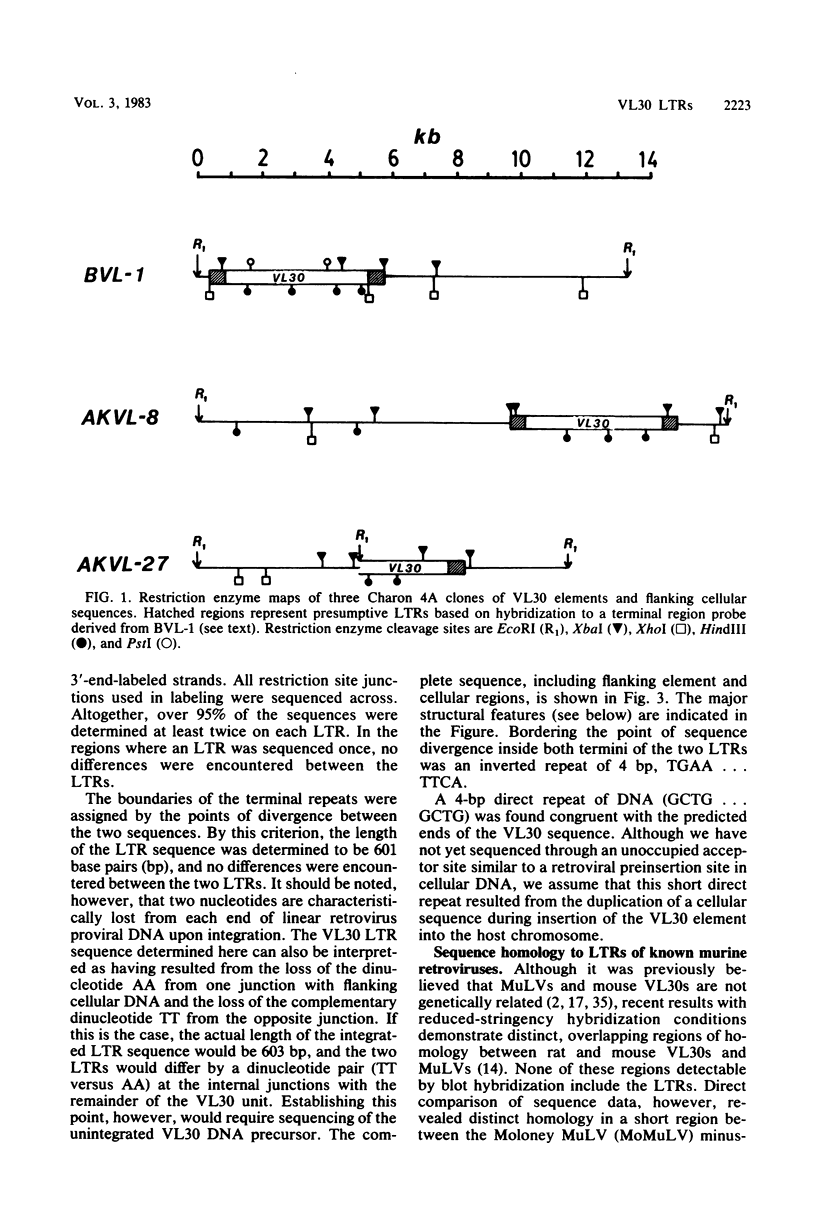
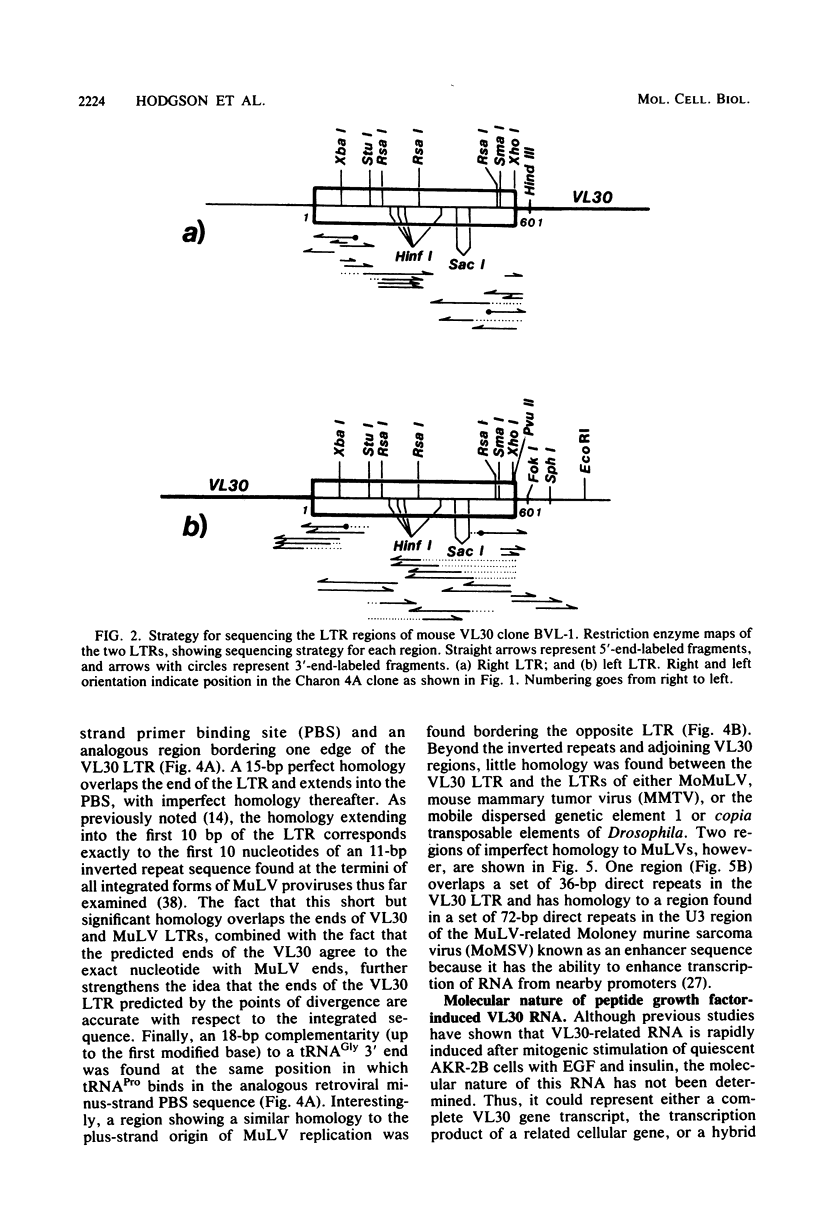
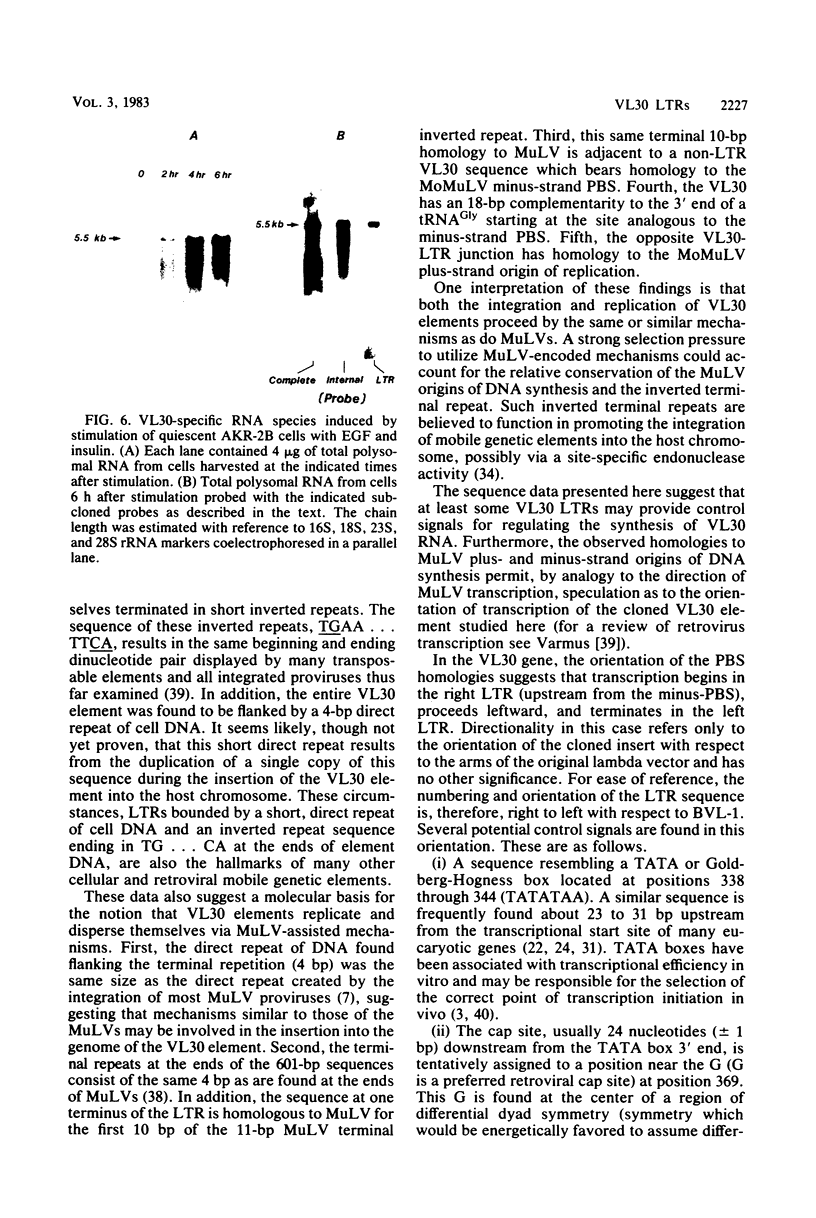
Abstract
DNA sequencing and blot hybridization analyses have been used to study the structure of a mouse VL30 gene and the molecular nature of VL30-related RNA which is induced upon the stimulation of cultured AKR mouse embryo cells with defined peptide growth factors. An integrated mouse VL30 gene was found to contain identical 601-base-pair long terminal repeats (LTRs) which were themselves terminated in short inverted repeats. The entire VL30 gene was flanked by a 4-base-pair direct repeat of cellular DNA. Thus, VL30 genes are structurally analogous to integrated forms of retrovirus proviruses and certain other classes of mobile genetic elements. The LTR sequence was found to contain putative promoter and polyadenylation signals and generally exhibited little sequence homology to murine leukemia virus proviral LTRs. Certain short regions of sequence conservation, however, were evident, including the inverted terminal repeat, LTR-adjacent regions corresponding to origins of murine leukemia virus proviral DNA synthesis, and a 36-base-pair direct repeat bearing homology to the 72-base-pair direct repeat (enhancer sequence) of the murine leukemia virus-related Moloney sarcoma virus. Upon mitogenic stimulation of quiescent cells with epidermal growth factor and insulin, a major 5.5-kilobase VL30-specific RNA complementary to both LTR and non-LTR sequences was rapidly induced. We conclude that a complete VL30 gene(s) is highly regulated by peptide growth factor binding to specific membrane receptors in these cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson G. R., Robbins K. C. Rat sequences of the Kirsten and Harvey murine sarcoma virus genomes: nature, origin, and expression in rat tumor RNA. J Virol. 1976 Feb;17(2):335–351. doi: 10.1128/jvi.17.2.335-351.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besmer P., Olshevsky U., Baltimore D., Dolberg D., Fan H. Virus-like 30S RNA in mouse cells. J Virol. 1979 Mar;29(3):1168–1176. doi: 10.1128/jvi.29.3.1168-1176.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Brutlag D. L., Clayton J., Friedland P., Kedes L. H. SEQ: a nucleotide sequence analysis and recombination system. Nucleic Acids Res. 1982 Jan 11;10(1):279–294. doi: 10.1093/nar/10.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney M. G., Elder P. K., Steffen D. L., Getz M. J. Evidence for an early evolutionary origin and locus polymorphism of mouse VL30 DNA sequences. J Virol. 1982 Aug;43(2):511–518. doi: 10.1128/jvi.43.2.511-518.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney M. G., Schmidt L. J., Getz M. J. Organization and expression of endogenous virus-like (VL30) DNA sequences in nontransformed and chemically transformed mouse embryo cells in culture. Cancer Res. 1982 Feb;42(2):569–576. [PubMed] [Google Scholar]
- Dhar R., McClements W. L., Enquist L. W., Vande Woude G. F. Nucleotide sequences of integrated Moloney sarcoma provirus long terminal repeats and their host and viral junctions. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3937–3941. doi: 10.1073/pnas.77.7.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C., Smith R., Peters G. In vitro synthesis of polypeptides encoded by the long terminal repeat region of mouse mammary tumour virus DNA. Nature. 1981 Jun 11;291(5815):511–513. doi: 10.1038/291511a0. [DOI] [PubMed] [Google Scholar]
- Donehower L. A., Huang A. L., Hager G. L. Regulatory and coding potential of the mouse mammary tumor virus long terminal redundancy. J Virol. 1981 Jan;37(1):226–238. doi: 10.1128/jvi.37.1.226-238.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Kafatos F. C., Maniatis T. The primary structure of rabbit beta-globin mRNA as determined from cloned DNA. Cell. 1977 Apr;10(4):571–585. doi: 10.1016/0092-8674(77)90090-3. [DOI] [PubMed] [Google Scholar]
- Foster D. N., Schmidt L. J., Hodgson C. P., Moses H. L., Getz M. J. Polyadenylylated RNA complementary to a mouse retrovirus-like multigene family is rapidly and specifically induced by epidermal growth factor stimulation of quiescent cells. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7317–7321. doi: 10.1073/pnas.79.23.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garel J. P., Keith G. Nucleotide sequence of Bombyx mori L. tRNA1Gly. Nature. 1977 Sep 22;269(5626):350–352. doi: 10.1038/269350a0. [DOI] [PubMed] [Google Scholar]
- Getz M. J., Elder P. K., Benz E. W., Jr, Stephens R. E., Moses H. L. Effect of cell proliferation on levels and diversity of poly(A)-containing mRNA. Cell. 1976 Feb;7(2):255–65. doi: 10.1016/0092-8674(76)90025-8. [DOI] [PubMed] [Google Scholar]
- Giri C. P., Hodgson C. P., Elder P. K., Courtney M. G., Getz M. J. Discrete regions of sequence homology between cloned rodent VL30 genetic elements and AKV-related MuLV provirus genomes. Nucleic Acids Res. 1983 Jan 25;11(2):305–319. doi: 10.1093/nar/11.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbüchle O., Bovey R., Young R. A. Tissue-specific expression of mouse-alpha-amylase genes: nucleotide sequence of isoenzyme mRNAs from pancreas and salivary gland. Cell. 1980 Aug;21(1):179–187. doi: 10.1016/0092-8674(80)90125-7. [DOI] [PubMed] [Google Scholar]
- Hobbs A. A., Rosen J. M. Sequence of rat alpha- and gamma-casein mRNAs: evolutionary comparison of the calcium-dependent rat casein multigene family. Nucleic Acids Res. 1982 Dec 20;10(24):8079–8098. doi: 10.1093/nar/10.24.8079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howk R. S., Troxler D. H., Lowy D., Duesberg P. H., Scolnick E. M. Identification of a 30S RNA with properties of a defective type C virus in murine cells. J Virol. 1978 Jan;25(1):115–123. doi: 10.1128/jvi.25.1.115-123.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. L., Ostrowski M. C., Berard D., Hager G. L. Glucocorticoid regulation of the Ha-MuSV p21 gene conferred by sequences from mouse mammary tumor virus. Cell. 1981 Dec;27(2 Pt 1):245–255. doi: 10.1016/0092-8674(81)90408-6. [DOI] [PubMed] [Google Scholar]
- Itin A., Keshet E. Nucleotide sequence analysis of the long terminal repeat of murine virus-like DNA (VL30) and its adjacent sequences: resemblance to retrovirus proviruses. J Virol. 1983 Sep;47(3):656–659. doi: 10.1128/jvi.47.3.656-659.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshet E., Itin A. Patterns of genomic distribution and sequence heterogeneity of a murine "retrovirus-like" multigene family. J Virol. 1982 Jul;43(1):50–58. doi: 10.1128/jvi.43.1.50-58.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshet E., Shaul Y., Kaminchik J., Aviv H. Heterogeneity of "virus-like" genes encoding retrovirus-associated 30S RNA and their organization within the mouse genome. Cell. 1980 Jun;20(2):431–439. doi: 10.1016/0092-8674(80)90629-7. [DOI] [PubMed] [Google Scholar]
- Keshet E., Shaul Y. Terminal direct repeats in a retrovirus-like repeated mouse gene family. Nature. 1981 Jan 1;289(5793):83–85. doi: 10.1038/289083a0. [DOI] [PubMed] [Google Scholar]
- Konkel D. A., Maizel J. V., Jr, Leder P. The evolution and sequence comparison of two recently diverged mouse chromosomal beta--globin genes. Cell. 1979 Nov;18(3):865–873. doi: 10.1016/0092-8674(79)90138-7. [DOI] [PubMed] [Google Scholar]
- Korn L. J., Brown D. D. Nucleotide sequence of Xenopus borealis oocyte 5S DNA: comparison of sequences that flank several related eucaryotic genes. Cell. 1978 Dec;15(4):1145–1156. doi: 10.1016/0092-8674(78)90042-9. [DOI] [PubMed] [Google Scholar]
- Lai E. C., Stein J. P., Catterall J. F., Woo S. L., Mace M. L., Means A. R., O'Malley B. W. Molecular structure and flanking nucleotide sequences of the natural chicken ovomucoid gene. Cell. 1979 Nov;18(3):829–842. doi: 10.1016/0092-8674(79)90135-1. [DOI] [PubMed] [Google Scholar]
- Laimins L. A., Khoury G., Gorman C., Howard B., Gruss P. Host-specific activation of transcription by tandem repeats from simian virus 40 and Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6453–6457. doi: 10.1073/pnas.79.21.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F., Mulligan R., Berg P., Ringold G. Glucocorticoids regulate expression of dihydrofolate reductase cDNA in mouse mammary tumour virus chimaeric plasmids. Nature. 1981 Nov 19;294(5838):228–232. doi: 10.1038/294228a0. [DOI] [PubMed] [Google Scholar]
- Levinson B., Khoury G., Vande Woude G., Gruss P. Activation of SV40 genome by 72-base pair tandem repeats of Moloney sarcoma virus. Nature. 1982 Feb 18;295(5850):568–572. doi: 10.1038/295568a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McReynolds L., O'Malley B. W., Nisbet A. D., Fothergill J. E., Givol D., Fields S., Robertson M., Brownlee G. G. Sequence of chicken ovalbumin mRNA. Nature. 1978 Jun 29;273(5665):723–728. doi: 10.1038/273723a0. [DOI] [PubMed] [Google Scholar]
- Nishioka Y., Leder P. The complete sequence of a chromosomal mouse alpha--globin gene reveals elements conserved throughout vertebrate evolution. Cell. 1979 Nov;18(3):875–882. doi: 10.1016/0092-8674(79)90139-9. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J. Complete 3' noncoding region sequences of rabbit and human beta-globin messenger RNAs. Cell. 1977 Apr;10(4):559–570. doi: 10.1016/0092-8674(77)90089-7. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Vass W. C., Howk R. S., Duesberg P. H. Defective retrovirus-like 30S RNA species of rat and mouse cells are infectious if packaged by type C helper virus. J Virol. 1979 Mar;29(3):964–972. doi: 10.1128/jvi.29.3.964-972.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro J. A. Molecular model for the transposition and replication of bacteriophage Mu and other transposable elements. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1933–1937. doi: 10.1073/pnas.76.4.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin S. A., Rapp U. R., Benveniste R. E., Sen A., Todaro G. J. Rescue of endogenous 30S retroviral sequences from mouse cells by baboon type C virus. J Virol. 1978 May;26(2):257–264. doi: 10.1128/jvi.26.2.257-264.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C. P., Cohen S. N. 3'-end labeling of DNA with [alpha-32P]cordycepin-5'-triphosphate. Gene. 1980 Jul;10(2):177–183. doi: 10.1016/0378-1119(80)90135-3. [DOI] [PubMed] [Google Scholar]
- Van Beveren C., Rands E., Chattopadhyay S. K., Lowy D. R., Verma I. M. Long terminal repeat of murine retroviral DNAs: sequence analysis, host-proviral junctions, and preintegration site. J Virol. 1982 Feb;41(2):542–556. doi: 10.1128/jvi.41.2.542-556.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E. Form and function of retroviral proviruses. Science. 1982 May 21;216(4548):812–820. doi: 10.1126/science.6177038. [DOI] [PubMed] [Google Scholar]
- Zarucki-Schulz T., Tsai S. Y., Itakura K., Soberon X., Wallace R. B., Tsai M. J., Woo S. L., O'Malley B. W. Point mutagenesis of the ovalbumin gene promoter sequence and its effect on in vitro transcription. J Biol Chem. 1982 Sep 25;257(18):11070–11077. [PubMed] [Google Scholar]