Abstract
Tritrichomonas foetus (TF) is a protozoan that infects the feline ileum and colon resulting in chronic diarrhea. Up to 30% of young purebred cats are infected with TF and the infection is recognized as pandemic. Only a single drug, characterized by a narrow margin of safety and emerging development of resistance, is effective for treatment. While the venereal pathogenicity of bovine TF is attributed to adherence to uterovaginal epithelium, the pathogenesis of diarrhea in feline TF infection is unknown. The aim of this study was to establish an in vitro model of feline TF adhesion to intestinal epithelium. Confluent monolayers of porcine intestinal epithelial cells (IPEC-J2) were infected with axenic cultures of feline TF that were labeled with [3H] thymidine or CFSE and harvested at log-phase. The effect of multiplicity and duration of infection, viability of TF, binding competition, formalin fixation and cytoskeletal inhibitors on adherence of feline TF to IPEC-J2 monolayers was quantified by liquid scintillation counting and immunofluorescence. [3H] thymidine and CFSE-labeled TF reproducibly adhered to IPEC-J2 monolayers. Clinical isolates of feline TF adhered to the intestinal epithelium in significantly greater numbers than Pentatrichomonas hominis, the latter of which is a presumably nonpathogenic trichomonad. Adhesion of TF required viable trophozoites but was independent of cytoskeletal activity. Based on saturation and competition binding experiments, adherence of feline TF to the epithelium occurred via specific receptor-ligand interactions. The developed model provides a valuable resource for assessing pathogenic mechanisms of feline TF and developing novel pharmacologic therapies for blocking the adhesion of feline TF to the intestinal epithelium.
Keywords: Tritrichomonas foetus, intestine, epithelium, adhesion, protozoa, diarrhea, model
1. Introduction
Tritrichomonas foetus (TF) is a protozoal pathogen that is recognized internationally as a common cause of colitis in domestic cats. The reported prevalence of TF among U.S. purebred cats is as high as 30% (Gookin et al, 2004) and distribution of the infection is acknowledged to be worldwide. Intestinal trichomonosis is a chronic infection in which symptomatic cats are afflicted by waxing and waning diarrhea (Gookin et al, 2001). Despite periods of clinical remission, it is likely that many cats remain infected for life (Foster et al, 2004). The nitroimidazole antibiotic, ronidazole, is currently the only effective antimicrobial treatment for feline trichomonosis. However, ronidazole has a narrow safety margin in cats and resistant strains have been recognized both clinically and in vitro (Gookin et al., 2010). In addition to a need for safer and more consistently effective drugs for killing TF, little is understood regarding how TF actually causes diarrhea. In cattle where TF infection is responsible for venereal disease, adherence of TF to the uterovaginal epithelium is thought to be a critical first step in TF pathogenicity (Singh et al., 1999). Further, adherence is known to be an essential prerequisite for intestinal colonization and cytopathogenicity by many enteric protozoal organisms including Giardia which uses its ventral adhesive disk to facilitate adherence to the small intestinal epithelium (Céu Sousa et al., 2001). We hypothesized that feline TF similarly adhere to the intestinal epithelium; an event that may be linked to its pathogenic effects and a potential pharmacological target for prevention or amelioration of clinical disease.
In these studies we demonstrate for the first time that feline TF adhere to intestinal epithelial monolayers. This adhesion requires TF viability but is independent of TF cytoskeletal integrity. Adherence of TF occurs via saturable and competitive binding kinetics that suggest specific receptor-ligand interactions with the epithelium. These studies provide supportive evidence that adhesion of feline TF to the intestinal epithelium may be pharmacologically inhibited. The developed model provides a valuable resource for determining the specific mechanisms mediating adhesion of feline TF to the intestinal epithelium and whether or not blockade of adhesion ameliorates the pathogenic effects of TF.
2. Materials and Methods
2.1 IPEC-J2 Cells
The porcine jejunal epithelial cell line (IPEC-J2) is a non-transformed primary cell line originally isolated from neonatal piglet jejunum and was obtained as a gift from Dr. Helen M. Berschneider. IPEC-J2 cells were grown in co-culture media which included Advanced Dulbecco’s minimal essential medium: Nutrient Mixture F-12 (DMEM/F12) supplemented with 5 µg/ml each of insulin, transferrin, and selenium, EGF (5 ng/ml), penicillin (50,000 IU/ml), streptomycin (50,000 mg/ml) and 5% fetal bovine serum and incubated at 37°C in 5% CO2. Prior to adhesion studies, IPEC-J2 cells were seeded onto permeable polycarbonate filters (0.4 µm pore size, 4.67cm2; Corning Incorporated, Lowell, MA) and cultivated until confluent (Transepithelial Electrical Resistance (TER) ≥ 2,000 Ω · 4.67cm2). For microscopic examination of adherent TF, IPEC-J2 cells were seeded onto Laboratory-Tek chamber slides (Nalg Nunc International, Rochester, NY) and grown to confluence over a period of 4 days prior to use. IPEC-J2 cells were used at passage numbers 38–50.
2.2 Trichomonads
Isolation and culture of TF and Pentatrichomonas hominis (PH) isolates were performed as previously described (Gookin et al., 2001). Each isolate was obtained from the feces of a naturally infected cat, cultured in modified Diamond’s media supplemented with antibiotics (penicillin, amphotericin B, streptomycin), and incubated at 37°C. After ≥ 5 passes in this media, axenic cultures were established in antibiotic-free Diamond’s medium. The identity of each isolate was confirmed by polymerase chain reaction (PCR) testing as previously described (Gookin et al., 2002). One P. hominis and six TF (F, A, C, Sti, M, Sta) isolates axenized from seven different naturally infected cats were used for comparative studies of adhesion.
2.3 Labeling of Trichomonads
Trichomonads were harvested in late log-phase of growth and inoculated into modified Diamond’s media containing 4 µCi/ml [3H] thymidine (10 Ci/mmol) (American Radiolabeled Chemicals or GE life sciences) or 2 µCi/ml [methyl-3H] thymidine (25 Ci/mmol) (GE life sciences). After 36 hours, radiolabeled trichomonads were washed three times by means of centrifugation (250×g) and reconstitution with sterile Hank’s Balanced Salt Solution (HBSS) to remove unassociated radioactive tracer. Trichomonads were counted using a hemocytometer and resuspended in IPEC-J2 media at the desired concentration. For fluorescence vital staining of trichomonads, isolates were labeled with Carboxy Fluorescein diacetate, Succinimidyl Ester (CFSE, 5–20 µM) (Life Technologies, Grand Island, NY) at 37°C in the dark. Prior to use, CFSE-labeled trichomonads were washed three times with HBSS to remove unincorporated label.
2.4 Co-culture adhesion assays
For co-culture adhesion assays, [3H] thymidine-labeled trichomonads were added to the apical media of IPEC-J2 cell monolayers grown to confluence on polycarbonate inserts. Co-cultures were incubated at 37°C in 5% CO2. Following adhesion, monolayers were washed twice with sterile HBSS to remove unbound trichomonads. The inserts were then excised using a scalpel blade and placed in 20 ml scintillation vials containing Econo 2 fluid (Fisher Scientific, Pittsburgh, PA). Radioactive emissions were counted using a Wallac 1209 liquid scintillation counter and expressed in counts per minute (CPM). Radioactive emissions measured from serial dilutions of the radiolabeled trichomonads were used for each assay to generate a standard curve of CPM per trichomonad. Number of trichomonads adhered to cell monolayers were calculated by applying the CPM measured to the standard curve.
For adhesion assays using vital stained trichomonads, TF were labeled with CFSE and added to monolayers of IPEC-J2 cells grown to confluence on chamber slides. Unbound trichomonads were removed by washing chamber slides twice with sterile HBSS. The epithelial cells and trichomonads were counterstained with the nuclear stain, DAPI, and adherent trichomonads in individual chamber wells were counted in six high power fields using an epifluorescence microscope. For evaluation of CFSE as a quantitative assay, serial dilutions of CFSE-labeled trichomonads were added to 96 well microplates (Greiner Bio One, Monroe, NC) and read with a fluorescence plate reader (Fluoroskan Ascent FL, MA) using excitation and emission wavelengths of 485 and 538 nm, respectively. Baseline differences in TF adhesion to IPEC-J2 cells between experiments were controlled for by inclusion of an untreated TF control group in each CFSE and radiolabeled TF adhesion assay.
For ultrastructural imaging of adherent TF, trichomonads were added to the apical media of IPEC-J2 cell monolayers grown to confluence on polycarbonate inserts and incubated as previously described. Following co-culture, inserts were gently washed twice with sterile HBSS to remove nonadherent trichomonads. Inserts were fixed in Trump’s 4F:1G fixative at 4°C. Samples were rinsed twice for 15 min each with 1.0 M Sorenson’s phosphate buffer (pH 7.2–7.4) and dehydrated in an ascending series of ethanol (50%, 75%, 95%, and 95%) for 15 min each, culminating in two washes in 100% ethanol for 30 min each. Samples were then dried in a Ladd critical-point dryer. Inserts were cut away and mounted with carbon tape on aluminum specimen stubs, and sputter coated with ~20 nm of gold-palladium using an Anatch Hummer VI sputter coater. Samples were viewed using a JEOL 6360 LV scanning electron microscope.
2.5 Adhesion characterization assays
Trichomonads were treated with the microtubule inhibitor colchicine (250 µM – 1.25 mM, Sigma-Aldrich, St. Louis, MO), the actin polymerization inhibitor cytochalasin B (10.5 µM, Sigma-Aldrich, St. Louis, MO) or their diluents, PBS and 0.5% DMSO for 1 hr at 37°C prior to co-culture. A growth curve of TF was evaluated in the presence of each drug or the appropriate diluent. To assess the cytolytic effect of treatments on TF, radiolabel liberation assays were performed. Following incubation with drug or vehicle for the designated time period, 5×106 TF were placed in a microcentrifuge tube and pelleted at 6000 × g for 2 min. Radioactivity released by the trichomonads was measured in the supernatant and values were compared to positive and negative controls (liquid nitrogen and PBS, respectively). To evaluate the dependence of TF adhesion on trophozoite viability, trichomonads were treated with 1% formalin or diluent (deionized H2O) for 30 seconds prior to co-culture with IPEC-J2 cells for a duration of six hours. Saturation binding of radiolabeled TF to IPEC-J2 monolayers was evaluated 6 hours post-addition of increasing numbers of trichomonads. Competition binding of TF to IPEC-J2 cells in co-culture was performed using a fixed number of radiolabeled TF in the presence of increasing numbers of non-radiolabeled trichomonads.
2.6 Statistical Analysis
Data were analyzed for normality (Kolmogorov-Smirnov) and variance (Levene median) using a statistical software package and tested for significance using parametric or non-parametric tests as appropriate (SigmaStat, Jandel Scientific). Parametric data were analyzed using a Student’s t test or one-way ANOVA. Non-parametric data were analyzed using Mann-Whitney rank sum test or Kruskal-Wallis ANOVA on Ranks. n = number of replicates. Results are reported as mean ± standard deviation. For all analyses, P ≤ 0.05 was considered significant.
3. Results
3.1 Radiolabel and vital staining of feline T. foetus
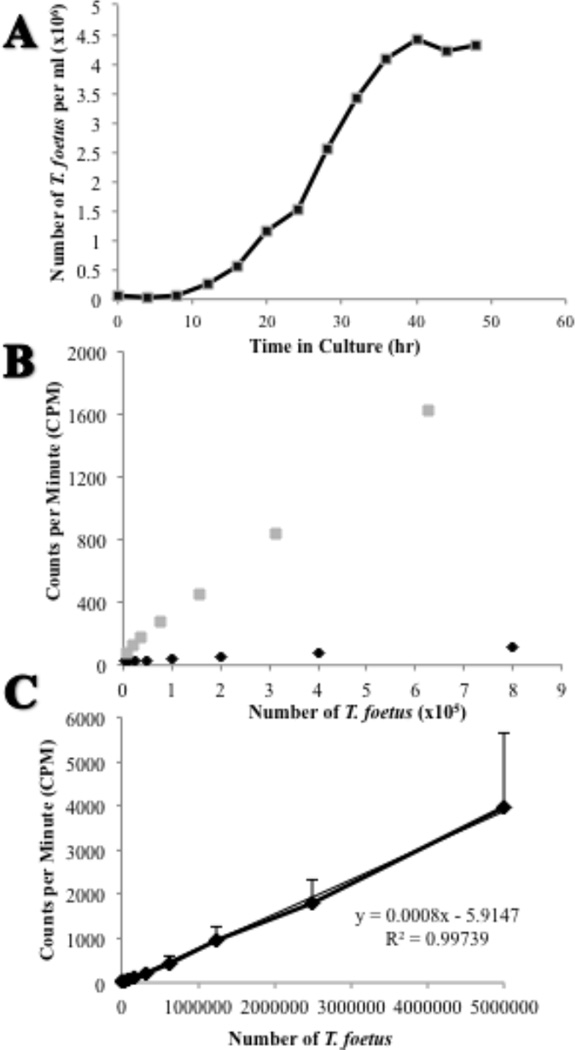
Following inoculation into fresh medium, feline TF (isolate F) exhibited logarithmic growth between 16–36 hrs of culture culminating in a peak concentration of approximately 4.5 × 106 cells/ml at 48 hrs (Fig. 1A). Based on this finding, TF were exposed to radiolabel beginning at the time of inoculation until their time of harvest at 36 hours of growth.
Figure 1. In vitro growth and comparison of radioisotopes for labeling of feline T. foetus.
(A) A typical growth curve of T. foetus following inoculation of 5×104 trichomonads into Advanced Diamond’s media. Each data point represents 3 replicates. (B) Standard dilution curve of TF following radiolabeling with either [3H] thymidine (gray square) or [methyl-3H] thymidine (black diamond). (C) Standard dilution curve of TF labeled with 4 µCi/ml [3H] thymidine. Each data point represents 3 replicates.
Two different radioisotopes were examined for labeling of TF DNA ([methyl-3H] thymidine) or DNA and RNA ([3H] thymidine). Superior labeling of TF was observed with [3H] thymidine compared to [methyl-3H] thymidine (Fig. 1B). Thus, radiolabeling of TF was performed using [3H] thymidine. A standard dilution curve of [3H] thymidine labeled-TF demonstrated a strong positive linear relationship between the number of TF and the radioactive emissions measured (Fig. 1C).
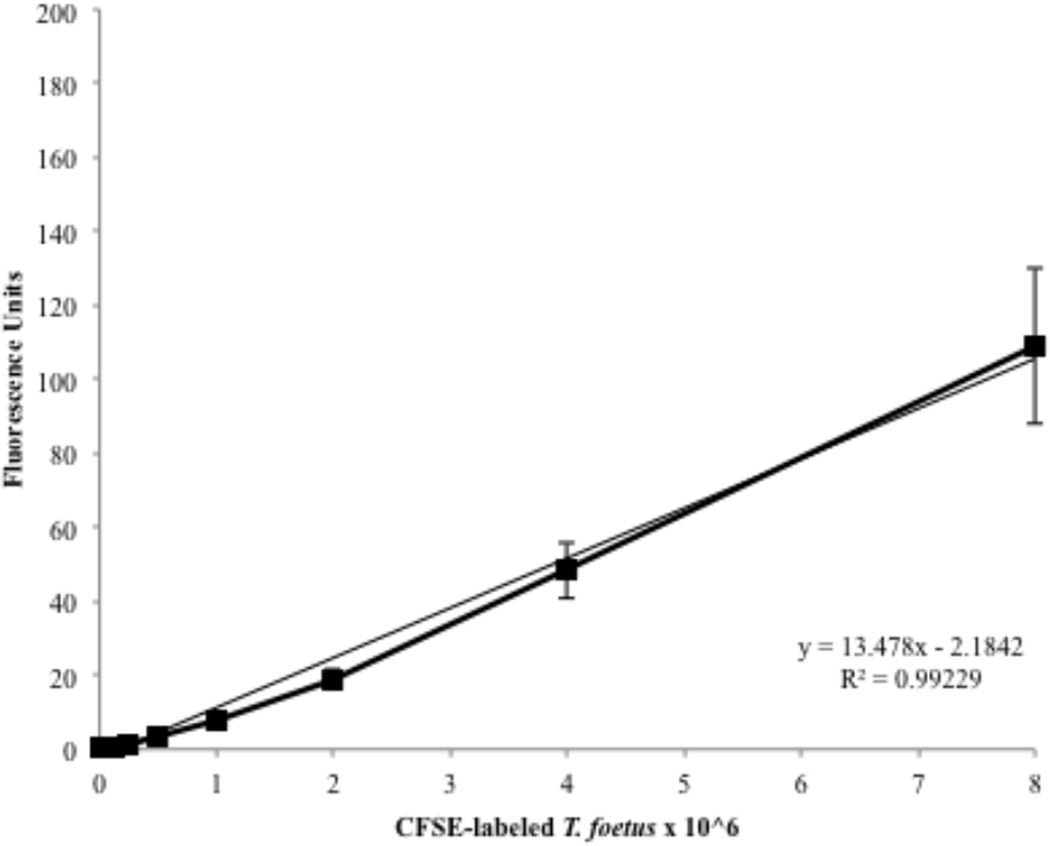
CFSE was examined as a vital stain for direct imaging of TF or as a non-radioactive approach to quantifying numbers of trichomonads. Optimal labeling of TF was achieved using 20 µM CFSE in PBS for a duration of 30 min. Lower concentrations of CFSE or shorter incubation periods of labeling led to weak fluorescence of the trichomonads. Labeling of TF with CFSE demonstrated a positive linear relationship between the number of TF and fluorescence emissions as measured using a fluorometer (Fig. 2). Labeling of TF using either radioisotope or CFSE had no effect on TF viability.
Figure 2. Fluorescence emission of CFSE-stained feline T. foetus.
TF labeled with 20 µM CFSE were serially diluted in 96 well microplates and emissions were recorded using a fluorescence plate reader. Each data point represents 3 replicates.
3.2 Feline T. foetus exhibit saturable adhesion to intestinal epithelial monolayers
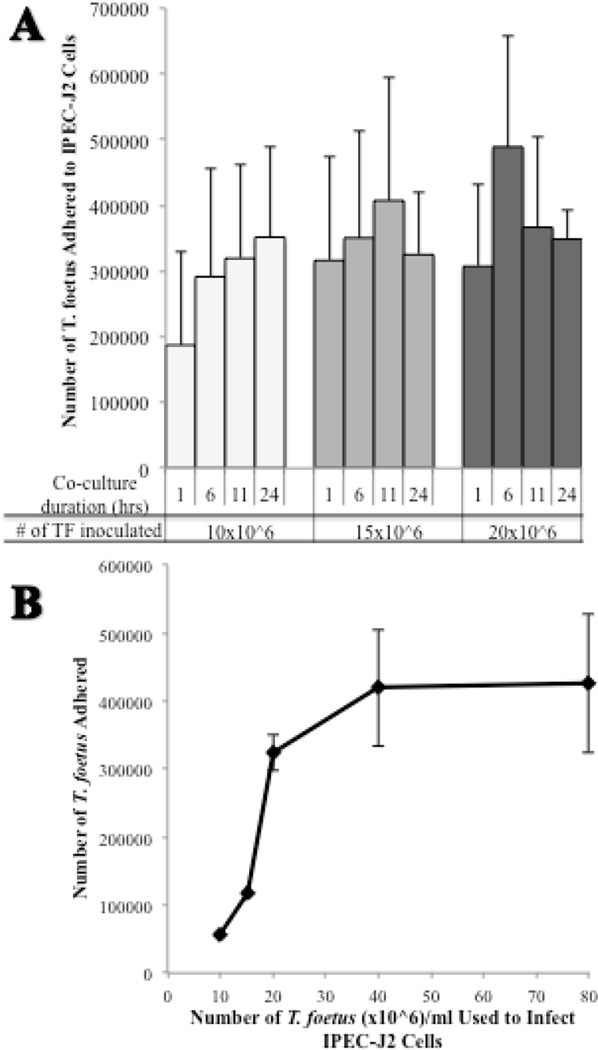
To determine if TF adhere to intestinal epithelium, monolayers of IPEC-J2 cells were infected with increasing numbers of [3H] thymidine labeled-TF and co-cultured for durations of time ranging from 1 to 24 hours. Adhesion was observed at all chosen concentrations of TF with maximum adhesion occurring earlier as the number of infecting TF was increased (Fig. 3A). A 6 hour duration of co-culture was chosen for performance of adhesion assays. Additionally, TF were determined to not significantly proliferate under co-culture conditions over this time period. In order to determine whether adhesion of TF to the intestinal epithelium was saturable, increasing numbers of [3H] thymidine labeled-TF were allowed to infect IPEC-J2 monolayers in co-culture for 6 hours. Adhesion of TF to IPEC-J2 cells was saturable at numbers ≥ 40 × 106 TF (Fig. 3B). An infection inoculum of 20 × 106 TF was identified as a sub-saturating number of TF that would be useful for examining the effect of pharmacological agents on TF adhesion. Infection of IPEC-J2 monolayers with 20 × 106 CFSE-labeled TF for 6 hours resulted in a multiplicity of infection of 2.26 ± 0.57 TF per IPEC-J2 cell.
Figure 3. Effect of time and number of feline T. foetus on adherence to IPEC-J2 cells.
(A) [3H] thymidine-labeled TF were added to IPEC-J2 monolayers at infection doses of 10, 15, and 20 × 106 trichomonads and co-cultured for 1, 6, 11, and 24 hours. Each data point represents 3–4 replicates. (B) The adherence of [3H] thymidine labeled-TF to IPEC-J2 cell monolayers (24 mm2 polycarbonate inserts) was saturable at infecting doses ≥ 40 × 106 TF per ml (highest dose of TF tested was 160 × 106 TF per ml). Each data point represents 3–4 replicates.
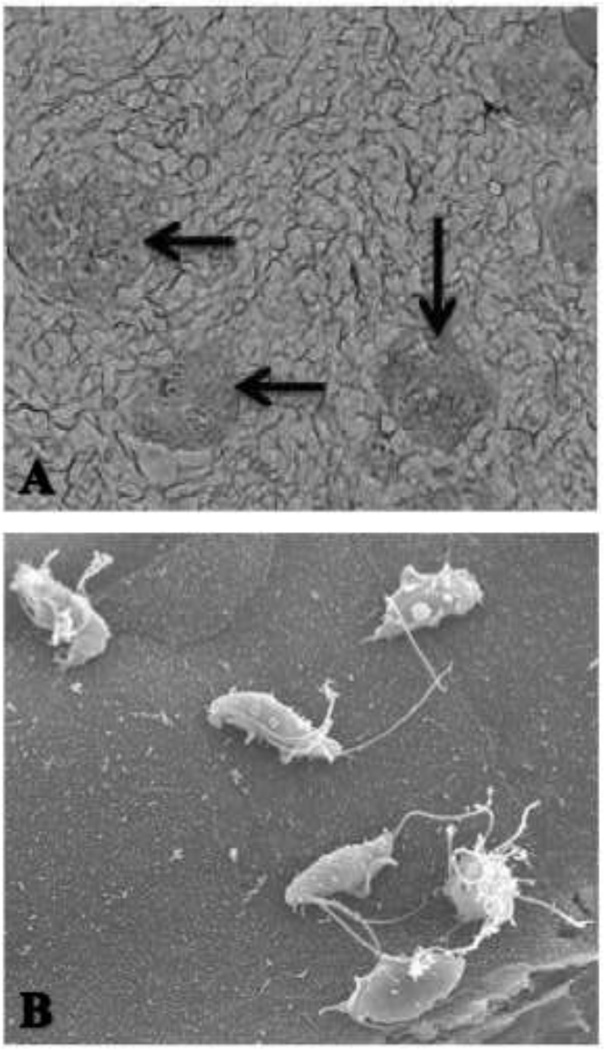
To verify that the estimated change in magnitude of TF adhesion using radiolabeled TF was comparable to direct visual counts, parallel assays of [3H] thymidine versus CFSE labeled-TF adhesion to IPEC-J2 cells were performed. The percent change in number of TF adhered between 1 and 6 hours of infection was comparable between visually counted CFSE-labeled TF (40% ± 12) and the radiolabel-estimated TF (39% ± 12). Examination of TF adhering to IPEC-J2 monolayers by means of scanning electron microscopy (SEM) revealed discrete multifocal regions of intensely infected IPEC-J2 cells adjoined by regions of largely uninfected epithelium (Fig. 4). No apparent breaches in epithelial monolayer continuity were observed by SEM following the six hour co-culture with TF.
Figure 4. Scanning electron microscopy of feline T. foetus adhesion to IPEC-J2 monolayers.
(A). Note the patchy distribution of large aggregates of trichomonads adhering to IPEC-J2 monolayers (35×). (B). Higher magnification of a cluster of six trichomonads adhering to the surface of a single IPEC-J2 cell (2300×).
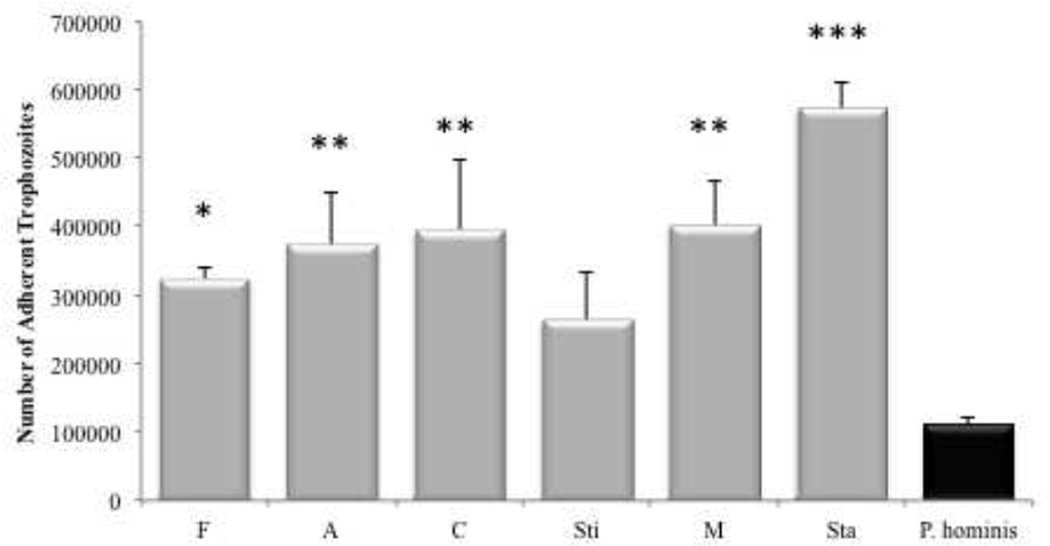
3.3 Isolates of feline T. foetus adhere more robustly to intestinal epithelium compared to feline P. hominis
To determine the magnitude and variability of adhesion among different clinical isolates of feline TF, the adhesion of six isolates of TF were compared. Adherence of TF isolates was also compared to a single clinical isolate of feline P. hominis as this species represents the only other known intestinal trichomonad of cats. All TF isolates adhered in greater numbers (mean ± SE, 389,202 ± 59,868 trophozoites) than P. hominis (109,784 ± 12,325 trophozoites) (Fig. 5). Parallel studies qualitatively examining the adhesion of CFSE-labeled TF and P. hominis to IPEC-J2 cells were in agreement with these findings.
Figure 5. Adhesion characteristics of multiple feline T. foetus and a single feline P. hominis isolate.
All TF isolates adhered in greater numbers to IPEC-J2 cell monolayers than did P. hominis. Each column represents 3–8 replicates per clinical isolate. *p<0.05 **p<0.01***p<0.001 compared to P. hominis (One Way ANOVA and post-hoc Holm-Sidak test).
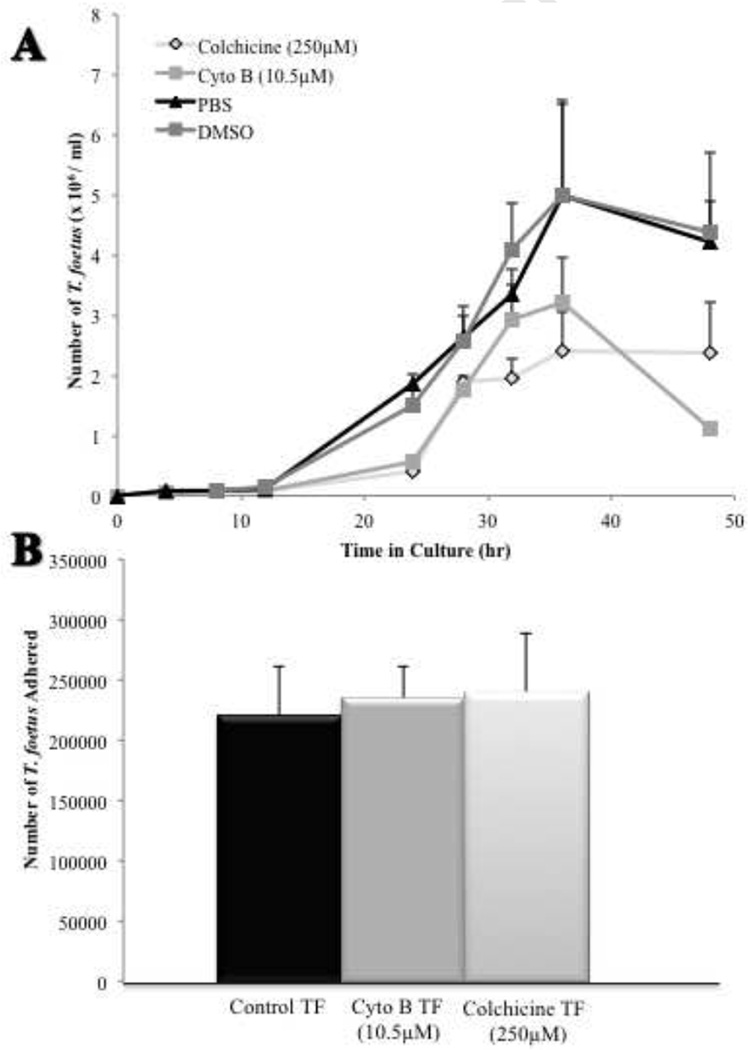
3.4 Adhesion of feline T. foetus to intestinal epithelium does not require cytoskeletal activity
The dependence of TF adhesion on actin filament or microtubule activity was evaluated by exposing trophozoites to cytoskeletal inhibitors prior to infection of IPEC-J2 cells. Incubation of TF in the presence of the actin polymerization inhibitor cytochalasin B (10.5 µM) or the microtubule inhibitor colchicine (250 µM) significantly inhibited replication of the trophozoites (Fig. 6A). Neither inhibitor had any effect on retention of radiolabel by the trophozoites or on adhesion of TF to IPEC-J2 cells (Fig. 6B). Examination of co-cultures using SEM disclosed no discernable effects of the cytoskeletal inhibitors on TF morphology or adhesion.
Figure 6. Proliferation of feline T. foetus but not adhesion is repressed by cytoskeletal inhibitors.
(A) Growth of TF in the presence of cytoskeletal inhibitors is reduced over time compared to vehicle-treated controls. n=3 replicates at each time point. (B) Adhesion of [3H] thymidine labeled-TF to IPEC-J2 cells was similar to control following exposure of TF to cytoskeletal inhibitors. Each column represents 8–10 replicates.
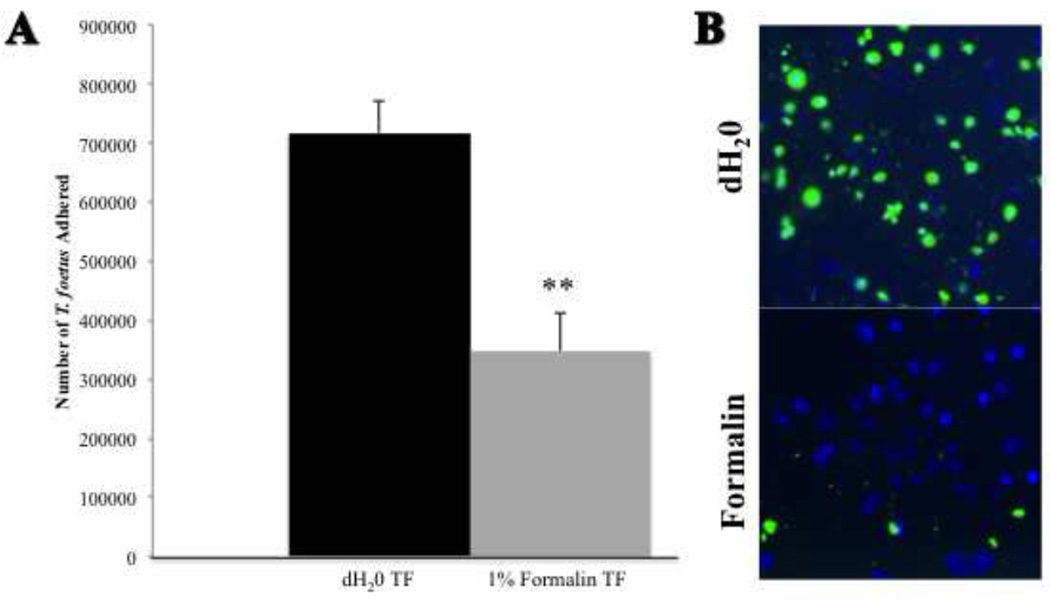
3.5 Adhesion of feline T. foetus to intestinal epithelium requires trophozoite viability
To determine if adhesion of TF to the intestinal epithelium was an active process, trophozoites were treated with 1% formalin prior to co-culture with IPEC-J2 cells. Formalin-fixation significantly reduced adhesion of TF to IPEC-J2 cells compared to vehicle-treated TF (Fig. 7A). Formalin-fixation had no cytolytic effect on the trichomonads based on their retention of radiolabel. Qualitative examination of CFSE-labeled TF adhesion to IPEC-J2 cells visually supported an inhibitory effect of formalin-fixation on TF adhesion (Fig. 7B).
Figure 7. Trophozoite viability is required for T. foetus adhesion.
(A) Adhesion of formalin-treated [3H] thymidine labeled-TF to IPEC-J2 cells is significantly reduced compared to vehicle treated (deionized water) TF. Each column represents 6 replicates. **p<0.01 compared to vehicle treated control (Student’s t test). (B) A representative, qualitative fluorescence microscopy image demonstrating CFSE-labeled TF (green) adhering to monolayers of porcine intestinal epithelial cells (IPEC-J2). IPEC-J2 cells can be identified by the nuclear counterstain, DAPI (blue). Adhesion is reduced following treatment of TF with 1% formalin (10× magnification).
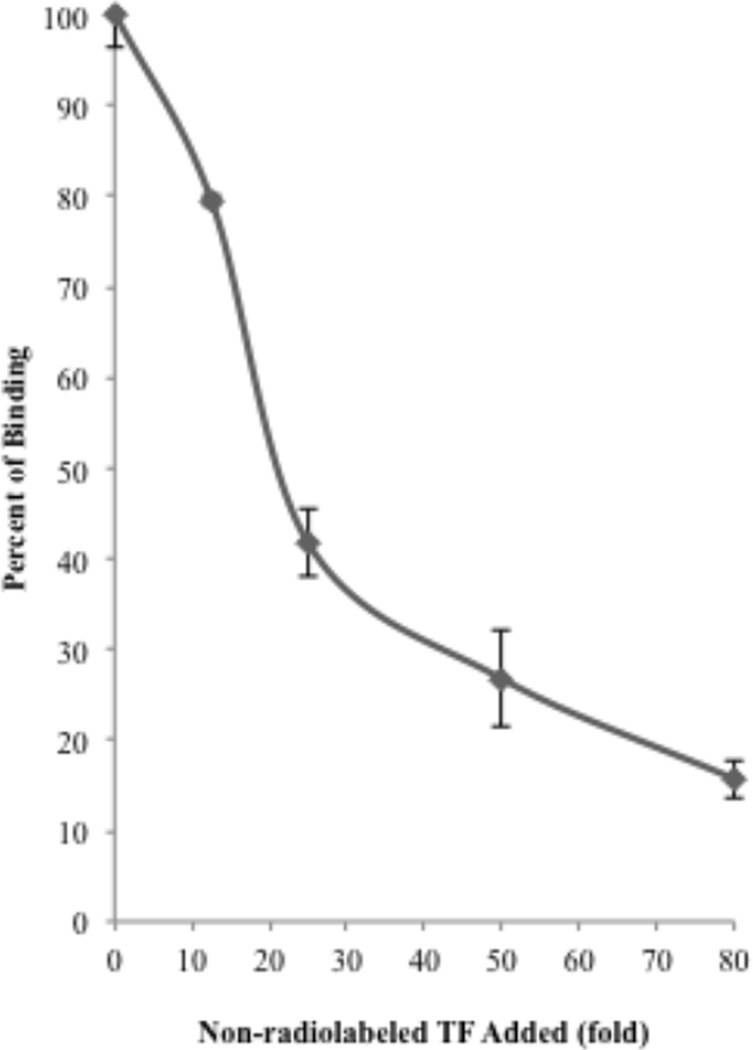
3.6 Feline T. foetus adhere to intestinal epithelium via specific receptor-ligand interaction
To determine if adhesion of TF to IPEC-J2 cells was receptor-specific and therefore could be outcompeted, we examined the effect of increasing numbers of non-radiolabeled TF on adhesion of [3H] thymidine labeled-TF. The addition of 80-fold excess of unlabeled TF outcompeted the binding of radiolabeled TF by 85 ± 1.9 % consistent with a receptor-mediated mechanism of TF adhesion (Fig. 8).
Figure 8. Non-radiolabeled TF outcompete [3H] thymidine labeled-TF for binding to IPEC-J2 cells.
Adhesion of [3H] thymidine labeled-TF to IPEC-J2 cells is inhibited by the addition of increasing numbers of non-radiolabeled TF. Each data point represents 3–4 replicates.
4. Discussion
To the authors’ knowledge there are no published studies investigating the mechanisms of adherence of feline TF to the intestinal epithelium. Based on the use of cell culture models of bovine TF and human Trichomonas vaginalis infections, adherence of trichomonads to the urogenital epithelium has been identified as a critical step in venereal pathogenicity (Singh et al., 1999; Silva Filho et al., 1988; Alderete et al, 1985). Studies of feline TF are similarly suited to a cell culture model approach as the organisms are lumen-dwellers that intimately associate with the intestinal epithelium and only rarely invade into the underlying lamina propria (Yaeger et al., 2005). Further, in experimentally infected cats, diarrhea precedes the host inflammatory response (Gookin et al., 2001) suggesting that the interaction between TF and the intestinal epithelium plays an early and key role in the genesis of diarrhea. In the present study, we sought to establish an in vitro model of feline TF infection that could be used to define the mechanisms of adhesion of feline TF to the intestinal epithelium. Because there are currently no feline intestinal epithelial cell lines available, we established a model system using non-transformed porcine intestinal epithelial cells (IPEC-J2). When IPEC-J2 cells are cultured to confluence on semi-permeable artificial basement membranes they form a polarized epithelial monolayer that mimics the native intestinal epithelium (Schierack et al., 2006). Both feline TF and the trichomonad of pigs, Tritrichomonas suis, are highly similar organisms and each demonstrates unique tropism for the gastrointestinal tract (Tachezy et al, 2002; Mostegl et al, 2011). Accordingly, porcine intestinal epithelial cells offer a particularly relevant alternative for the study of feline TF adherence to the intestinal epithelium. We demonstrated that multiple different clinical isolates of feline TF adhered robustly to IPEC-J2 monolayers in co-culture. Ultrastructural analysis demonstrated that adherence of feline TF to IPEC-J2 monolayers occurred in a patchy distribution consisting of large aggregates of trichomonads attached to the epithelium. This observation supports light microscopic descriptions of the infection in vivo where segmental foci of trichomonads are found to occur along the colonic epithelium (Yaeger et al., 2005).
Using the described co-culture model system, we first sought to determine whether adherence of feline TF was an active or passive event. Significant inhibition of feline TF adherence by gentle formalin fixation of the trophozoites supports the existence of an active process that requires cellular viability. Participation of cytoskeletal elements during the adhesion process has been demonstrated in other enteric protozoal infections (Katelaris et al, 1995; López-Revilla, R., 1982). Thus, we considered an active role for actin or tubulin in adherence of feline TF. Cytochalasin B and colchicine had a modest inhibitory effect on growth of TF, however adherence was not affected. These findings suggest that adherence of TF is a metabolically active process that is not critically dependent on dynamic cytoskeletal rearrangement.
Adherence of feline TF to the intestinal epithelium may be a key step in establishing infection and inducing diarrhea. While the cytopathogenic effects of feline TF on the intestinal epithelium was not a focus of this particular study, we found it particularly interesting that feline isolates of TF adhered to the intestinal epithelium in significantly greater numbers than did feline P. hominis, a presumably nonpathogenic trichomonad. Future studies will be required to determine if there is any causal relationship between the magnitude of adhesion and epithelial cytopathogenicity among different TF isolates or between TF and P. hominis. To determine if adhesion of feline TF was receptor-specific and therefore could be outcompeted, we studied the interaction kinetics between TF and the intestinal epithelium. The addition of increasing numbers of non-radiolabeled TF displaced adherence of a fixed number of radiolabeled TF by approximately 85%. These results, along with our observation that adherence of radiolabeled TF was saturable, provide compelling evidence for the involvement of specific receptor-ligand interactions between TF and the intestinal epithelium. The identity of these ligands may represent an important step toward development of novel therapies for feline trichomonosis. Several studies of bovine venereal TF have highlighted cellular proteases and host cell surface carbohydrates as important for adherence and cytopathogenicity (Singh et al., 2005, Singh et al., 1999, Bonilha et al., 1995). Therefore, future aims will be focused on defining the role of these and other specific molecules in adhesion of TF to the intestinal epithelium.
In summary, the present study has established a unique in vitro model system to study the adherence of feline TF to the intestinal epithelium. Adhesion of feline TF requires that the trichomonads are viable but does not depend on a dynamic rearrangement of the actin or tubulin cytoskeleton. Further, adherence of feline TF to the intestinal epithelium is mediated by specific receptor-ligand interactions, which provide an encouraging focus for the development of pharmacologic inhibitors of TF adhesion. In future studies, the developed model will be used to evaluate the pathogenic effects of feline TF on the intestinal epithelium, to identify novel molecular inhibitors of adhesion and to determine if pharmacological inhibition of adhesion ameliorates epithelial pathogenicity.
Acknowledgments
This work was supported by a grant from the Morris Animal Foundation (grant no. D08FE-04) and the North Carolina Veterinary Medical Foundation’s Support for T. foetus Research Innovation and Veterinary Education (STRIVE) fund. M.K. Tolbert is supported by a Ruth L. Kirschstein National Research Service Award (T32 RR024394) as part of North Carolina State University’s Comparative Medicine and Translational Research Training Program. Scanning electron microscopy was performed in the Laboratory for Advanced Electron and Light Optical Microscopy in the College of Veterinary Medicine at North Carolina State University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alderete JF, Garza GE. Specific nature of Trichomonas vaginalis parasitism of host cell surfaces. Infect and Immun. 1985;50:701–708. doi: 10.1128/iai.50.3.701-708.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha VL, Ciavaglia MC, Souza W de, Silva Filho FC. The involvement of terminal carbohydrates of the mammalian cell surface in the cytoadhesion of trichomonads. Parasitol Res. 1995;81:121–126. doi: 10.1007/BF00931616. [DOI] [PubMed] [Google Scholar]
- Céu Sousa M, Gonçalves CA, Bairos VS, Poiares-da-Silva J. Adherence of Giardia lamblia trophozoites to Int-407 human intestinal cells. Clin Diag Lab Immun. 2001;8:258–265. doi: 10.1128/CDLI.8.2.258-265.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DM, Gookin JL, Poore MF, Stebbins ME, Levy MG. Outcome of cats with diarrhea and Tritrichomonas foetus infection. J Am Vet Med Assoc. 2004;225:888–892. doi: 10.2460/javma.2004.225.888. [DOI] [PubMed] [Google Scholar]
- Gookin JL, Levy MG, Law JM, Papich MG, Poore MF, Breitschwerdt EB. Experimental infection of cats with Tritrichomonas foetus. Am J Vet Res. 2001;62:1690–1697. doi: 10.2460/ajvr.2001.62.1690. [DOI] [PubMed] [Google Scholar]
- Gookin JL, Birkenheuer AJ, Breitschwerdt EB, Levy MG. Single-tube nested PCR for detection of Tritrichomonas foetus in feline feces. J Clin Microbiol. 2002;40:4126–4130. doi: 10.1128/JCM.40.11.4126-4130.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gookin JL, Stebbins ME, Hunt E, Burlone K, Fulton M, Hochel R, Talaat M, Poore M, Levy MG. Prevalence of and risk factors for feline Tritrichomonas foetus and Giardia infection. J Clin Microbiol. 2004;42:2707–2710. doi: 10.1128/JCM.42.6.2707-2710.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gookin JL, Stauffer SH, Dybas D, Cannon DH. Documentation of in vivo and in vitro aerobic resistance of feline Tritrichomonas foetus isolates to ronidazole. J Vet Intern Med. 2010;24:1003–1007. doi: 10.1111/j.1939-1676.2010.0534.x. [DOI] [PubMed] [Google Scholar]
- Katelaris PH, Naeem A, Farthing MJ. Attachment of Giardia lamblia trophozoities to a cultured human intestinal cell line. Gut. 1995;37:512–518. doi: 10.1136/gut.37.4.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Revilla R, Cano-Mancera R. Adhesion of Entamoeba histolytica trophozoites to human erythrocytes. Infect Immun. 1982;37:281–285. doi: 10.1128/iai.37.1.281-285.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostegl MM, Richter B, Nedorost N, Maderner A, Dinhopl N, Weissenböck H. Investigations on the prevalence and potential pathogenicity of intestinal trichomonads in pigs using in situ hybridization. Vet Parasitol. 2011;178:58–63. doi: 10.1016/j.vetpar.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schierack P, Nordhoff M, Pollmann M, Weyrauch KD, Amasheh S, Lodemann U, Jores J, Tachu B, Kleta S, Blikslager A, Tedin K, Wieler LH. Characterization of a porcine intestinal epithelial cell line for in vitro studies of microbial pathogenesis in swine. Histochem Cell Biol. 2006;125:293–305. doi: 10.1007/s00418-005-0067-z. [DOI] [PubMed] [Google Scholar]
- Silva Filho FC, Souza W. The interaction of Trichomonas foetus and Tritrichomonas vaginalis with epithelial cells in vitro. Cell Structure and Function. 1988;13:301–310. doi: 10.1247/csf.13.301. [DOI] [PubMed] [Google Scholar]
- Singh BN, Lucas JJ, Beach DH, Shin ST, Gilbert RO. Adhesion of Tritrichomonas foetus to bovine vaginal epithelial cells. Infect and Immun. 1999;67:3847–3854. doi: 10.1128/iai.67.8.3847-3854.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh BN, Hayes GR, Lucas JJ, Beach DH, Gilbert RO. In vitro cytopathic effects of a cysteine protease of Tritrichomonas foetus on cultured bovine uterine epithelial cells. Am J Vet Res. 2005;66:1181–1186. doi: 10.2460/ajvr.2005.66.1181. [DOI] [PubMed] [Google Scholar]
- Tachezy J, Tachezy R, Hampl V, Sedinová M, Vanacová S, Vrlík M, Van Ranst M, Flegr J, Kuldaa J. Cattle pathogen Tritrichomonas foetus (Riedmüller, 1928) and pig commensal Tritrichomonas suis (Gruby & Delafond, 1843) belong to the same species. J Eukaryot Microbiol. 2002;49:154–163. doi: 10.1111/j.1550-7408.2002.tb00360.x. [DOI] [PubMed] [Google Scholar]
- Yaeger MJ, Gookin JL. Histologic features associated with Tritrichomonas foetus-induced colitis in domestic cats. Vet Path. 2005;42:797–804. doi: 10.1354/vp.42-6-797. [DOI] [PubMed] [Google Scholar]