Abstract
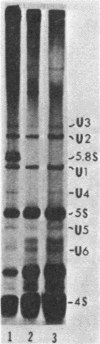
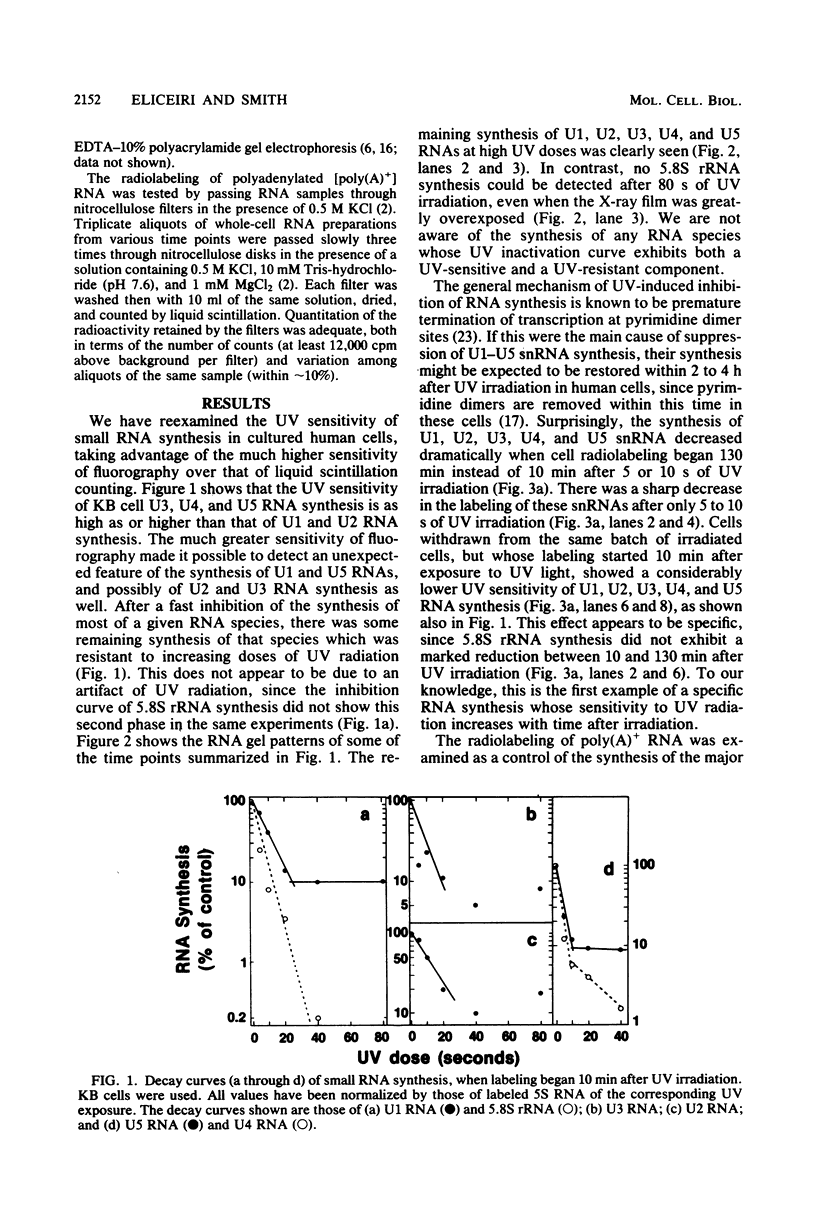
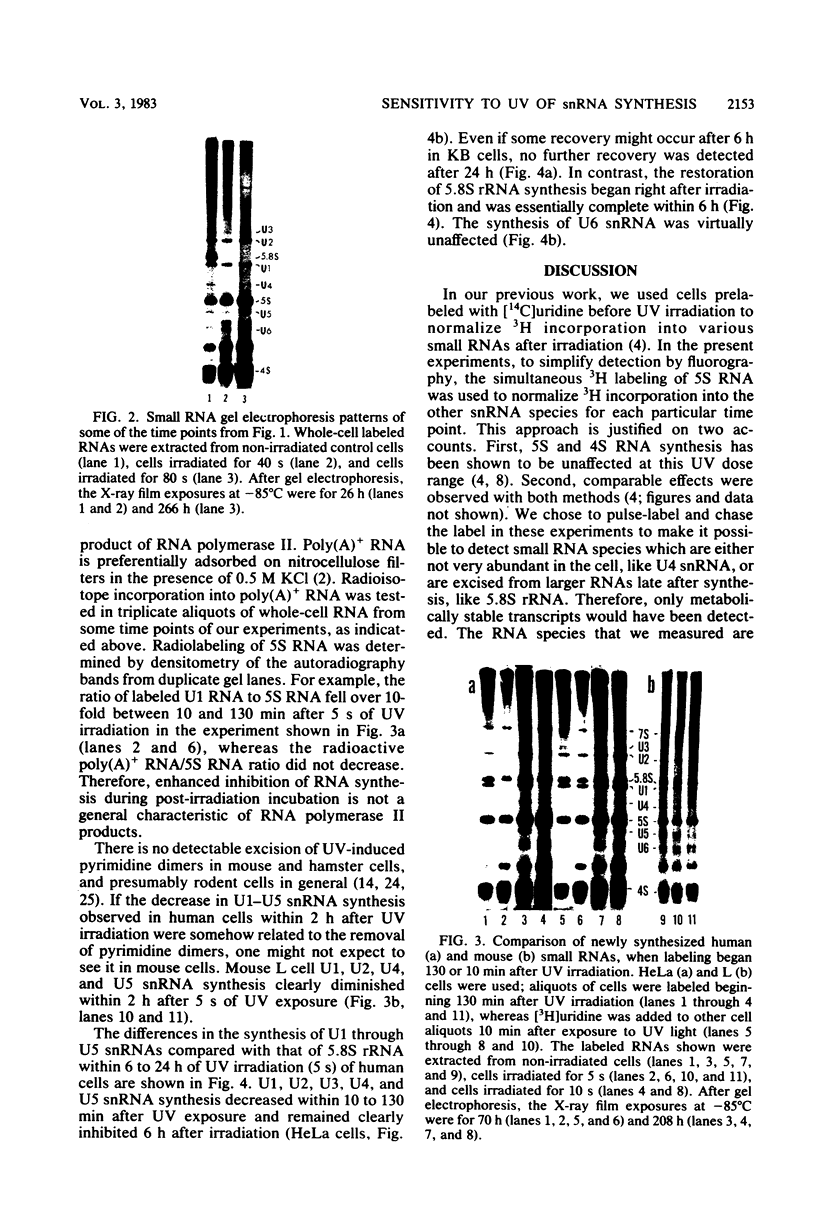
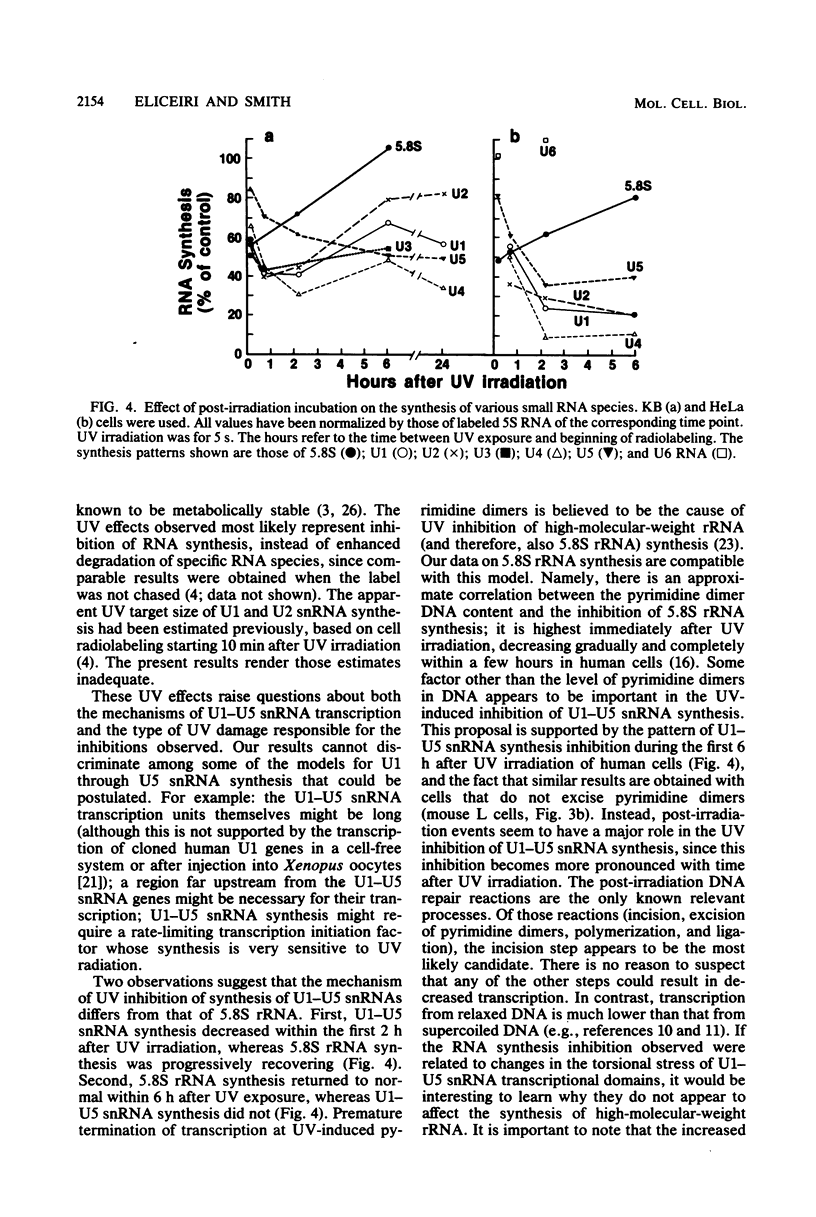
It was demonstrated previously that the synthesis of small nuclear RNA (snRNA) species U1 and U2 in human cells is very sensitive to UV radiation. In the present work, the UV sensitivity of U3, U4, and U5 snRNA synthesis is shown to be also high. The synthesis of U1, U2, U3, U4, and U5 snRNAs progressively decreased during the first 2 h after UV irradiation (this was not observed in polyadenylated RNA) and had not returned to normal rates 6 h after UV exposure. In contrast, the restoration of 5.8S rRNA synthesis began immediately after UV irradiation and was essentially complete 6 h later. A small fraction of U1 and U5 (and possibly U2 and U3) snRNA synthesis remained unaffected by high UV doses, when cell radiolabeling began 10 min after UV irradiation. The present data suggest that a factor other than the level of pyrimidine dimers in DNA (possibly, steps in the post-irradiation DNA repair process) plays an important role in the mechanism of UV-induced inhibition of U1-U5 snRNA synthesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brawerman G., Mendecki J., Lee S. Y. A procedure for the isolation of mammalian messenger ribonucleic acid. Biochemistry. 1972 Feb 15;11(4):637–641. doi: 10.1021/bi00754a027. [DOI] [PubMed] [Google Scholar]
- Busch H., Reddy R., Rothblum L., Choi Y. C. SnRNAs, SnRNPs, and RNA processing. Annu Rev Biochem. 1982;51:617–654. doi: 10.1146/annurev.bi.51.070182.003153. [DOI] [PubMed] [Google Scholar]
- Eliceiri G. L., Sayavedra M. S. Small RNAs in the nucleus and cytoplasm of HeLa cells. Biochem Biophys Res Commun. 1976 Sep 20;72(2):507–512. doi: 10.1016/s0006-291x(76)80070-8. [DOI] [PubMed] [Google Scholar]
- Eliceiri G. L. Sensitivity of low molecular weight RNA synthesis to UV radiation. Nature. 1979 May 3;279(5708):80–81. doi: 10.1038/279080a0. [DOI] [PubMed] [Google Scholar]
- Frederiksen S., Hellung-Larsen P., Gram Jensen E. The differential inhibitory effect of alpha-amanitin on the synthesis of low molecular weight RNA components in BHK cells. FEBS Lett. 1978 Mar 15;87(2):227–231. doi: 10.1016/0014-5793(78)80338-x. [DOI] [PubMed] [Google Scholar]
- Goldberg S., Schwartz H., Darnell J. E., Jr Evidence from UV transcription mapping in HeLa cells that heterogeneous nuclear RNA is the messenger RNA precursor. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4520–4523. doi: 10.1073/pnas.74.10.4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon J. B., Melton D. A. Gene transfer in amphibian eggs and oocytes. Annu Rev Genet. 1981;15:189–218. doi: 10.1146/annurev.ge.15.120181.001201. [DOI] [PubMed] [Google Scholar]
- Harland R. M., Weintraub H., McKnight S. L. Transcription of DNA injected into Xenopus oocytes is influenced by template topology. Nature. 1983 Mar 3;302(5903):38–43. doi: 10.1038/302038a0. [DOI] [PubMed] [Google Scholar]
- Hellung-Larsen P., Kulamowicz I., Frederiksen S. Synthesis of low molecular weight RNA components in cells with a temperature-sensitive polymerase II. Biochim Biophys Acta. 1980 Aug 26;609(1):201–204. doi: 10.1016/0005-2787(80)90213-0. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Bonner J. Preparation, molecular weight, base composition, and secondary structure of giant nuclear ribonucleic acid. Biochemistry. 1973 Jun 5;12(12):2330–2338. doi: 10.1021/bi00736a023. [DOI] [PubMed] [Google Scholar]
- Jensen E. G., Hellung-Larsen P., Frederiksen S. Synthesis of low molecular weight RNA components A, C and D by polymerase II in alpha-amanitin-resistant hamster cells. Nucleic Acids Res. 1979 Jan;6(1):321–330. doi: 10.1093/nar/6.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klímek M. Thymine dimerization in L-strain mammalian cells after irradiation with ultraviolet light and the search for repair mechanisms. Photochem Photobiol. 1966 Aug;5(8):603–607. doi: 10.1111/j.1751-1097.1966.tb05806.x. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Steitz J. A. Antibodies to small nuclear RNAs complexed with proteins are produced by patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5495–5499. doi: 10.1073/pnas.76.11.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas C. J. Immunological demonstration of the disappearance of pyrimidine dimers from nuclei of cultured human cells. Exp Cell Res. 1972 Oct;74(2):480–486. doi: 10.1016/0014-4827(72)90404-1. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Efstratiadis A. Fractionation of low molecular weight DNA or RNA in polyacrylamide gels containing 98% formamide or 7 M urea. Methods Enzymol. 1980;65(1):299–305. doi: 10.1016/s0076-6879(80)65040-x. [DOI] [PubMed] [Google Scholar]
- Manser T., Gesteland R. F. Characterization of small nuclear RNA U1 gene candidates and pseudogenes from the human genome. J Mol Appl Genet. 1981;1(2):117–125. [PubMed] [Google Scholar]
- Manser T., Gesteland R. F. Human U1 loci: genes for human U1 RNA have dramatically similar genomic environments. Cell. 1982 May;29(1):257–264. doi: 10.1016/0092-8674(82)90110-6. [DOI] [PubMed] [Google Scholar]
- Murphy J. T., Burgess R. R., Dahlberg J. E., Lund E. Transcription of a gene for human U1 small nuclear RNA. Cell. 1982 May;29(1):265–274. doi: 10.1016/0092-8674(82)90111-8. [DOI] [PubMed] [Google Scholar]
- Roop D. R., Kristo P., Stumph W. E., Tsai M. J., O'Malley B. W. Structure and expression of a chicken gene coding for U1 RNA. Cell. 1981 Mar;23(3):671–680. doi: 10.1016/0092-8674(81)90430-x. [DOI] [PubMed] [Google Scholar]
- Sauerbier W., Hercules K. Gene and transcription unit mapping by radiation effects. Annu Rev Genet. 1978;12:329–363. doi: 10.1146/annurev.ge.12.120178.001553. [DOI] [PubMed] [Google Scholar]
- TROSKO J. E., CHU E. H., CARRIER W. L. THE INDUCTION OF THYMINE DIMERS IN ULTRAVIOLET-IRRADIATED MAMMALIAN CELLS. Radiat Res. 1965 Apr;24:667–672. [PubMed] [Google Scholar]
- Weinberg R., Penman S. Metabolism of small molecular weight monodisperse nuclear RNA. Biochim Biophys Acta. 1969 Sep 17;190(1):10–29. doi: 10.1016/0005-2787(69)90150-6. [DOI] [PubMed] [Google Scholar]