Abstract
A new indolizidine alkaloid, named Δ1,6-juliprosopine (1), together with previously known indolizidine analogs (2–6), was isolated from the leaves of Prosopis glandulosa var. glandulosa, collected from Nevada, USA; while two other known indolizidines juliprosopine (6) and juliprosine (7) were isolated from P. glandulosa leaves collected in Texas, USA. The structures of compound 1 and 7 were determined using a combination of NMR and MS techniques. Compound 7 exhibited potent antiplasmodial activity against Plasmodium falciparum D6 and W2 strains with IC50 values of 170 and 150 ng/mL, respectively, while 1 was found to be less active (IC50 values 560 and 600 ng/mL). Both the compounds were devoid of VERO cells toxicity up to a concentration of 23800 ng/mL. The antileishmanial activity of indolizidines was evaluated against Leishmania donovani promastigotes, axenic amastigotes and amastigotes in THP1 macrophage cultures. When tested against macrophage cultures, the tertiary bases (1, 3, 6) were found to be more potent than quaternary salts (2, 5, 7), displayed IC50 values between 0.8–1.7 μg/mL and 3.1–6.0 μg/mL, respectively. In addition, compound 7 showed potent antifungal activity against Cryptococcus neoformans and antibacterial activity against Mycobacterium intracellulare, while 1 was potent only against C. neoformans and weakly active against other organisms.
Keywords: Prosopis glandulosa; Fabaceae; indolizidine alkaloid; Δ1,6-juliprosopine; antimicrobial; antiparasitic
Introduction
Prosopis glandulosa Torrey var. glandulosa (Fabaceae), commonly known as Honey Mesquite, is a medium-sized flowering tree native to the southwestern United States and Mexico [1, 2]. The plant is a folk remedy for dyspepsia, eruptions, eyes, hernias, skin ailments, sore throat and umbilical ailments [3, 4]. Earlier investigations on this plant reported various types of biological activities such as antibacterial [5, 6], antifungal [7], anti-infective and antiparasitic activities [8, 9], which are attributed to piperidinyl indolizidine alkaloids. We have previously reported [8] several potent antiparasitic and antimicrobial diastereoisomeric tertiary and quaternary indolizidine alkaloids from P. glandulosa collected in Nevada. In continuation of our previous investigation, we now have isolated a new tertiary indolizidine alkaloid, named Δ1,6- juliprosopine (1), and large amounts of five known indolizidines (2–6) [8]. In addition, we also have examined a sample of P. glandulosa var. glandulosa, obtained from Texas, which yielded two known indolizidines, namely the tertiary juliprosopine (6) and the quaternary juliprosine (7), and interestingly devoid of compounds 1–5. We herein report the isolation and structure elucidation of 1, as well as the antiplasmodial and antimicrobial activities of 1 and 7, and a detailed antileishmanial activity of isolated compounds (1–3, 5–7) against Leishmania donovani promastigotes, axenic amastigotes and amastigotes in THP1 macrophage cultures.
Materials and Methods
General
Optical rotations were measured in CHCl3 or MeOH using an AUTOPOL IV instrument at ambient temperature. IR spectra were obtained using a Bruker Tensor 27 instrument. NMR spectra were acquired on a Varian Mercury 400 spectrometer (Varian Inc.; Palo Alto, CA) with standard pulse sequences operating at 400 MHz in 1H NMR and 100 MHz in 13C NMR in CDCl3 or CD3OD (Cambridge Isotope Laboratories, Inc.) using the residual solvent as internal standard. TLC was carried out on aluminum oxide IB-F plates (Baker-flex) using CH2Cl2-MeOH-NH3·H2O (8:2:0.1) as solvent. Centrifugal preparative TLC (CPTLC, Chromatotron®, Harrison Research Inc., model 8924) was carried out on 1, 2 and 4 mm alumina (Analtech, Inc.) rotors, using CH2Cl2-MeOH-NH3·H2O as solvent. The compounds were visualized by observing under UV light at 254 or 365 nm, followed by spraying with Dragendorff’s or 1% vanillin-H2SO4 spray reagents. All positive controls for biological experiments are commercially available with declared purity from known vendors.
Plant Material
The leaves of P. glandulosa var. glandulosa were collected from Nevada, USA (voucher # NIX 230506/1/A) in May 2006 and Texas (voucher # 3794/11062008/PRGLM) in June 2008, and were identified by Dr. Vaishali C. Joshi, NCNPR, School of Pharmacy, University of Mississippi. Voucher specimens are deposited at the Herbarium of NCNPR, University of Mississippi.
Extraction and isolation
The air dried powdered leaves of P. glandulosa (Nevada, 1.8 kg) were extracted by percolation (95% EtOH, 2 L × 3, 48 h) and resulted EtOH extracts was evaporated to dryness (273 g). The dried extracts (130 g) was dissolved in aqueous 0.1 N HCl and partitioned successively with n-hexane (1L × 3) followed by CH2Cl2 (600 mL × 3). The aqueous acidic layer was then basified with 0.1 N NH4OH to pH 11 (based on pKa 10.2 of tryptamine), followed by partitioning with CH2Cl2 (500 mL × 4). The pH of the remaining basic layer was then adjusted to 12 by NH4OH, followed by partitioning successively with CH2Cl2 (500 mL × 3) and EtOAc (300 mL × 3). The CH2Cl2 fraction (2.8 g) was subjected to CPTLC, using 4 mm Al2O3 rotor with CH2Cl2-MeOH-NH3 ·H2O as solvent to yield 13 fractions. Further purification by repeated CPTLC afforded compound 1 (37 mg), 2 (60 mg), 3 (53 mg), 4 (35 mg) 5 (94 mg) and 6 (84 mg) (Fig. 4S in Supporting Information). Compounds 2–6 were identified by 1H and 13C NMR spectral data, as well as by direct comparison with authentic samples of prosopilosidine, prosopilosine, isoprosopilosine, isoprosopilosidine and juliprosopine, respectively, previously isolated from the same plant [8].
A sample of P. glandulosa (1 Kg, air dried) leaves collected in Texas was extracted (95% EtOH, 1.5 L × 3, 48 h) and evaporated to dryness (yield 149 g). The EtOH extract was fractionated using acid/base treatment to give alkaloidal enriched fraction (67 g), and then processed according to procedure as described previously [8]. The TLC characteristic of alkaloidal fraction was found to be significantly different to those of the samples from Nevada. CPTLC separation of alkaloidal fraction, using above method, yielded compound 6 (200 mg) and juliprosine (7; 225 mg). The identity of compound 6 was established by 1H and 13 NMR spectral data and direct comparison with an authentic sample of juliprosopine.
Δ1,6-juliprosopine (1)
Colorless gum; [α]20D +6.9 (MeOH, c 0.5), Rf 0.63 [Al2O3/CH2Cl2-MeOH-NH3·H2O (96:4:0.1)]; UV (CH3OH) λmax (log ε) 287.0 (2.81), 204.5 (3.48); IR (film) νmax, cm−1 3270 (OH, NH), 2924, 2852, 1462 (C-H) and 1657 (C=N); 1H and 13C NMR, see table 1; HRESIMS m/z 626.5628 [M + H]+ (calcd for C40H71N3O2, 626.5624).
Table 1.
1H and 13C NMR Data with HMBC correlation (J values in Hz, in parenthesis) for 1.
| Position | δH | δCa | HMBC (H-C) | Position | δH | δCa | HMBC (H-C) |
|---|---|---|---|---|---|---|---|
| 2, 2′ | 3.53 br s | 56.1 d | C-4/4′, C-6/6′, C-7/7′ | 9‴ | 1.40-1.2b | 27.1 t | C-6‴′, C-10‴ |
| 3, 3′ | 3.83 br s | 66.3 d | C-4/4′, C-5/5′, C-7/7′ | 10″ | 1.94 t (7) | 35.1 t | C-9″, C-5‴′, C-6‴′, C-7‴′ |
| 4, 4′ | 1.82 m, 1.73m | 25.6 t | C-3/3′, C-6/6′ | 10‴ | 1.4-1.2b | 27.9 t | -b |
| 5, 5′ | 2.27m, 2.31m | 29.5 t | C-6/6′, C-3/3′, C-1‴ | 1‴′ | 1.4-1.15 b m | 33.1 t | C-3‴′, C-8‴′, C-8a‴′ |
| 6, 6′ | - | 171.1 s | - | 2‴′ | 1.83 m; 1.73 m | 21.4 t | C-1‴′, C-8a‴′ |
| 7, 7′ | 1.23b | 17.5 q | C-2/2′, C-3/3′ | 3‴′ | 3.15 m, 2.10 m | 54.5 t | C-1‴′, C-5‴′, C-8a‴′ |
| 1″, 1‴ | 2.15 t (7.2) | 40.7 t | C-6/6′, C-5/5′ | 5‴′ | 3.28 d (15.2) 2.60 d (15.2) |
55.2 t | C-3‴′, C-6‴′, C-7‴′, C-8a‴′, C-10″ |
| 2″-8″, 2‴-8‴ | 1.40-1.2 b | 29.3, 29.4 (x 2), 29.5 (x 2), 29.6, 30.1 (all triplets) | -b | 6‴′ | - | 136.0 s | - |
| 7‴′ | 5.32 s | 123.9 d | C-5‴′, C-8‴′, C-8a‴′, C-10″ | ||||
| 8‴′ | 1.99 m | 42.5 d | C-6‴′, C-7‴′, C-8a‴′ | ||||
| 9″ | 27.1 t | C-10″, C-8‴′ | 8a‴′ | 1.77 m | 65.5 d | - |
Spectra recorded in CDCl3 at 400 MHz (1H) and 100 MHz (13C).
Multiplicities were determined by DEPT and HSQC experiments.
Overlapped signals.
Juliprosine (7)
Colorless gum; [α]20D +5 (CHCl3, c 0.5) [Lit. 21: [α]D 11, CHCl3], Rf 0.22 [Al2O3/CH2Cl2-MeOH-NH3·H2O (96:4:0.1)]; UV (CH3OH) λmax (log ε) 277 (3.81), 226 (3.79), 222 (3.85); IR (film) νmax, cm−1 3291 (OH, NH), 2925, 2853, 1458 (C-H), and 1506 (C-C); The 1H and 13C NMR spectral data were in agreement with those reported previously by Daetwyler et al. [21], HRESIMS: m/z 628.5854 [M]+ (calcd. for C20H27O4, m/z 628.5854).
Biological activity
Antileishmanial assay
The in vitro antileismanial assay was done on a culture of Leishmania donovani promastigotes and axenic amastigotes by Alamar Blue assay [13]. In a 96 well microplate the samples with appropriate dilution were added to the leishmania promastigotes/axenic amastigote culture (2×106 cell/mL). The compounds were tested in duplicates at six concentrations ranging from 40 to 0.0128 μg/mL. The plates were incubated at 26 °C for 72 hours (37 °C for amastigote) and growth of leishmania promastigotes/amastigote was determined. IC50 and IC90 values were computed from the dose response curves. The in vitro amastigote assay in macrophages is described in Fig. 5S, Supporting Information Section. The compounds were also tested against VERO cells and PMA transformed THP1 cells by Neutral Red assay [22].
Antiplasmodial assay
The in vitro antiplasmodial activity, as described previously [8], was measured by a colorimetric assay that determines the parasitic lactate dehydrogenase (pLDH) activity [14]. All experiments were carried out in duplicates.
Antimicrobial assay
All microorganisms were obtained from the ATCC (Manassas, VA) and include the fungi Candida albicans (ATCC 90028), C. krusei (ATCC 6258), C. glabrata (ATCC 90030), Cryptococcus neoformans (ATCC 90113), and Aspergillus fumigatus (ATCC 204305) and the bacteria Staphylococcus aureus (ATCC 29213), methicillin-resistant S aureus (MRSA, ATCC 33591), and Mycobacterium intracellulare (ATCC 23068). For all organisms, excluding M. intracellulare and A. fumigatus, susceptibility testing was performed using a modified version of the CLSI (formerly NCCLS) methods [15, 16] and optical density was used to monitor growth. Media supplemented with 5% Alamar Blue (BioSource International, Camarillo, CA) was utilized for growth detection of M. intracellulare [17] and A. fumigatus [18]. Concentrations that afford 50% inhibition (IC50s) relative to controls were calculated using XLfit 4.2 software (IDBS, Alameda, CA) using fit model 201 based on duplicate readings. Minimum fungicidal or bactericidal concentrations were determined by removing 5 μL from each clear well, transferring to agar, and incubating until growth was seen. Drug controls [Ciprofloxacin (ICN Biomedicals, Ohio, 99.3% purity) for bacteria and Amphotericin B (ICN Biomedicals, Ohio, 94.8% purity) for fungi] were included in each assay.
Results and Discussion
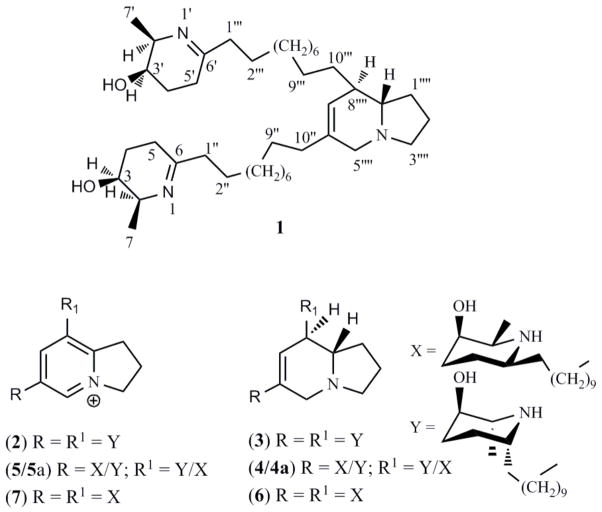
Compound 1 (colorless gum) was analyzed for a molecular ion of m/z 626.5628 [M+H]+ (calcd. 626.5624) by HRESIMS for C40H71N3O2. The IR (film) spectrum showed the presence of OH/NH group(s) (νmax 3270 cm−1) and C=N (νmax 1657 cm−1) absorption bands. The NMR spectra of 1 revealed the presence of a 6,7-dehydroindolizidine nucleus [10, 11, 19, 20] consisting of four triplets, three doublets, and a singlet (Table 1, Fig. 2S in supporting information). In addition, NMR spectra displayed oxymethine, methine adjacent to nitrogen, methylene, and methyl signals at δC 66.3, 56.1, 25.6, 29.5, and 17.5, respectively, assigned to two piperidinyl rings, as well as 10 methylenes for two decanyl moieties. Comparison of NMR spectra of 1 with spectra of other dehydroindolizidines such as juliprosopine [8, 12] suggested close similarities with that of juliprosopine (6). However, there is only one notable difference for the piperidinyl rings of 1 (δC 56.1, 66.3, 25.6, 29.5, 171.1, and 17.5; C-2/2′-C-7/7′) with that of juliprosopine (δC 57.2, 67.8, 26.1, 25.8, 55.8, and 18.4; C-2/2′-C-7/7′) at C-6/6′. The downfield value observed for C-6/6′ (δC 171.1, quaternary carbon) when compared with juliprosopine (δC 55.8, tertiary carbon) suggested the presence of a double bond between 1 and 6 position (nitrogen and carbon), which allowed us to conclude that the piperidinyl ring was dehydrogenated at this position. The HMBC experiment of 1 (Table 1) showed correlations between H-7‴′ and C-5‴′, C-8‴′, C-8a‴′, and C-10″; H-10″ and C-5‴′, C-6‴′, and C-7‴′; H-8‴ and C-6‴′, C-7‴′, and C-8a‴′ confirming a 6,8-dialkylated 6,7-dehydroindolizidine nucleus of juliprosopine (6). The NOESY experiment showed correlation between H-2/2′ and H-3/3′, suggesting that these protons were cis and on the α-face of the molecule, thereby the C-2/2′ methyl group and C-3/3′ OH group were on the β face (3R) of the molecule, hence two 2β-methyl-3β-hydroxy-6-decanylpiperidine moiety with the same configuration for both ring was confirmed. On the basis of above discussion, the structure of 1 was deduced as shown (Figure 1), and it was named as Δ1,6-juliprosopine.
Figure 1.
Compounds (1–7) isolated from P. glandulosa.
During the course of isolation of 1, five other indolizidines 2-6 (Fig. 1) were isolated from P. glandulosa from Nevada. Their structures were confirmed by NMR [8] and direct comparison with authentic samples (available in our laboratory) as prosopilosidine (2), prosopilosine (3), isoprosopilosine (4), isoprosopilosidine (5) and juliprosopine (6). Examination of EtOH extracts of P. glandulosa leaves, collected from Texas, showed weak in vitro antiinfective and antiparasitic activities. Its alkaloidal profile, by TLC, was found to be substantially different with those observed for Nevada sample. This prompted us to carry out an acid/base fractionation. The bioactivity of the EtOH extract was significantly increased in the alkaloid enriched CH2Cl2 fraction. A CPTLC of the CH2Cl2 fraction resulted in the isolation of compounds 6 [8] and 7 [21], in the yields of 0.044% and 0.050%, respectively. The structure of compound 7 was confirmed by comparing its physical and spectral data (1H and 13C NMR and MS) with those reported previously for juliprosine [21].
Δ1,6-juliprosopine (1) and juliprosine (7) were tested for in vitro antiplasmodial, antileishmanial, antibacterial and antifungal activities. The dihydroindolizinium salt (7) exhibited potent antiplasmodial activity and high SI (Selectivity Index) (Table 2) against P. falciparum (IC50 values of 170 and 150 ng/mL, against chloroquine sensitive (D6) and chloroquine resistant (W2) strains, respectively). The activity of 7 was found to be similar to those of chloroquine (IC50= 150 and 140 ng/mL) against P. falciparum W2 strain. However, the antiplasmodial activity of 7 was found to be less potent compared to its diastereoisomer prosopilosidine (2), previously isolated from same plant [8]. On the other hand, the dehydroindolizidine base Δ1,6-juliprosopine (1) was less potent (IC50 = 560 and 600 ng/mL against D6 and W2 strains) than 7 and juliprosopine (6) [8], with no toxicity against mammalian VERO cells like 7, and less toxic than 6 (IC50 = >23800 ng/mL for both 1 and 7 vs. 5000 ng/mL for 6). Therefore, 2 and 7 exhibited higher SI’s against P. falciparum D6 and W2 strains (SI >610, >250 and SI >140, >159, respectively) than 1 and 6 (SI >43, 23 and SI 40, 13 respectively), confirming our previous observation that the quaternary alkaloids (i.e., 2, 7) are better candidates than the tertiary bases (i.e., 1, 6) for further antiplasmodial studies.
Table 2.
Antiplasmodial activities of 1 and 7 and two analogues.
| Compound | P. falciparum (ng/mL) | VERO (ng/mL) | |||
|---|---|---|---|---|---|
| D6a | W2b | IC50 | |||
| IC50 | SIc | IC50 | SIc | ||
| 1 | 560±5 | >43 | 600±5 | >40 | >23800 |
| 2d | 39±2 | >610 | 95±2 | >250 | >23800 |
| 6d | 220±25 | 23 | 380±50 | 13 | 5000 |
| 7 | 170±35 | >140 | 150±60 | >159 | >23800 |
| Chloroquine | 17 | 140 | >23800 | ||
| Artemisinin | 16 | 17 | >23800 | ||
Chloroquine-sensitive clone;
Chloroquine-resistant clone;
Selectivity index= IC50 VERO cells/IC50 P. falciparum;
Data reported previously [8], and used here for comparison with 1 and 7.
The antileishmanial activities of tertiary (1, 3, 6) and quaternary (2, 5, 7) indolizidines were evaluated against promastigotes and axenic amastigotes [13], and amastigotes in macrophage cultures of L. donovani (Table 3). The promastigotes/amastigotes activity of 3 and 6 were found to be equally potent to standard drug pentamidine (IC50= 0.18/1 and 0.3/2 μg/mL for 3 and 6 vs. 0.8/2.1 μg/mL for pentamidine). However, the quaternary salts (2, 5 and 7) were found to be less potent, but also less toxic towards VERO cells than tertiary bases (3 and 6). Interestingly, the presence of a C-1,2 double bond in both piperidine rings, as observed in 1, retained potent antileishmanial activity (IC50= 0.8 and 1.8 μg/mL) against both L. donovanii promastigotes and axenic amastigotes, respectively, while decreased its VERO toxicity, compared to 3 and 6. When tested against L. donovanii in THP1 macrophage cultures, the tertiary bases (1, 3, 6) were found to be more potent than quaternary salts (2, 5, 7), displayed IC50 values between 0.8-1.7 μg/mL vs. 3.1–6.0 μg/mL. The activities of tertiary bases in THP1 were also superior to those of pentamidine (IC50 = 3.0 μg/mL).
Table 3.
Antileishmanial activities of P. glandulosa extracts and compounds 1–7.
| Extract/Compound | Leishmania donovani | Cytotoxicity (Transformed THP1 cells) | ||||||
|---|---|---|---|---|---|---|---|---|
| Promastigotes | Axenic Amastigotes | Macrophage-Amastigotes | ||||||
| IC50 | IC90 | IC50 | IC90 | IC50 | IC90 | IC50 | IC90 | |
| P. glandulosa (from Nevada, EtOH extract) | 44.2 ± 7.3 | 91.4 ± 5.2 | NT | NT | NT | NT | NT | NT |
| P. glandulosa (from Texas, EtOH extract) | 15.4 ± 2.1 | 31.9 ± 3.5 | NT | NT | NT | NT | NT | NT |
| P. glandulosa (From Nevada, alkaloid fr.) | 18.7 ± 1.9 | 34.7 ± 2.2 | NT | NT | NT | NT | NT | NT |
| 1 | 0.83 ± 0.11 | 1.67 ± 0.21 | 1.83 ± 0.24 | 5.89 ± 0.76 | 1.67 ± 0.23 | 2.48 ± 0.62 | 8.47 ± 1.32 | >20 |
| 2 | 0.91 ± 0.17 | 4.17 ± 0.54 | 2.08 ± 0.30 | 25.4 ± 5.3 | 3.23 ± 0.54 | 8.74 ± 1.33 | 9.49 ± 2.25 | >20 |
| 3 | 0.17 ± 0.05 | 6.33 ± 1.07 | 0.98 ± 0.12 | 6.49 ± 0.84 | 1.08 ± 0.21 | 1.65 ± 0.22 | 2.33 ± 0.51 | 3.89 ± 0.51 |
| 5 | 1.22 ± 0.21 | 4.52 ± 0.74 | 2.14 ± 0.32 | 24.8 ± 3.7 | 5.87 ± 0.76 | 9.49 ± 1.14 | 7.92 ± 1.22 | 19 |
| 6 | 0.29 ± 0.05 | 1.14 ± 0.21 | 1.92 ± 0.19 | 6.25 ± 0.47 | 0.79 ± 0.11 | 1.34 ± 0.24 | 3.43 ± 0.32 | 7.89 ± 1.2 |
| 7 | 1.38 ± 0.23 | 5.85 ± 0.72 | 2.58 ± 0.37 | 29.8 ± 4.3 | 3.08 ± 0.43 | 7.89 ± 1.91 | 19.5 ± 1.0 | >20 |
| Pentamidine | 1.06 ± 0.32 | 3.86 ± 0.41 | 2.09 ± 0.37 | 14.7 ± 2.2 | 2.97 ± 0.44 | 6.87 ± 2.11 | >20 | >20 |
| Amphotericin B | 0.09 ± 0.01 | 0.24 ± 0,04 | 0.32 ± 0.05 | 0.97 ± 0.14 | 0.14 ± 0.02 | 1.74 ± 0.22 | 3.34 ± 0.41 | 8.05 ± 1.33 |
The results (IC50/IC90) are presented as μg/mL. NT= not tested. Values are mean ± S.D. of three observations.
Finally, compound 7 showed potent in vitro antifungal activity against C. neoformans, and antibacterial activity against M. intracellulare with IC50/MIC values of 0.17/0.63, and 0.80/1.25 μg/mL, and 1 against C. neoformans (IC50/MIC = 0.66/1.25 μg/mL, respectively) (Table 4). The minimum fungicidal concentration (MFC) of 7 against C. neoformans was equipotent to amphotericin B. Both the compounds showed weak activities against S. aureus and MRSA, while 1 also displayed weak activity against C. albicans, C. krusei and M. intracellulare. The antifungal activity of a mixture of juliprosine (7) and isojuliprosine were reported previously against various pathogenic fungi [7], however juliprosine had not been reported previously for biological activity as a single pure compound.
Table 4.
Antimicrobial activity of 1 and 7.
| Compound | IC50/MIC/MFC or MBC (μg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| C. albicans | C. glabrata | C. krusei | C. neoformans | A. fumigatus | S. aureus | MRSA | M. intracellulare | |
| 1 | 6.7/20/20 | -/-/- | 5.4/10/10 | 0.66/1.25/1.25 | -/-/- | 5.58/10/20 | 5.36/10/10 | 9.72/10/20 |
| 7 | -/-/- | -/-/- | -/-/- | 0.17/0.63/0.63 | -/-/- | 3.0/5.0/10 | 3.03/5.0/10 | 0.80/1.25/5 |
| Amphotericin B | 0.13/0.63/0.63 | 0.14/0.63/0.63 | 0.54/1.25/1.25 | 0.32/1.25/1.25 | 0.72/1.25/2.5 | NT | NT | NT |
| Ciprofloxacin | NT | NT | NT | NT | NT | 0.1/0.5/- | 0.08/0.25/1 | 0.25/0.5/- |
IC50 is the concentration that affords 50% inhibition of growth; MIC (minimum inhibitory concentration) is the lowest test concentration that allows no detectable growth; MFC/MBC (minimum fungicidal/bactericidal concentration) is the lowest test concentration that kills the organism; NT = Not tested; (-) = Not active at the highest test concentration of 20 μg/mL.
This appears to be the first report of Δ1,6-juliprosopine (1) from either a natural or synthetic sources. Compound 1 is a new addition to our previously reported indolizidines (2–6) [8] from P. glandulosa, collected in Nevada. During this investigation, we also analyzed P. glandulosa sample from Texas, which afforded only 6 and 7 and devoid of indolizidines 1–5. This work suggests that the qualitative and quantitative nature of the bioactive alkaloidal profile in P. glandulosa varies significantly due to geographical location.
Supplementary Material
Acknowledgments
The authors sincerely thank Dr. Mark Blumenthal, American Botanical Council, and Mr. Elray Nixon for supplying plant material from Texas and Nevada, respectively, Dr. Bharathi Avula, NCNPR, for recording mass spectra, and Ms. Marsha Wright and Mr. John Trott for assistance in biological work. This work was supported by US Department of Defense CDMRP grant # W81XWH-09, and in part by USDA Agricultural Research Service Specific Cooperative Agreement No. 58-6408-2-0009 and NIH, NIAID, Division of AIDS, Grant No. AI 27094.
Footnotes
Part III. For part II, see Samoylenko et al., (2009).
The isolation and purification of compounds, 1H, 13C NMR and HMBC spectra for compound 1, and antileishmanial macrophage amastigote assay are available in Supporting Information.
References
- 1.Hilu YW, Boyd S, Felker P. Morphological diversity and taxonomy of California mesquites (Prosopis, Lepminosae) Madrono. 1982;29:237–254. [Google Scholar]
- 2.Burkhart A. A monograph of the genus Prosopis. J Arnold Arboretum. 1976;57:450–525. [Google Scholar]
- 3.Kay MA. Healing with Plants in the American and Mexican West. Tuscon: The University of Arizona Press; 1996. pp. 221–224. [Google Scholar]
- 4.Powell AM. Trees and shrubs of Trans-Pecos and adjacent areas. Austin: The University of Texas Press; 1998. pp. 177–179. [Google Scholar]
- 5.Kanthasamy A, Subramanian S, Govindasamy S. Bactericidal and fungicidal effects of Prosopis juliflora alkaloidal fraction. Indian Drugs. 1989;26:390–394. [Google Scholar]
- 6.Ahmad A, Khan KA, Ahmad VU, Qazi S. Antibacterial Activity of Juliflorine Isolated from Prosopis juliflora. Planta Medica. 1986;52:285–288. [PubMed] [Google Scholar]
- 7.Ahmad A, Khursheed AK, Sabiha Q, Viqaruddin A. Antifungial activity of some hydrosoluble Prosopis juliflora alkaloids. Fitoterapia. 1989;60:86–89. [Google Scholar]
- 8.Samoylenko VP, Ashfaq MK, Jacob MR, Tekwani BL, Khan SI, Manly SP, Joshi VC, Walker LA, Ilias M. Indolizidine, antiinfective and antiparasitic compounds from Prosopis glandulosa var. glandulosa. J Nat Prod. 2009;72:92–98. doi: 10.1021/np800653z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bero J, Quetin-Leclercq J. Natural products published in 2009 from plants traditionally used to treat malaria. Planta Medica. doi: 10.1055/s-0030-1250405. advance online publication 10 October 2010. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad VU, Sultana A, Qazi S. Alkaloids from the leaves of Prosopis juliflora. J Nat Prod. 1989;52:497–501. [Google Scholar]
- 11.Samoylenko VP, Dunbar DC, Jacob MR, Joshi VC, Ashfaq MK, Ilias M. Two new alkylated piperidine alkaloids from western honey mesquite: Prosopis glandulosa Torr. var. torreyana . Nat Prod Commun. 2008;3:35–40. [Google Scholar]
- 12.Ahmad VU, Qazi SJ. Studies on the structure of Julifloricine. J Chem Soc Pak. 1985;7:347–350. [Google Scholar]
- 13.Mikus J, Steverding D. A simple colorimetric method to screen drug cytotoxicity against Leishmania using the dye Alamar Blue. Parasitol Int. 2000;48:265–269. doi: 10.1016/s1383-5769(99)00020-3. [DOI] [PubMed] [Google Scholar]
- 14.Makler MT, Ries JM, Williams JA, Bancroft JE, Piper RC, Gibbins BL, Hinrichs DJ. Parasite lactate dehydrogenase as an assay for Plasmodium falciparum drug sensitivity. Am J Trop Med Hyg. 1993;48:739–741. doi: 10.4269/ajtmh.1993.48.739. [DOI] [PubMed] [Google Scholar]
- 15.NCCLS. Approved standard. 2. Vol. 22. National Committee for Clinical Laboratory Standards; 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts; pp. 1–51. [Google Scholar]
- 16.NCCLS. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. NCCLS Document M7-A5 National Committee for Clinical Laboratory Standards. 2000;20:1–58. [Google Scholar]
- 17.NCCLS. Susceptibility testing of mycobacteria, nocardia, and other aerobic actinomycetes. Tentative standard M24-T2. National Committee for Clinical Laboratory Standards. (2) 2000;20:1–81. [PubMed] [Google Scholar]
- 18.NCCLS. Reference method for broth dilution antifungal susceptibility testing of conidium forming filamentous fungi. Proposed standard M38-P National Committee for Clinical Laboratory Standards. 1998;18:1–39. [Google Scholar]
- 19.Ott-Longoni R, Viswanathan N, Hesse M. Die konstitution des alkaloides juliprosopin aus Prosopis juliflora A. Dc. 176. Mitteilung über organische. Naturstoffe Helv Chim Acta. 1980;7:2119–2129. [Google Scholar]
- 20.Snider BB, Neubert BJ. Syntheses of ficuseptine, juliprosine, and juliprosopine by biomimetic intramolecular chichibabin pyridine syntheses. Org Lett. 2005;7:2715–2718. doi: 10.1021/ol050931l. [DOI] [PubMed] [Google Scholar]
- 21.Daetwyler P, Ott-Longoni R, Schoepp E, Hesse M. Uber Juliprosin, ein weiteres Alkaloid aus Prosopis juliflora A. DC Helv Chim Acta. 1981;64:1959–1963. [Google Scholar]
- 22.Babich H, Borenfreund E. Cytotoxicity of T2 toxin and its metabolites with the neutral red cell viability assay. App Envt Microbiol. 1991;57:2101–2103. doi: 10.1128/aem.57.7.2101-2103.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.