Abstract
Renal plasmacytoma is extremely rare, presenting diagnostic challenges due to its unusual location and non-specific or absent symptoms. To the best of our knowledge, only 24 cases of renal plasmacytoma have been reported in the literature. The present study reports a case of primary renal plasmacytoma in a 46-year-old female patient. Computed tomography (CT) revealed that the mass was located in the lower pole of the left kidney and metastasis was detected in an enlarged para-aortic lymph node. Following careful preparation, a partial nephrectomy was performed and the retroperitoneal lymph node was resected. A pathological examination revealed a renal parenchyma with lymph node involvement; this was confirmed by immunohistochemistry and nested polymerase chain reaction (PCR). Consequently, a diagnosis of a renal extramedullary plasmacytoma (EMP) was proposed. Following this unexpected diagnosis, various examinations were performed, but there was no evidence of systemic plasma cell disease. The patient refused further therapy, including external beam radiotherapy and chemotherapy. Abdominal CT was performed three months post-surgery and did not reveal any relapse. The patient remains disease-free at nine months post-surgery. The current study also presents a review of the literature. Although the general prognosis and outcome of EMP is good, a follow-up examination is recommended due to the possibility of relapse or progression to plasma cell neoplasm (PCN).
Keywords: extramedullary plasmacytoma, kidney, multiple myeloma
Introduction
Extramedullary plasmacytoma (EMP) is a rare malignant neoplasm that develops due to uncontrolled plasma cell proliferation and monoclonal plasmacytic infiltration (1). The majority of EMPs are detected in the head and neck (2), and the occurrence of an EMP in the kidney is extremely rare. This study presents a case of renal EMP and reviews the existing literature concerning EMPs, as well as multiple myelomas (MMs). EMPs may present as the main symptom of MM, or develop during the course of MM or occasionally occur as solitary tumors. To the best of our knowledge, there are only 24 cases of renal plasmacytoma reported previously (Table I). We report the case of a patient with renal EMP, who may have been diagnosed and treated incorrectly. The study was approved by the Ethics Committee of Sun Yat-sen University Cancer Center, Guangzhou, China. Written informed consent was obtained from the patient.
Table I.
Clinical data from 24 cases of renal plasmacytoma.
| Authors, year (Ref.) | Age (years)/gender | Tumor location | SPE/IE | Bence-Jones protein | Previously diagnosed PCN | Type of EMP | Clinical manifestations | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Knudsen O, 1937 (5) | 46/F | NA | NA | NA | None | P | Palpable mass | Radical nephrectomy | NA |
| Farrow GM et al, 1968 (6) | 53/M | L | NA | NA | NA | P | NA | Radical nephrectomy, radiotherapy | Succumbed after 16 years |
| Solomito VL et al, 1972 (7) | 64/M | L | NA | NA | Nasopharynx | S | Palpable mass, fatigue | Radical nephrectomy, ileocolectomy | Alive after 4.5 years |
| Catalona WJ et al, 1974 (8) | 52/F | R | β ↑ | + | Temporal lobe | S | Palpable mass | Biopsy, radiotherapy, chemotherapy | NA |
| Siemers PT et al, 1977 (9) | 56/M | L | Normal | NA | None | P | Anorexia, fatigue, palpable mass | Radical nephrectomy, radiotherapy, hemodialysis | Alive after 3 months |
| Morris SA et al, 1977 (10) | 50/M | L | IgM ↑ | + | Skull, scapula | S | Gross hematuria | Radical nephrectomy, radiotherapy | NA |
| Silver TM et al, 1977 (11) | 40/M | L | Normal | - | None | P | Gross hematuria, flank pain | Radical nephrectomy | NA |
| Kandel LB et al, 1984 (12) | 55/M | R | γ ↑ | + | None | p | Burning feeling, | Radical nephrectomy, radiotherapy | NA |
| Jaspan T et al, 1984 (13) | 75/F | L | IgG ↑ | + | None | P | Back pain | Biopsy | Succumbed after biopsy |
| Kanoh T et al, 1987 (14) | 50/M | R | IgA ↑ | NA | MM | S | Microscopic hematuria, abdominal fullness | Chemotherapy, radiotherapy | Succumbed after 2 years |
| Igel TC et al, 1991 (15) | 64/M | L | IgM ↑ | NA | None | P | Burning feeling, weight loss | Radical nephrectomy, radiotherapy, chemotherapy | NA |
| Kanoh T et al, 1992 (16) | 76/F | NA | NA | NA | None | P | NA | Radical nephrectomy | Succumbed after 3 months |
| Kanoh T et al, 1993 (17) | 43/M | R | NA | + | Spinal bones | S | Paraplegia | Radiotherapy | NA |
| Rebelakos AG et al, 1995 (18) | 52/F | R | NA | NA | T9 vertebra | S | Gross hematuria, back pain, palpable mass | Radical nephrectomy | Alive after 6 months |
| Shustik C et al, 1995 (19) | 31/M | NA | IgG ↑ | NA | None | P | Asymptomatic | Radical nephrectomy | Alive after 33 months |
| Manseck A et al, 1997 (20) | 64/NA | NA | NA | - | None | P | NA | Radical nephrectomy | NA |
| Tejido Sanchez A et al, 2001 (21) | 59/NA | R | NA | NA | None | P | NA | Chemotherapy | Succumbed after 1 year |
| Kim SH et al, 2003 (22) | 44/F | R | NA | NA | MM | S | Palpable mass | Surgery, chemotherapy, radiotherapy | Alive after 3 months |
| Fan F et al, 2005 (23) | 61/F | R | NA | NA | None | P | Back pain | Partial nephrectomy, chemotherapy | Alive after 2.5 year |
| Park SY et al, 2007 (24) | 39/M | L | Normal | NA | None | P | NA | Radical nephrectomy | Succumbed after 34 months |
| Yazici S et al, 2009 (25) | 67/F | L | α2 ↑, γ ↑ | NA | None | P | Asymptomatic | Radical nephrectomy | Alive after 6 months |
| Mongha R et al, 2010 (26) | 58/M | R | Normal | - | None | P | Lumbar pain | Radical nephrectomy, radiotherapy | Alive after 1 year |
| Yang GF et al, 2010 (27) | 76/F | L | NA | NA | None | P | Back pain | Radical nephrectomy | NA |
| Zhong Y et al, 2010 (28) | 41/M | L | NA | - | None | P | Epigastric discomfort | Radical nephrectomy | NA |
| Present case | 46/F | L | γ ↑, α1 ↑ | + | None | P | NA | Radical nephrectomy | Alive after 6 months |
F, female; M, male; L, left kidney; R, right kidney; NA, not available; SPE, serum protein electrophoresis; IE, immunoelectrophoresis;
↑, increased;
+, positive;
−, negative; PCN, plasma cell neoplasm; MM, multiple myeloma; EMP, extramedullary plasmacytoma; P, primary; S, secondary.
Case report
A mass in the left kidney was detected in a 46-year-old female patient who underwent ultrasonography as part of a routine physical checkup. Computed tomography (CT) revealed that the mass showed enhancement, was located in the lower pole of the left kidney and measured 38×30 mm. Furthermore, metastasis was detected in an enlarged (20 mm) para-aortic lymph node (Fig. 1). The radiologist suspected a diagnosis of renal cell carcinoma and advised a partial nephrectomy. However, the pre-operative work-up revealed the following: white blood cell (WBC) count, 2.7×109/l; percentage of neutrophils (NE%), 61.8; red blood cell (RBC) count, 3.58×1012/l; hemoglobin (HGB) level, 101.1 g/l; platelet (PLT) count, 187.6×109/l; and serum creatinine, calcium and phosphorus levels within normal ranges.
Figure 1.
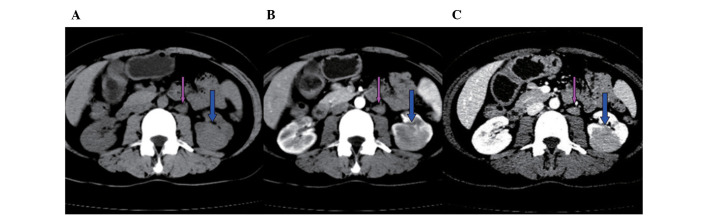
CT scans reveal that the mass (blue arrow) showed enhancement, was located in the lower pole of the left kidney and measured 38×30 mm. Metastasis was detected in an enlarged (20 mm) para-aortic lymph node (pink arrow). CT values; (A) plain scan, 40–60; (B) arterial phase, 71–89; (C) venous phase, 90–120. CT, computed tomography.
The patient had a 19-year history of hyperthyroidism and an 11-year history of Henoch-Schönlein purpura. The patient had no known drug allergies and their family history was non-contributory. A bone marrow examination was performed next, but it did not reveal any evidence of lymphoma or a plasma cell neoplasm (PCN). The patient was subcutaneously injected with 300 μg recombinant human granulocyte colony-stimulating factor and the complete blood count (CBC) was repeated. The new CBC results were as follows: WBC count, 6.2×109/l; NE%, 84.7; RBC count, 4.01×1012/l; HGB level, 110.0 g/l; and PLT count, 133.0×109/l. Subsequently, a partial nephrectomy was performed and the retroperitoneal lymph node was resected.
The surgically resected specimen consisted of a segment of renal parenchyma and a 30-mm soft subcapsular mass anchored firmly to the parenchymal part of the kidney, with clear surgical margins. The section cut from the tumor was a light white and contained hemorrhagic foci (Fig. 2). Histological examination revealed a 30×25×10 mm unencapsulated mass showing a diffuse infiltration of plasmacytoid cells that were of different sizes and at various degrees of differentiation (Fig. 3). Immunohistochemical analysis demonstrated that the tumor cells were positive for monoclonal κ light chains, CD38, MUM-1, CD138 and VS38C, while certain cells were positive for monoclonal λ light chains. The cells were negative for CD79a, CD5, CD10, L26, UCHL-1, Pax-5, BCL-2, CK and IgD. Ki-67 staining revealed a high cell proliferation rate (>30% immunoreactive cells), indicating the malignant nature of the lesion. Nested polymerase chain reaction (PCR) revealed that the tumor was negative for the rearrangement of the B-cell lymphoma, IgH, and T cell lymphoma, TCRγ, genes. The retroperitoneal lymph node was also involved. Therefore, a diagnosis of a renal EMP was proposed.
Figure 2.
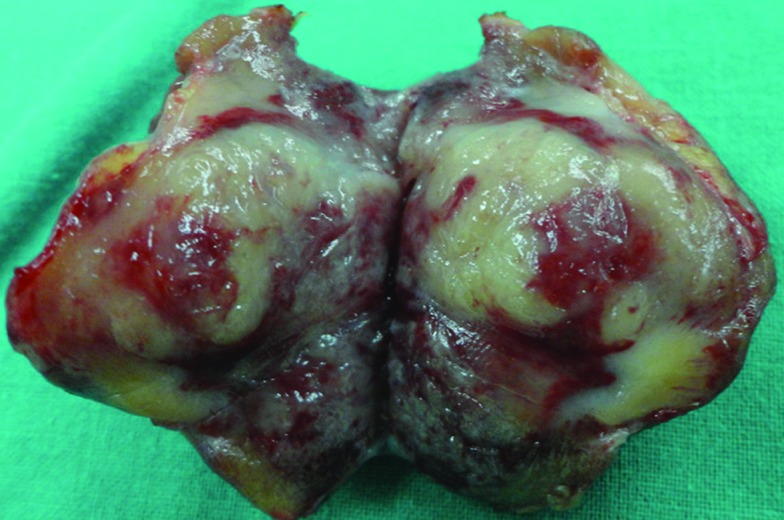
The surgically resected specimen consisted of a segment of renal parenchyma and a 30-mm soft subcapsular mass anchored firmly to the parenchymal section of the kidney, with clear surgical margins. The tumor was a light white and contained hemorrhagic foci.
Figure 3.
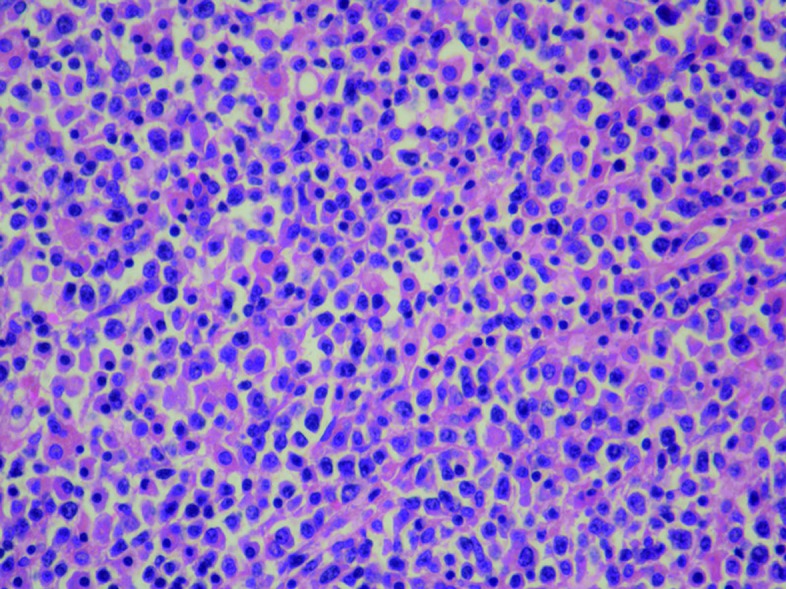
Histological examination showing diffuse infiltration of plasmacytoid cells, which were of different sizes and at various degrees of differentiation. Hematoxylin-eosin staining; magnification, ×400.
Following this unexpected diagnosis, a skeletal survey was performed to complete the tumor staging. The survey did not reveal any lytic bone lesions or evidence of active malignant disease elsewhere. In addition, the urinalysis was positive for Bence-Jones protein. Serum protein electrophoresis excluded MMs and there was no evidence of systemic plasma cell disease.
All these observations were consistent with a diagnosis of an EMP involving the kidney. The patient refused further therapy, including external beam radiotherapy and chemotherapy. Abdominal CT was performed three months post-surgery and did not reveal any relapse. The results of follow-up blood tests were normal and no evidence of hematological disease was noted. The patient remains disease-free at nine months post-surgery.
Discussion
EMPs may coexist with MM; they may present as the main symptom of MM, develop during the course of MM or occasionally occur as solitary tumors. Laso et al considered EMPs and MM to be part of a continuous spectrum of PCNs rather than separate entities (1). The majority of EMPs involve the head and neck region, particularly the upper respiratory tract (2). Others involve diverse anatomical sites, including the gastrointestinal tract, central nervous system, thyroid, breasts, parotid gland, lymph nodes, skin, lungs, pleura, muscle, liver, spleen and pancreas (3,4).
To the best of our knowledge, only 24 cases of renal plasmacytoma have been reported in the literature (Table I) (5–28). However, more cases may have occurred, as this tumor is underdiagnosed and underreported. The clinical suspicion of isolated plasmacytoma is infrequent in patients, with the exception of those with systemic diseases.
Although secondary EMP is much more frequent than primary EMP, almost all cases of renal EMPs registered so far have been primary in nature, that is, without any evidence of an associated PCN.
In cases of renal EMPs, the tumor mass is often confined to this anatomical area and only two cases have shown other organ involvement (7,8). This may reflect the indolent course of renal EMPs.
The diagnosis of an EMP is complex and requires radiological, hematological, biochemical and histological investigation. Primary renal plasmacytomas are not distinguishable from other renal tumors in pre-operative imaging tests. In the present patient, an EMP was diagnosed on the basis of diffuse monoclonal plasma cell infiltration at a single site observed in the immunohistochemical staining for the κ and λ light chains and following the exclusion of a diagnosis of MM (29).
There have been two cases in which an initial diagnosis of renal plasmacytoma was later revised to MM (12,23).
A renal mass in a patient previously diagnosed with EMP is markedly suggestive of tumor recurrence involving the kidney. Among the 24 cases of renal EMPs reported in the literature, five were cases of a renal recurrence of an EMP that had previously occurred at a different site (7,8,10,17,18).
Further tests to rule out MM should include a CBC, serum and urine protein electrophoresis, immunoelectrophoresis, skeletal survey and bone marrow examination (30).
As a result of the lack of typical clinical symptoms and evidence from specific laboratory tests, a diagnosis may be delayed, with potentially disastrous consequences for the patient. In the present study, the patient was initially misdiagnosed with clear cell carcinoma. However, the surgical observations and post-operative pathology confirmed the final diagnosis. Once the diagnosis has been confirmed, the EMP may be staged: stage I, tumors confined to the primary site; stage II, tumors showing local extension or lymph node involvement; and stage III, systemic spread (18,31).
To the best of our knowledge, there are no guidelines for the treatment of renal plasmacytoma. Treatment options for renal plasmacytomas include surgery, chemotherapy and radiotherapy, either alone or in combination (17). Local radiotherapy is the preferred therapeutic modality for EMP owing to its documented radiosensitivity (32). If adjacent nodal involvement is observed, radiation should be applied to these zones as well (33). Kanoh et al reported that radiotherapy alone was sufficient to treat their patient (17). In the case of non-renal EMPs, definitive radiation therapy yielded five-year local control rates of >80% and local recurrence rates of <10% (2,34).
Optimal treatment strategies for renal EMPs are difficult to formulate owing to the rarity of the tumors. At present, there is no standard treatment for EMPs involving the kidney, but the current reported experiences of treating primary EMPs indicate that combined therapy (surgery and radiotherapy) is an accepted treatment, depending on the resectability of the lesion. A combination treatment may provide the best results (3).
EMP has a relatively good prognosis, but local recurrence and metastasis develop in 30 and 40% of patients, respectively (35). The five-year survival rate of EMP is excellent at 90%, and previous data suggest that local regression does not necessarily indicate a worse prognosis (31). However, progression to MM does imply this. Three of the reported literature cases of renal plasmacytoma recurred following surgery (23–25), one of which was finally diagnosed as MM.
The periodic evaluation of patients with EMP is necessary due to the possibility of relapse and progression to MM. Physical examinations coupled with laboratory tests, including CBC, renal function tests, analyses of blood calcium, serum albumin and immunoglobulin levels, serum protein electrophoresis, free light chain assays and radiographic studies of the skeleton are required for follow-up.
EMP of the kidney is a rare clinical entity, presenting diagnostic challenges due to its unusual location and nonspecific or absent symptoms. Imaging evaluations may illustrate the existence, size and location of the tumor but are unable to indicate a specific diagnosis. A review of the literature shows that there is currently no widely established standard treatment for EMP of the kidney. If the tumor is located in an area with restricted surgical access, a treatment regimen of local surgery, local radiotherapy or a combination of the two may be initiated. Surgery in combination with radiotherapy may be the best treatment. Although the general prognosis and outcome for EMP is good, a follow-up examination is recommended due to the possibility of relapse or progression to PCN.
Acknowledgments
The present study was supported by the Biobank of Complex Diseases in Shenzhen, China (CXC201005260001A).
References
- 1.Laso FJ, Tabernero MD, Iglesias-Osma MC. Extramedullary plasmacytoma: a localized or systemic disease? Ann Intern Med. 1998;128:156. doi: 10.7326/0003-4819-128-2-199801150-00018. [DOI] [PubMed] [Google Scholar]
- 2.Reed V, Shah J, Medeiros LJ, et al. Solitary plasmacytomas: outcome and prognostic factors after definitive radiation therapy. Cancer. 2011;117:4468–4474. doi: 10.1002/cncr.26031. [DOI] [PubMed] [Google Scholar]
- 3.Alexiou C, Kau RJ, Dietzfelbinger H, Kremer M, Spiess JC, Schratzenstaller B, Arnold W. Extramedullary plasmacytoma: tumor occurrence and therapeutic concepts. Cancer. 1999;85:2305–2314. [PubMed] [Google Scholar]
- 4.Dimopoulos MA, Kiamouris C, Moulopoulos LA. Solitary plasmacytoma of bone and extramedullary plasmacytoma. Hematol Oncol Clin North Am. 1999;13:1249–1257. doi: 10.1016/s0889-8588(05)70124-6. [DOI] [PubMed] [Google Scholar]
- 5.Knudsen O. A case of plasmacytoma of the kidney. Nord Med Tidskr. 1937;14:1493–1495. [Google Scholar]
- 6.Farrow GM, Harrison EG, Jr, Utz DC. Sarcomas and sarcomatoid and mixed malignant tumors of the kidney in adults. II. Cancer. 1968;22:551–555. doi: 10.1002/1097-0142(196809)22:3<551::aid-cncr2820220309>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 7.Solomito VL, Grise J. Angiographic findings in renal (extra-medullary) plasmacytoma. Case report Radiology. 1972;102:559–560. doi: 10.1148/102.3.559. [DOI] [PubMed] [Google Scholar]
- 8.Catalona WJ, Biles JD., 3rd Therapeutic considerations in renal plasmacytoma. J Urol. 1974;111:582–583. doi: 10.1016/s0022-5347(17)60020-x. [DOI] [PubMed] [Google Scholar]
- 9.Siemers PT, Coel MN. Solitary renal plasmacytoma with palisading tumor vascularity. Radiology. 1977;123:597–598. doi: 10.1148/123.3.597. [DOI] [PubMed] [Google Scholar]
- 10.Morris SA, Vaughan ED, Jr, Makoui C. Renal plasmacytoma. Urology. 1977;9:303–306. doi: 10.1016/0090-4295(77)90353-3. [DOI] [PubMed] [Google Scholar]
- 11.Silver TM, Thornbury JR, Teears RJ. Renal peripelvic plasmacytoma: unusual radiographic findings. AJR Am J Roentgenol. 1977;128:313–315. doi: 10.2214/ajr.128.2.313. [DOI] [PubMed] [Google Scholar]
- 12.Kandel LB, Harrison LH, Woodruff RD, Williams CD, Ahl ET., Jr Renal plasmacytoma: a case report and summary of reported cases. J Urol. 1984;132:1167–1169. doi: 10.1016/s0022-5347(17)50081-6. [DOI] [PubMed] [Google Scholar]
- 13.Jaspan T, Gregson R. Extra-medullary plasmacytoma of the kidney. Br J Radiol. 1984;57:95–97. doi: 10.1259/0007-1285-57-673-95. [DOI] [PubMed] [Google Scholar]
- 14.Kanoh T, Ohno T, Ohnaka T, Uchino H. Renal plasmacytoma. Nihon Ketsueki Gakkai Zasshi. 1987;50:906–910. [PubMed] [Google Scholar]
- 15.Igel TC, Engen DE, Banks PM, Keeney GL. Renal plasmacytoma: Mayo Clinic experience and review of the literature. Urology. 1991;37:385–389. doi: 10.1016/0090-4295(91)80274-b. [DOI] [PubMed] [Google Scholar]
- 16.Kanoh T, Yago K, Iwata H, Tei K, Higashino T. IgM-producing renal plasmacytoma. Urology. 1992;40:484–488. doi: 10.1016/0090-4295(92)90471-8. [DOI] [PubMed] [Google Scholar]
- 17.Kanoh T, Katoh H, Izumi T, Tsuji M, Okuma M. Renal plasmacytoma. Rinsho Ketsueki. 1993;34:1470–1473. (In Japanese). [PubMed] [Google Scholar]
- 18.Rebelakos AG, Papanastasiou K, Apostolikas N. Renal plasmacytoma. Br J Urol. 1995;75:562. doi: 10.1111/j.1464-410x.1995.tb07291.x. [DOI] [PubMed] [Google Scholar]
- 19.Shustik C, Jamison BM, Alfieri C, Scherer S, Loertscher R. A solitary plasmacytoma of donor origin arising 14 years after kidney allotransplantation. Br J Haematol. 1995;91:167–168. doi: 10.1111/j.1365-2141.1995.tb05263.x. [DOI] [PubMed] [Google Scholar]
- 20.Manseck A, Flössel C, Günther H, Wirth M. Primary renal plasmacytoma. A case report. Urologe A. 1997;36:369–373. doi: 10.1007/s001200050114. (In German). [DOI] [PubMed] [Google Scholar]
- 21.Tejido Sánchez A, Hernández Martínez E, Ortíz MC, Garcia de la Torre JP, Pamplona Casamayor M, de la Rosa Kherman F, Leiva Galvis O. Renal plasmacytoma. Report of a new case. Arch Esp Urol. 2001;54:718–722. (In Spanish). [PubMed] [Google Scholar]
- 22.Kim SH, Kim ES, Hur JW, Lee JH, Chang SH, Kim YS, Eo WK. A case of relapsed renal plasmacytoma after complete remission of multiple myeloma. Korean J Med. 2003;64:114–118. (In Korean). [Google Scholar]
- 23.Fan F, Deauna-Limayo D, Brantley Thrasher J, Damjanov I. Anaplastic plasmacytoma of the kidney. Histopathology. 2005;47:432–433. doi: 10.1111/j.1365-2559.2005.02123.x. [DOI] [PubMed] [Google Scholar]
- 24.Park SY, Lee JW, Son YW, Moon HS, Park MH, Lee TY. Primary renal plasmacytoma. Korean J Urol. 2007;48:878–880. [Google Scholar]
- 25.Yazici S, Inci K, Dikmen A, Ergen A, Bilen CY. Port site and local recurrence of incidental solitary renal plasmacytoma after retroperitoneoscopic radical nephrectomy. Urology. 2009;73:210.e15–210.e17. doi: 10.1016/j.urology.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 26.Mongha R, Narayan S, Dutta A, Sharma S, Uttara C, Kundu AK. Plasmacytoma of the kidney. Saudi J Kidney Dis Transpl. 2010;21:931–934. [PubMed] [Google Scholar]
- 27.Yang GF, Zhu H, Zhang LJ, Lu GM. Primary renal plasmacytoma: Case report and literature review. J Cancer Res Exp Oncol. 2010;2:43–46. [Google Scholar]
- 28.Zhong Y, Chen S, Liu X, Hu Y, Li X, Zhang J, An N. Renal extramedullary plasmacytoma - One case report. Clin Oncol Cancer Res. 2010;7:380–382. [Google Scholar]
- 29.Dimopoulos MA, Hamilos G. Solitary bone plasmacytoma and extramedullary plasmacytoma. Curr Treat Options Oncol. 2002;3:255–259. doi: 10.1007/s11864-002-0015-2. [DOI] [PubMed] [Google Scholar]
- 30.Avila A, Villalpando A, Montoya G, Luna MA. Clinical features and differential diagnoses of solitary extramedullary plasmacytoma of the thyroid: a case report. Ann Diagn Pathol. 2009;13:119–23. doi: 10.1016/j.anndiagpath.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Waldron J, Mitchell DB. Unusual presentations of extramedullary plasmacytoma in the head and neck. J Laryngol Otol. 1988;102:102–104. doi: 10.1017/s002221510010413x. [DOI] [PubMed] [Google Scholar]
- 32.Chao MW, Gibbs P, Wirth A, Quong G, Guiney MJ, Liew KH. Radiotherapy in the management of solitary extramedullary plasmacytoma. Intern Med J. 2005;35:211–215. doi: 10.1111/j.1445-5994.2005.00804.x. [DOI] [PubMed] [Google Scholar]
- 33.Mayr NA, Wen BC, Hussey DH, Burns CP, Staples JJ, Doornbos JF, Vigliotti AP. The role of radiation therapy in the treatment of solitary plasmacytomas. Radiother Oncol. 1990;17:293–303. doi: 10.1016/0167-8140(90)90003-f. [DOI] [PubMed] [Google Scholar]
- 34.Krause S, Hillengass J, Goldschmidt H, Debus J, Neuhof D. Radiotherapy of solitary plasmacytoma. Ann Hematol. 2011;90:1093–1097. doi: 10.1007/s00277-011-1190-7. [DOI] [PubMed] [Google Scholar]
- 35.Holland J, Trenkner DA, Wasserman TH, Fineberg B. Plasmacytoma. Treatment results and conversion to myeloma. Cancer. 1992;69:1513–1517. doi: 10.1002/1097-0142(19920315)69:6<1513::aid-cncr2820690633>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]


