Abstract
We report two cases of anti-glutamic acid receptor (anti-GluR) antibody-positive encephalitis in males with symptoms such as Parkinsonism, urinary retention, and paralytic ileus. Although non-herpetic encephalitis typically shows magnetic resonance imaging (MRI) lesions in the limbic system during early stages, the present cases showed MRI lesions during later stages in the bilateral claustrum and pons. In both cases, anti-GluRɛ2 and δ2 antibodies were later shown to be positive in the cerebrospinal fluid but negative in the serum. Although early detection of anti-GluR antibodies is essential, early treatment may be significantly more important.
Keywords: anti-GluR antibody-positive encephalitis, MRI, Parkinsonism, autonomic failure
Introduction
The cause of non-herpetic limbic encephalitis is often uncertain, but it has recently been suggested that anti-GluR antibody-positive encephalitis may contribute to development of the condition.1–3 Symptoms such as Parkinsonism and autonomic failure have been reported in non-herpetic limbic encephalitis.4 The lesions present in non-herpetic limbic encephalitis are typically detected by magnetic resonance imaging (MRI) during the early phase. There are several case reports regarding MRI patterns of anti-N-methyl-D-aspartate receptor (NMDAR) and anti-voltage gated potassium channel (VGKC) antibody encephalitis.5,6 However, case reports regarding anti-GluR antibody-positive encephalitis MRI are uncommon, and the relationship between anti-GluR and anti-NMDAR remains unclear. Here, we report two unique cases in males with Parkinsonism and autonomic failure, as well as atypical MRI legions during a later phase.
Case Report
Case 1
A 75-year-old previously healthy man developed arthralgia in both distal interphalangeal joints. Forty-eight days later, he experienced hearing loss and tinnitus in the left ear; by day 58, his walking had become unsteady. His cognition gradually became impaired and he was transferred to our hospital 77 days after symptom onset.
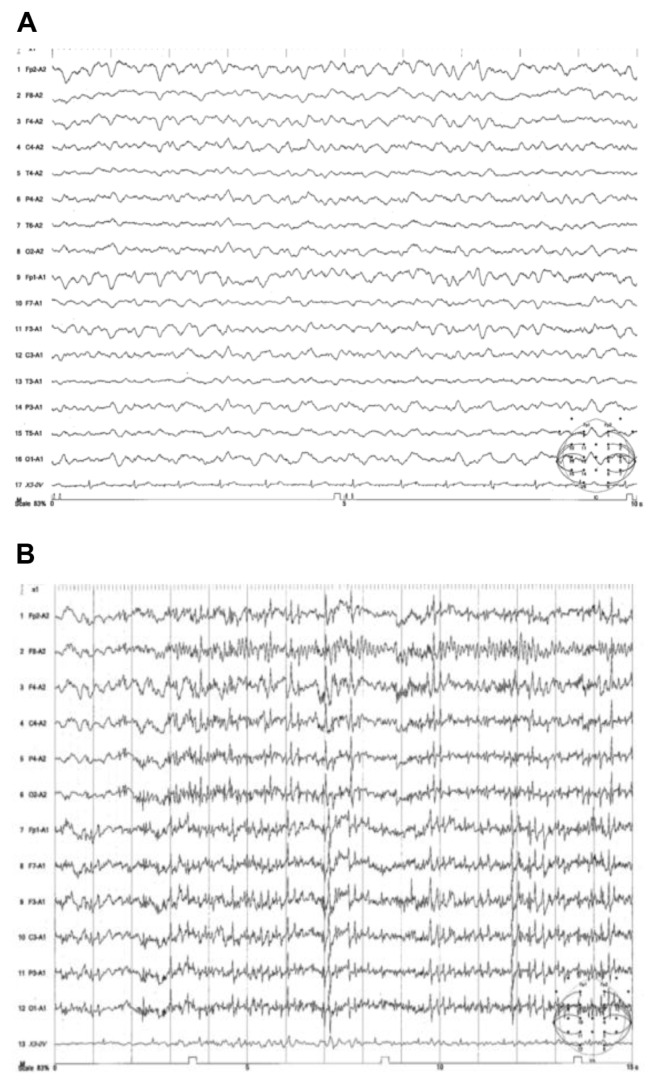
His consciousness was stupor with Glasgow coma scale (GCS) at 11 (E3V3M5). Cogwheel rigidity was observed in his bilateral upper limbs, although he was not taking any medication known to cause Parkinsonism. His gait was wide-based and unstable, and nuchal rigidity and Kernig sign were evident. Laboratory examination showed a white cell count of 11,610/μL and a serum reactive C-protein of 0.49 mg/dL. Erythrocyte sedimentation rate was elevated at 65 mm/h. Cartinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) were normal (3.68 ng/mL and 1.2 U/mL, respectively). Electroencephalogram (EEG) showed a diffuse slow wave of 3–4 Hz (Fig. 1A). An MRI during the early clinical phase (day 78 and 95) was unremarkable (Fig. 2A). Single photon emission computed tomography (SPECT) with 99mTc-ethylcysteinate dimer (99mTc-ECD) showed a mild decrease in bilateral frontal and left parietal cerebrocortical blood flow. Chest and abdominal computed tomography (CT) showed no space-occupying lesion.
Figure 1.
(A) EEG of case 1 showing widespread continuous slow-wave abnormalities. (B) EEG of case 2 showing polyspike.
Figure 2.
(A) Axial fluid attenuated inversion recovery (FLAIR) MR images of case 1. FLAIR MR image at 78 (a), 95 (b), 120 (c), 127 (d), and 182 (e) days after onset showing high signal intensity in the bilateral claustrum, medial of the anterior lobe, and periventricular legions (arrows) only at 120 and 127 days after onset, which then disappeared 182 days after onset. Mini mental state examination (MMSE). (B) FLAIR MR image of case 2 at 8 (f), 19 (g), 27 (h), 57 (i), and 91 (j) days after onset showing high signal intensity in the bilateral basal ganglia (arrows) at 19 days after onset and in the pons at 57 days after onset, but both disappeared 91 days after onset.
A lumbar puncture on the first hospital day revealed a cell count of 63/μL (monocyte 48/μL, polynuclear cell 15/μL), an elevated protein level of 72 mg/dL, and a sugar level of 50 mg/dL compared with a serum level of 86 mg/dL. Bacterial and viral cultures of the cerebrospinal (CSF) were negative. Real-time PCR of CSF herpes simplex virus was negative. Interleukin-6 in serum was elevated to 519 pg/mL and in CSF to 911 pg/mL. Autoimmune encephalitis was suspected, and methylprednisolone pulse therapy (1,000 mg/day) was performed twice: at 78–80 days and 85–87 days from onset. His consciousness improved with an increase in GCS to 14 (E4V4M6). Oral prednisolone 60 mg/day (1 mg/kg/day) was initiated, but his consciousness worsened with GCS, dropping to 8 (E2V1M5) at 98 days. Myoclonus appeared in his right upper and lower limbs, and his Parkinsonism worsened. He was not able to sit by himself. Although the deep tendon reflex was normal on the day of admission, it became hyperreflexia, and bilateral Babinski and Chaddock reflexes became positive at 98 days. In an attempt to improve his encephalitis, plasma exchange therapy was performed at 99, 101, 103, and 105 days. This was followed by two doses of methylprednisolone pulse therapy, which gradually improved his symptoms. During the course of symptom improvement, MRI signal abnormalities became evident in the bilateral claustrum, medial of the frontal lobe, with periventricular lesions, at 120 days (Fig. 2A). When he finally returned home after 167 days, his consciousness was alert with GCS of 15 (E4V5M6) and MMSE of 24. Anti-GluRɛ2 and δ2 antibodies were positive in the CSF on the first hospital day, but not in serum after his illness improved (Table 1). Anti-VGKC antibody was negative.
Table 1.
Anti-GluR antibodies measured by ELISA.
| GluRɛ2-NT2 | GluRɛ2-CT1 | GluRδ2-NT | GluRδ2-CT | |
|---|---|---|---|---|
| Serum | ||||
| Control | 0.639 | 0.637 | 0.721 | 0.752 |
| Case 1 (1st hospital day) | 0.384 | 0.389 | 0.462 | 0.454 |
| Case 2 (1st hospital day) | 0.316 | 0.358 | 0.37 | 0.34 |
| CSF | ||||
| Non-inflammation partial seizure | 0.293 | 0.317 | 0.395 | 0.442 |
| Non-inflammation mental disorder | 0.276 | 0.359 | 0.373 | 0.361 |
| Non-inflammation generalized seizure | 0.247 | 0.352 | 0.413 | 0.337 |
| Case 1 (1st hospital day) | 0.840 | 0.915 | 1.144 | 1.143 |
| Case 2 (1st hospital day) | 0.562 | 0.606 | 0.742 | 0.669 |
Notes: Serum control of anti-glutamic acid receptor (anti-GluR) antibodies showed negative control and CSF control showed disease control. In both of case 1 and 2, serum anti-GluR receptor antibodies were negative and CSF anti-GluR antibodies were positive.
Abbreviation: CSF, Cerebrospinal fluid.
Case 2
A 52-year-old previously healthy man developed a fever of 39.5 °C and a headache, and four days later gait disturbance appeared accompanied by pollakiuria (approximately 16 times per day) and impaired cognition. He was transferred to our hospital 8 days after symptom onset.
Physical examination showed the absence of abdominal bowel sounds with abdominal distension. Complete urinary retention developed with urethral catheter placement. His consciousness was stupor with GCS at 12 (E4V4M4), and nuchal rigidity and Kernig sign were evident. Serum osmotic pressure was 243 mOsm/kg · H2O, serum sodium 118 mEq/L, urinary osmotic pressure 799 mOsm/kg · H2O, and urinary sodium 164 mEq/L. His serum vasopressin level increased to 7.9 pg/mL. He was diagnosed with syndrome of inappropriate secretion of antidiuretic hormone (SIADH). CEA and CA19-9 were normal (3.12 ng/mL and 0.8 U/mL, respectively). Abdominal X-ray showed colon expansion and the patient was diagnosed with paralytic ileus. EEG showed polyspike (Fig. 1B). A cranial MRI during the early clinical phase (day 8) showed relatively high intensity of the right posterior cortex (Fig. 2B). The SPECT with 99mTc-ECD showed a severe decrease in diffuse cerebrocortical blood flow. Chest and abdominal CT showed no space-occupying lesion.
A lumbar puncture on the first hospital day revealed a cell count of 68/μL (monocyte 68/μL, polynuclear 0/μL), an elevated protein level of 279 mg/dL, and a sugar level of 50 mg/dL compared with a serum level of 78 mg/dL. Real-time PCR of CSF herpes simplex virus was negative. Because autoimmune encephalitis was also suspected, methylprednisolone pulse therapy (1,000 mg/day) was performed for 3 days from days 9 to 11. His cognition began to improve from day 12, and cranial MRI showed abnormal intensities in the bilateral basal ganglia at day 19 and in the pons at day 57 (Fig. 2B). He was able to care for himself at home with his wife and he resumed working. Anti-GluRɛ2 and δ2 antibodies were positive in CSF on the first hospital day, but not in the serum after his illness improved (Table 1).
Discussion
We report two cases of encephalitis presenting with positive anti-GluR antibodies. In addition to disturbance of recognition, Parkinsonism, and myoclonus appeared in case 1 and urinary retention, paralytic ileus, and SIADH appeared in case 2. In case 2, whether pons inflammation involved hyponatremia remains unknown since the abnormal intensity of the MRI appeared after hyponatremia had diminished. EEG showed a slow wave in case 1 (Fig. 1A), but polyspike in case 2 (Fig. 1B). Methylprednisolone pulse therapy was effective, but case 1 required additional plasma exchange therapy. Therefore, there may be several clinical types of anti-GluR antibody-positive encephalitis.
Both cases showed slightly increased MRI intensity during the early phase, which later became evident at 120 days (case 1) and 57 days (case 2). Non-herpetic limbic encephalitis frequently shows MRI abnormalities in the limbic system, but the present cases showed later appearance of abnormal intensities in bilateral claustrum and the pons. The relationship between the symptom and MRI abnormal area of anti-GluR antibody-positive encephalitis may clear the mechanism of encephalitis. These cases demonstrate the importance of considering anti-GluR antibody-positive encephalitis even when there is no abnormal intensity in the cranial MRI in the early phase.
Acknowledgements
We appreciate the cooperation of the patients and their families. Written consent for reproduce information or photographs appearing in this work was obtained from the patients and families. We thank Dr. Yukitoshi Takahashi, National Epilepsy Center, Shizuoka Institute of Epilepsy and Neurological Disorders, Shizuoka, Japan.
Footnotes
Author Contributions
Conceived and designed the experiments: KM. Analysed the data: TK, SD, TY, KD, YI. Wrote the first draft of the manuscript: KM. Contributed to the writing of the manuscript: KM. Agree with manuscript results and conclusions: KA. Jointly developed the structure and arguments for the paper: KA. Made critical revisions and approved final version: KA. All authors reviewed and approved of the final manuscript.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
Funding
This work was partly supported by a Grant-in-Aid for Scientific Research (B) 21390267 from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and by Grants-in-Aid from the Research Committee of CNS Degenerative Diseases (to Nakano I), and grants (to Mizusawa H, Nishizawa M, Sasaki H, and Sobue G) from the Ministry of Health, Labour and Welfare of Japan.
References
- 1.Kamei S, Kuzuhara S, Ishihara M, et al. Nationwide survey of acute juvenile female non-herpetic encephalitis in Japan: relationship to anti-N-methyl-D-aspartate receptor encephalitis. Intern Med. 2009;48(9):673–9. doi: 10.2169/internalmedicine.48.1898. [DOI] [PubMed] [Google Scholar]
- 2.Wakamoto H, Takahashi Y, Ebihara T, et al. An immunologic case study of acute encephalitis with refractory, repetitive partial seizures. Brain Dev. 2012;34(9):763–7. doi: 10.1016/j.braindev.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi Y, Kubota Y, Yamasaki E, Matsuda K. Rasmussen encephalitis and non-herpetic acute limbic encephalitis. Rinsho Shinkeigaku. 2008;48(3):163–72. doi: 10.5692/clinicalneurol.48.163. [DOI] [PubMed] [Google Scholar]
- 4.Dale RC, Irani SR, Brilot F, et al. N-methyl-D-aspartate receptor antibodies in pediatric dyskinetic encephalitis lethargica. Annals of Neurology. 2009;66(5):704–9. doi: 10.1002/ana.21807. [DOI] [PubMed] [Google Scholar]
- 5.Geschwind MD, Tan KM, Lennon VA, et al. Voltage-gated potassium channel autoimmunity mimicking creutzfeldt-jakob disease. Arch Neurol. 2008;65(10):1341–6. doi: 10.1001/archneur.65.10.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iizuka T, Yoshii S, Kan S, et al. Reversible brain atrophy in anti-NMDA receptor encephalitis: a long-term observational study. J Neurol. 2010;257(10):1686–91. doi: 10.1007/s00415-010-5604-6. [DOI] [PMC free article] [PubMed] [Google Scholar]