study in 24 patients. Cancer. 1993;71(8):2624–2630. doi: 10.1002/1097-0142(19930415)71:8<2624::aid-cncr2820710830>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 41.Ruutiainen A T, Soulen M C, Tuite C M. et al. Chemoembolization and bland embolization of neuroendocrine tumor metastases to the liver. J Vasc Interv Radiol. 2007;18(7):847–855. doi: 10.1016/j.jvir.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 42.Strosberg J R, Choi J, Cantor A B, Kvols L K. Selective hepatic artery embolization for treatment of patients with metastatic carcinoid and pancreatic endocrine tumors. Cancer Contr. 2006;13(1):72–78. doi: 10.1177/107327480601300110. [DOI] [PubMed] [Google Scholar]
- 43.Therasse E, Breittmayer F, Roche A. et al. Transcatheter chemoembolization of progressive carcinoid liver metastasis. Radiology. 1993;189(2):541–547. doi: 10.1148/radiology.189.2.7692465. [DOI] [PubMed] [Google Scholar]
- 44.Varker K A, Martin E W, Klemanski D, Palmer B, Shah M H, Bloomston M. Repeat transarterial chemoembolization (TACE) for progressive hepatic carcinoid metastases provides results similar to first TACE. J Gastrointest Surg. 2007;11(12):1680–1685. doi: 10.1007/s11605-007-0235-7. [DOI] [PubMed] [Google Scholar]
- 45.Wängberg B, Westberg G, Tylén U. et al. Survival of patients with disseminated midgut carcinoid tumors after aggressive tumor reduction. World J Surg. 1996;20(7):892–899; discussion 899. doi: 10.1007/s002689900136. [DOI] [PubMed] [Google Scholar]
- 46.Yao K A, Talamonti M S, Nemcek A. et al. Indications and results of liver resection and hepatic chemoembolization for metastatic gastrointestinal neuroendocrine tumors. Surgery. 2001;130(4):677–682; discussion 682–685. doi: 10.1067/msy.2001.117377. [DOI] [PubMed] [Google Scholar]
- 47.Kim Y H, Ajani J A, Carrasco C H. et al. Selective hepatic arterial chemoembolization for liver metastases in patients with carcinoid tumor or islet cell carcinoma. Cancer Invest. 1999;17(7):474–478. doi: 10.3109/07357909909032856. [DOI] [PubMed] [Google Scholar]
- 48.de Baere T, Deschamps F, Teriitheau C. et al. Transarterial chemoembolization of liver metastases from well differentiated gastroenteropancreatic endocrine tumors with doxorubicin-eluting beads: preliminary results. J Vasc Interv Radiol. 2008;19(6):855–861. doi: 10.1016/j.jvir.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 49.Gaur S K, Friese J L, Sadow C A. et al. Hepatic arterial chemoembolization using drug-eluting beads in gastrointestinal neuroendocrine tumor metastatic to the liver. Cardiovasc Intervent Radiol. 2011;34(3):566–572. doi: 10.1007/s00270-011-0122-1. [DOI] [PubMed] [Google Scholar]
- 50.Dong X D, Carr B I. Hepatic artery chemoembolization for the treatment of liver metastases from neuroendocrine tumors: a long-term follow-up in 123 patients. Med Oncol. 2011;28 01:S286–S290. doi: 10.1007/s12032-010-9750-6. [DOI] [PubMed] [Google Scholar]
- 51.Murthy R, Nunez R, Szklaruk J. et al. Yttrium-90 microsphere therapy for hepatic malignancy: devices, indications, technical considerations, and potential complications. Radiographics. 2005;25 01:S41–S55. doi: 10.1148/rg.25si055515. [DOI] [PubMed] [Google Scholar]
- 52.Murthy R, Kamat P, Nunez R. et al. Yttrium-90 microsphere radioembolotherapy of hepatic metastatic neuroendocrine carcinomas after hepatic arterial embolization. J Vasc Interv Radiol. 2008;19(1):145–151. doi: 10.1016/j.jvir.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 53.Salem R, Thurston K G. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: Technical and methodologic considerations. J Vasc Interv Radiol. 2006;17(8):1251–1278. doi: 10.1097/01.RVI.0000233785.75257.9A. [DOI] [PubMed] [Google Scholar]
- 54.Mahvash A, Zaer N, Shaw C, Chasen B, Avritscher R, Murthy R. Temporary balloon occlusion of the common hepatic artery for administration of yttrium-90 resin microspheres in a patient with patent hepatoenteric collaterals. J Vasc Interv Radiol. 2012;23(2):277–280. doi: 10.1016/j.jvir.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 55.Kennedy A S, Dezarn W A, McNeillie P. et al. Radioembolization for unresectable neuroendocrine hepatic metastases using resin 90Y-microspheres: early results in 148 patients. Am J Clin Oncol. 2008;31(3):271–279. doi: 10.1097/COC.0b013e31815e4557. [DOI] [PubMed] [Google Scholar]
- 56.McStay M K, Maudgil D, Williams M. et al. Large-volume liver metastases from neuroendocrine tumors: hepatic intraarterial 90Y-DOTA-lanreotide as effective palliative therapy. Radiology. 2005;237(2):718–726. doi: 10.1148/radiol.2372041203. [DOI] [PubMed] [Google Scholar]
- 57.King J, Quinn R, Glenn D M. et al. Radioembolization with selective internal radiation microspheres for neuroendocrine liver metastases. Cancer. 2008;113(5):921–929. doi: 10.1002/cncr.23685. [DOI] [PubMed] [Google Scholar]
- 58.Rhee T K, Lewandowski R J, Liu D M. et al. 90Y Radioembolization for metastatic neuroendocrine liver tumors: preliminary results from a multi-institutional experience. Ann Surg. 2008;247(6):1029–1035. doi: 10.1097/SLA.0b013e3181728a45. [DOI] [PubMed] [Google Scholar]
- 59.Kalinowski M, Dressler M, König A. et al. Selective internal radiotherapy with yttrium-90 microspheres for hepatic metastatic neuroendocrine tumors: a prospective single center study. Digestion. 2009;79(3):137–142. doi: 10.1159/000209849. [DOI] [PubMed] [Google Scholar]
- 60.Memon K, Lewandowski R J, Mulcahy M F. et al. Radioembolization for neuroendocrine liver metastases: safety, imaging, and long-term outcomes. Int J Radiat Oncol Biol Phys. 2012;83(3):887–894. doi: 10.1016/j.ijrobp.2011.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saxena A, Chua T C, Bester L, Kokandi A, Morris D L. Factors predicting response and survival after yttrium-90 radioembolization of unresectable neuroendocrine tumor liver metastases: a critical appraisal of 48 cases. Ann Surg. 2010;251(5):910–916. doi: 10.1097/SLA.0b013e3181d3d24a. [DOI] [PubMed] [Google Scholar]
- 62.Kennedy A S, McNeillie P, Dezarn W A. et al. Treatment parameters and outcome in 680 treatments of internal radiation with resin 90Y-microspheres for unresectable hepatic tumors. Int J Radiat Oncol Biol Phys. 2009;74(5):1494–1500. doi: 10.1016/j.ijrobp.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 63.Krenning E P, Kwekkeboom D J, Bakker W H. et al. Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med. 1993;20(8):716–731. doi: 10.1007/BF00181765. [DOI] [PubMed] [Google Scholar]
- 64.Krenning E P Teunissen J J Valkema R deHerder W W deJong M Kwekkeboom D J Molecular radiotherapy with somatostatin analogs for (neuro-)endocrine tumors J Endocrinol Invest 20052811, Suppl International146–150. [PubMed] [Google Scholar]
- 65.McStay M K, Maudgil D, Williams M. et al. Large-volume liver metastases from neuroendocrine tumors: hepatic intraarterial 90Y-DOTA-lanreotide as effective palliative therapy. Radiology. 2005;237(2):718–726. doi: 10.1148/radiol.2372041203. [DOI] [PubMed] [Google Scholar]
- 66.Limouris G S, Chatziioannou A, Kontogeorgakos D. et al. Selective hepatic arterial infusion of In-111-DTPA-Phe1-octreotide in neuroendocrine liver metastases. Eur J Nucl Med Mol Imaging. 2008;35(10):1827–1837. doi: 10.1007/s00259-008-0779-0. [DOI] [PubMed] [Google Scholar]
- 67.Takeuchi I, Ishida H, Suzuki T, Nakada H, Tada M, Idezuki Y. A case of liver metastases of rectal carcinoid successfully treated with hepatic arterial infusion of methotrexate and 5-fluorouracil [in Japanese] Gan To Kagaku Ryoho. 1999;26(12):1929–1932. [PubMed] [Google Scholar]
- 68.Christante D, Pommier S, Givi B, Pommier R. Hepatic artery chemoinfusion with chemoembolization for neuroendocrine cancer with progressive hepatic metastases despite octreotide therapy. Surgery. 2008;144(6):885–893; discussion 893–894. doi: 10.1016/j.surg.2008.08.037. [DOI] [PubMed] [Google Scholar]