Abstract
Cervical cancer is still a major contributor to cancer-related mortality amongst women living in poor, rural communities of developing countries. The objective of this study is to establish the clinical presentation of cervical cancer and the management challenges encountered in Abakaliki, southeast Nigeria, with a view to finding intervention strategies. This study is a retrospective descriptive assessment of cases of clinically diagnosed cervical cancer managed at a state teaching hospital over six years. Of 76 cases managed, 61 (80.3%) cases notes were available for study. The mean age and parity of patients were 53.8 years and 6.8 years, respectively. The majority (75.4%) were illiterate. All had been married, but 42.6% were widowed. The main occupations were farming or petty trading. One patient (1.6%) had had a single Pap smear in her life. The major presenting complaints were abnormal vaginal bleeding (86.9%), offensive vaginal discharge (41.0%), and weight loss. Twenty patients (32.8%) were lost to follow-up prior to staging. Of the remaining 41 patients, 16 (39.0%) had stage III disease and 17.1% stage IV. Fifteen patients (24.6%) with late stage disease accepted referral, and were referred for radiotherapy. Those who declined were discharged home on request, though 4 (9.8%) died in the hospital. There was no feedback from referred patients confirming that they went and benefitted from the referral. The presentation followed known trends. Illiteracy, poverty, early marriages, high parity, widowhood, non-use of screening methods, late presentation, non-acceptance of referral, and lack of communication after referral were some of the major challenges encountered. These underscore the needs for health education and awareness creation, women educational and economic empowerment, legislation against early marriages and in protection of widows, and creation of a well-staffed and well-equipped dedicated gynecologic oncology unit to forestall further referral.
Keywords: cervical cancer, late presentation, cancer-related deaths, management challenges, referral
Introduction
Globally, cervical cancer constitutes about 12% of all cancers in women,1 and reflects striking health inequity more than any other cancer.2 It is the second most common cancer in women worldwide, but is the most common among women in developing countries.1–3 More than 85% of approximately 529,800 cases diagnosed annually occur in developing countries, a proportion that is expected to increase to 90% by 2020.2,4 In addition, cervical cancer is the leading cause of cancer deaths among women in low-resource settings.1,2 For instance, every two minutes a woman dies from the disease, equating to more than 275,000 cancer deaths annually, of which more than 87% occur in developing countries.4 It also affects relatively young women and is the largest single cause of years of life lost to cancer in the developing world.2 The deaths of these women who are in their most productive years have devastating effects on the well-being of their families, and results, for example, in decreases in school attendance and nutritional status among their children.2,5 Cancer of the cervix affects women at a time of life when they are critical to social and economic stability.6
Although mortality from cancer of the cervix has declined in many developed countries,7–9 such as the United Kingdom and Sweden, the story is different with the sub-Saharan African region. The sub- Saharan African region posts the highest incidence rates for cervical cancer in the world.4,6 Here, cervical cancer is not only the most frequently occurring cancer among middle-aged women, but also a leading cause of death.10 The disproportionately high burden of cervical cancer in this region and elsewhere in medically underserved populations is largely due to a lack of screening that allows detection of precancerous and early stage cervical cancer.6,10 So, while about 75% of cases present early in developed countries thereby making expectation of cure realistic, in developing countries, the majority of cases present with advanced disease.6 For instance, Ibrahim et al10 found that about 72% of the cervical cancer cases in a Sudanese study conducted in 2007 were diagnosed at an advanced stage.
In Nigeria, cancer management is domiciled in teaching hospitals located in urban cities, and reported incidence rates for cervical cancer are institution-based. Cervical cancer is the second most common cancer in females and the most common female genital tract cancer, constituting about 62.3%–70.5% of all gynecological cancers.11–14 Literature reveals a peak mean age incidence range of 43.5–59.7 years,12–19 endorsing the assertion that women are afflicted at an age in life when they are critical to social and economic stability.6 Other risk factors reported in the patients include poverty and low socio-economic status, grand multi-parity, limited educational attainment, early age at onset of sexual intercourse, polygamous marriages, and history of multiple sex partners.10–13,20 The documented clinical features7,12–15 include abnormal vaginal bleeding (inter-menstrual, post-coital, post-menopausal bleeding) in about 51.9%–100% of cases, offensive vaginal discharge in some 25%–79% of cases, and pain from nerve involvement on pelvic side wall. Majority of the cases (75%–86%) tended to present very late, with advanced disease,13,14,19 and these later patients were apt to have weight loss, backache, leg pain, edema, and haematuria.12,13,16,17 This assemblage of problems may increase the challenges care givers faced in the management of cervical cancer in Nigeria.
This study aimed to establish the clinical presentation of cervical cancer at a tertiary hospital in Abakaliki, southeast Nigeria, and to identify the challenges care givers faced in its management with a view to finding strategies for intervention.
Methodology
The Ebonyi State University Teaching Hospital (EBSUTH) was a state-owned teaching hospital and one of the two tertiary hospitals in Abakaliki, the Ebonyi State capital. EBSUTH offered tertiary healthcare services. The major beneficiaries of the services were Ebonyi State indigenes. Patients also came from the other neighboring states of Abia, Akwa Ibom, Benue, Cross River, Enugu, and Imo. The main occupations of the major beneficiaries included farming, fishing, and petty trading. Illiteracy was rife amongst them and their health seeking behavior poor.
EBSUTH had a well-developed Department of Obstetrics and Gynecology (O&G), but did not have a dedicated oncology unit. It also lacked facilities for radiotherapy. And although the department had a designated Oncology Team, cases of gynecological oncology were not usually referred to this team. Rather, they were managed by other teams as they Clinical presentation and management challenges of cervical cancer deemed best, based on their experience and the facilities at their disposal.
This was a six-year (January 2005 to December 2010 inclusive) retrospective descriptive study of all the cases of cervical cancer clinically diagnosed and managed at EBSUTH, Abakaliki. Data on patient’s age, parity, education, marital status, presenting complaints, management and outcome, and referral were collected from patients’ case notes, theatre records, and pathological records. Analysis was done using simple percentages and inferential statistics.
The research was approved by the Hospital’s Research Ethics Committee.
Results
Of the 76 cases clinically diagnosed as cervical cancer, 61 case notes (80.3%) were able to be retrieved and studied. Table 1 shows their demographic characteristics. Their ages ranged from 32 to 78 years, the modal age range was 60–69 years, and the mean age (±SD) was 54.0 (±12.7) years. Also, their parity ranged from 0 to 16 with a mean of 7.0 (±3.2); 47 of them (77.0%) were grand multiparous. Illiteracy was high as 22 (36.1%) had no formal education and 39.3% had only primary education. All the women had been previously married, but 26 (42.6%) were widows at presentation. Also, 15 (24.6%) married early (before menarche), 16.4% were in polygamous marriages, and 6.6% remarried. Their occupations were basically petty and some had more than one occupation: 37 (60.7%) were farmers, and 37.7% traders, while the rest were dependent either on their children and/or other relations, or were retirees. The sexual histories also showed that 45.9% had single sexual partners, 26.2% had multiple sex partners, and in 27.9% the number of sex partners was not documented. Only one of the patients (1.6%) had done Pap smear once in the past and the result was negative.
Table 1.
Demographic characteristics of patients who were clinically diagnosed with cervical cancer (N = 61).
| Item | Frequency (%) | Range | Mean (±standard deviation) |
|---|---|---|---|
| Age (years) | 32–78 | 54.0 (±12.7) | |
| ≤39 | 8 (13.1) | ||
| 40–49 | 16 (26.2) | ||
| 50–59 | 12 (19.7) | ||
| 60–69 | 17 (27.9) | ||
| ≥70 | 8 (13.1) | ||
| Parity | 0–16 | 7.0 (±3.2) | |
| 0 | 1 (1.6) | ||
| 1–4 | 13 (21.4) | ||
| ≥5 | 47 (77.0) | ||
| Educational status | |||
| No formal education | 22 (36.1) | ||
| Primary school | 24 (39.3) | ||
| Secondary school | 14 (23.0) | ||
| Tertiary | 1 (1.6) | ||
| Marital status and marriage characteristics | |||
| Married | 35 (57.4) | ||
| Widowed | 26 (42.6) | ||
| Early marriage | 15 (24.6) | ||
| Polygamous marriage | 10 (16.4) | ||
| Remarried | 4 (6.6) | ||
| Occupation | |||
| Farming | 37 (60.7) | ||
| Trading | 23 (37.7) | ||
| Dependent | 22 (36.1) | ||
| Retired | 4 (6.6) | ||
| Sexual partners | |||
| Multiple partners | 16 (26.2) | ||
| Single partner | 28 (45.9) | ||
| Undocumented | 17 (27.9) | ||
Table 2 depicts the patients’ presenting complaints, the initial medical care offered to them, and their acceptance and rejection of further care. The most common presenting complaint, abnormal vaginal bleeding, was documented in 88.5% of the patients. Other common complaints were offensive vaginal discharge (42.6%), weight loss (39.3%), and pain (Table 2). Twenty-nine (46.8%) of the patients were admitted in the first instance and transfused with between 2 to 6 units of blood to correct anemia and stabilize their clinical state prior to further management. Following counseling for examination under anesthesia (EUA), staging, and definitive care, 20 patients (32.8%) rejected further medical care. Of those who rejected further care, 15 (75%) benefited from blood transfusion and then signed against medical advice, while five (25%) ceased coming to the clinic before staging could be done. One of the patients who rejected further care died in the hospital while waiting to go home.
Table 2.
Presenting complaints and medical care offered to patients clinically diagnosed with cervical cancer (N = 61).
| Item | Frequency (%) |
|---|---|
| Presenting complaints | |
| Abnormal vaginal bleeding | 54 (88.5) |
| Offensive vaginal discharge | 26 (42.6) |
| Weight loss | 24 (39.3) |
| Pain | 14 (23.0) |
| Constipation | 5 (8.2) |
| Haematuria | 3 (4.9) |
| Leg swelling | 2 (3.3) |
| Haemoptysis | 1 (1.6) |
| Blood transfusion/acceptance of further medical care | |
| Transfusion | 29 (46.8) |
| Rejected further care | 20 (32.8) |
| Accepted | 41 (67.2) |
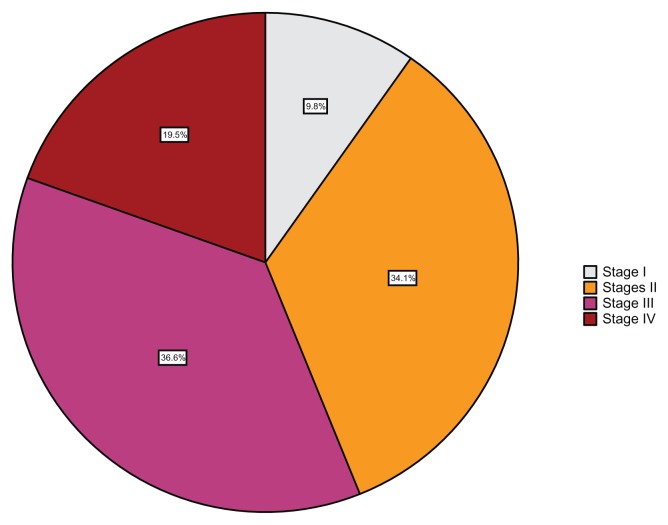
Table 3 shows the stages of the disease amongst the cases and the further medical care offered to the 41 patients who consented to post-counseling. The majority, 23 cases (56.1%), had advanced stage disease (39.0% stage III and 17.1% stage IV), while stage I comprised only 9.8% (Fig. 1). Two of the patients with late stage disease had had previous hysterectomies in peripheral hospitals for unstated gynecological indications, but the specimens did not undergo histopathological analysis. Eventually, 40 patients (97.6%) underwent EUA and staging and afterwards, 26.8% had a hysterectomy while 48.8% were referred for radiotherapy. One of the patients considered to have stage IV disease was referred for radiotherapy without undergoing EUA. None of the patients were tested for human papilloma virus (HPV) as the HPV test was not readily available in the hospital. Table 4 shows disease stage-specific interventions and outcomes. Twenty-one of the patients (51.2%), 14 of whom belonged to stages III and IV, had biopsy during EUA, while 11 had hysterectomy (all the patients with stage I and 50% of the patients with stage II). Twenty (48.8%) of the patients, of which 31.7% had late stage disease, were referred for radiotherapy. No back referral was recorded for any of the referred cases. Eleven patients, 26.8%, signed against medical advice during the course of management, 14.6% became lost to follow-up, while 9.8% died in care.
Table 3.
Stage of disease amongst and further management of patients with cervical cancer who accepted care (N = 41).
| Item | Frequency (%) |
|---|---|
| Stage | |
| Stage I | 4 (9.8) |
| Stage II | 14 (34.1) |
| Stage III | 15 (36.6) |
| Stage IV | 8 (19.5) |
| Further management | |
| EUA/staging | 40 (97.6) |
| Hysterectomy | 11 (26.8) |
| Referred | 20 (48.8) |
Abbreviation: EUA, Examination under anesthesia.
Figure 1.
Stage of disease at presentation amongst patients clinically diagnosed with and managed for cervical cancer (N = 41).
Table 4.
Stage of disease, stage-specific management offered and outcome (N = 41).
| Item | Stage I | Stage II | Stage III | Stage IV | Overall (%) |
|---|---|---|---|---|---|
| EUA/staging | 4 | 14 | 15 | 7 | 40 (97.6) |
| Biopsy | – | 7 | 10 | 4 | 21 (51.2) |
| Hysterectomy | 4 | 7 | – | – | 11 (26.8) |
| Referred | 2 | 5 | 7 | 6 | 20 (48.8) |
| Back referral | – | – | – | – | – (0.0) |
| Signed against | – | 2 | 4 | 5 | 11 (26.8) |
| Lost to follow up | – | 2 | 4 | – | 6 (14.6) |
| Died | – | – | 2 | 2 | 4 (9.8) |
Abbreviation: EUA, Examination under anesthesia.
Discussion
Cervical cancer has continued to have a devastating impact on women’s health globally, and particularly in developing countries like Nigeria where it has remained the most common cancer of the female genital tract.12,14,15,17–19
The demographic characteristics of the patients in this study share similarities with results from several other centers Nigeria. For instance, the mean age of patients in this study falls within the peak mean age range of about 43.5–54.5 years,11–16,21 and the high mean parity agrees with the results from other studies.11,16,20,21 These findings further affirm that cervical cancer afflicts women at a time when they are vital to social and economic stability6 and that grand multiparity is an important causal risk factor.
One of the major challenges and risk factors found in the study is high rate of illiteracy or low educational attainment.12–14 A low education level increases the risk of invasive cervical cancers and illiteracy has been shown to greatly increase this risk.22 Other risk factors identified were significant levels of early marriages, polygamous marriages, repeat marriages, and multiple sexual partners.12–14 And, although most cervical cancers (94%–100%) are associated with sexually transmitted genital infection by HPV,23 testing for HPV could not be performed on the patients in this series as the service was not available in the hospital. This is not surprising as the HPV testing for a study conducted at Ibadan, Nigeria, by Okolo et al was done in The Netherlands.24 The study confirmed that HPV16 and 18 are the two most frequently detected types among invasive cervical cancer in Nigeria, and that they account for 78% of HPV positive invasive cervical cancer.24 Fundamentally, unprotected coitus encourages HPV infection. So, early marriages, polygamy, remarriages, and multiple sexual partners are high risk factors25,26 as they increase the duration of exposure to and the probability of contracting HPV infection. Basically, these findings support the causal association between sexual activity and cervical cancer.25
More than 40% of the patients in this study were widows. Widows are vulnerable in the society because they are likely to be sexually exploited.26 Widowhood has been found to be at increased risk of HPV infection and cervical cancer than married women. Poverty, as shown by the petty occupations documented in this study, and widowhood could catalyze the likelihood of women succumbing to sexual advances by multiple sexual partners, thereby increasing the chances of HPV transmission. Poverty makes women unduly dependent on their husbands and partners for financial support and sustenance, and can lead to sexual promiscuity with its attendant short- and long-term cervical cancer-related risks.27 Poverty and/or widowhood also contribute to late presentation for obvious reasons. Poverty, early marriage, polygamy, grand multiparity, and illiteracy have all been found to be significantly associated with increased risk of occurrence of cervical cancer and could contribute to the high incidence of late presentation.17
Although cervical screening services are poorly developed in Nigeria,27 the available ones are also poorly utilized: only 1.6% of the patients in this case series was screened. Unfortunately, this finding compares favorably with the 0.6% documented amongst gynecological clinic attendees is southeast Nigeria,27 and confirms that cervical cancer screening uptake is very low in Nigeria, especially amongst the poor and illiterate women. Non-use of screening methods implies that cases are more likely to present or be discovered late, at a time when they are less probable to benefit from the commonly available management options.
The presenting complaints were essentially similar to the reports in the literatures reviewed. The incidence of abnormal vaginal bleeding was marginally higher than the 55.9%–84.0%11,13–15,18,19 reported in literature, thus affirming that abnormal vaginal bleeding is an important sign in cervical cancer. Abnormal vaginal bleeding normally occurs as post-coital, inter-menstrual, or postmenopausal bleeding. Should every case of abnormal vaginal bleeding be promptly investigated, cervical cancers would be diagnosed in early stages, at a time when there could be hopes of cure. However, due to the amalgamation of illiteracy, poverty, ignorance, non-utilization of screening services, and a host of others factors particular to the underserved in the poor rural communities of southeast Nigeria, the majority of the women present in late stage disease.11,13,15,16,19 Offensive vaginal discharge and weight loss were two other presenting complaints that occurred in about 40.0% of the cases in this study. Both of these complaints have been report with varying frequencies in association with cervical cancer.11,13–16,19
One of the major management challenges encountered was late presentation, as more than 56.0% of the cases presented in stages III and IV. Though less than 75.0% reported from Ilorin,13 85.5% from Zaria, 86.0% from Abuja,16 78.6% from Sagamu,15 and 89.3% from Nnewi,11 the results generally affirm that cervical cancer patients present very late to tertiary hospitals in Nigeria. Patients’ delay in seeking healthcare and care providers’ delay in referring patients to a tertiary hospital have been shown to contribute immensely to the late presentation.19 Patients that presented late tended to have multiple complaints and complications (Table 2). They were often anemic and needed blood transfusion and other forms of resuscitation prior to definitive investigations and treatment. This was a huge challenge in our centre as more than 46% needed transfusion, and blood for transfusion is not readily available, such that the blood bank operators had to often recourse to the poor relatives of the poor patients for blood, without success.28
Another frustrating challenge was that after the initial resuscitation, some 33% of the patients rejected further medical care, and an additional 14.6% of the patients that accepted further medical care became lost to follow-up later on. Their reasons were not known, but may not be unconnected with illiteracy, poverty, ignorance, and societal myths and beliefs. There is need for further research on this. Umezulike et al16 working in Abuja, Nigeria, reported a 44.4% lost to follow-up rate.
Approximately 98% of the patients that consented to further medical care had EUA and staging, and around 27% finally had a hysterectomy as they were adjudged to have early stage diseases. The rest, considered to have late stage diseases, were counseled and subsequently referred for radiotherapy. Regrettably, not every patient that was counseled and referred finally acceded to the referral. Moreover, amongst the patients that left promptly following their referral, there were no means of ascertaining if they actually heeded the referral, and there were no back referrals to confirm that patients complied with the referrals. So, although the patients were referred for optimal care, one cannot categorically state that they benefited from such referrals.
A cancer-related mortality rate of approximately 10% was recorded in this study amongst the patients who accepted further medical care and remained in the hospital long enough for the mortality to occur. There were no records of the outcomes of the patients that refused further medical care after the initial resuscitation and those who refused medical advice. Indeed, taken holistically, the cancer-related death rate could be much higher as most of the patients signed against further medical care and the majority of those referred were expected to succumb to the disease sooner if they did not receive further treatment. Unfortunately, there were no ways of tracking the patients.
By and large, cervical cancer still remains a major cause of cancer-related deaths in women from developing countries1,2,4 including Nigeria, with illiteracy, poverty, early marriages, polygamy, remarriages, grand multiparity, and late presentation as facilitators.29 In India, Thulaseedharan et al30 also found that illiterate women of increasing age and having many pregnancies were at significantly increased risk of cervical cancer.
Conclusion
Cervical cancer most often afflicts women in developing countries, like Nigeria. It reflects gross imbalance that results from inequalities in social and economic development, and in the infrastructural and human resources required for care.31 The risk factors for cervical cancer and management challenges identified in this study include high incidences of high parity, early marriages, polygamy, remarriages and widowhood, multiple sexual partners, poverty, illiteracy, and low educational attainment. Others factors include low cervical screening uptake, late presentation, lack of a dedicated gynecological oncology unit, poorly functioning blood bank, rejection of medical care, loss to follow-up, inability to perform HPV testing on cervical specimens collected from patients with cervical cancer, no-tracking of referrals, and absence of back referrals. To stem the incidence of cervical cancer in women, there is a need to curb these factors and challenges.
Sustained health education and awareness creation could mitigate high parity, early marriages, polygamy, multiple sexual partners, illiteracy, and late presentation, and improve uptake of cervical screening. Education and economic empowerment of women and girls would palliate illiteracy, early marriages, polygamy, high parity, multiple sexual partners, and late presentation, as well as improve cervical screening uptake. There is need for legislation in favor of widows and against early marriages. Tertiary hospitals should be encouraged to create dedicated gynecologic oncology units with appropriate services in place such as HPV testing of cervical specimens. Experiences pooled from such units could improve patient care and record keeping. They should also have functional blood banks to cushion the blood transfusion requirements of late presenters. Additionally, there is need for a patient tracking system to help recognize the whereabouts and outcomes of patients presenting with cervical cancer as a means of forestalling the dilemma of loss to follow-up.
In the interim, treatment for cervical cancer needs to be free or highly subsidized to make the care and benefit received by the patients independent of their financial abilities. Truly, reductions in cervical cancer rates can be achieved by reducing inequalities in socioeconomic conditions, availability of preventive health services, and improving women’s social status.31 To achieve these, there is a need for team work between the hospitals, government, non-governmental organizations, philanthropists, and the people. The Federal Government of Nigeria has commenced vaccination of young girls aged 9–15 years with HPV vaccines as part of the long-term preventive measures for cervical cancers in Nigeria. However, this vaccination may remain inaccessible to many without international assistance with funds,32 therefore amplifying the need for team work.
Acknowledgements
The authors are grateful to the doctors who helped in the collection of the data.
Footnotes
Author Contributions
Conceived and designed the experiments: JNE. Analyzed the data: JNE, ENE-I. Wrote the first draft of the manuscript: JNE. Contributed to the writing of the manuscript: JNE, ENE-I. Agree with manuscript results and conclusions: JNE, ENE-I, FOE. Jointly developed the structure and arguments for the paper: JNE, ENE-I. Made critical revisions and approved final version: JNE, ENE-I, FOE. All authors reviewed and approved of the final manuscript.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
Funding
Author(s) disclose no funding sources.
References
- 1.Denny L. The prevention of cervical cancer in developing countries. BJOG. 2005;112(9):1204–12. doi: 10.1111/j.1471-0528.2005.00713.x. [DOI] [PubMed] [Google Scholar]
- 2.Agosti JM, Goldie SJ. Introducing HPV vaccine in developing countries— key challenges and issues. N Engl J Med. 2007;356(19):1908–10. doi: 10.1056/NEJMp078053. [DOI] [PubMed] [Google Scholar]
- 3.Ali F, Kuelker R, Wassie B. Understanding cervical cancer in the context of developing countries. Ann Trop Med Public Health. 2012;5(1):3–15. [Google Scholar]
- 4.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 5.Saleh JA, Yusuph H, Zailani SB, Aji BM. Role of HPV vaccine in the prevention of cervical cancer. J Interdiscipl Histopathol. 2013;1(4):2146–8362. [Google Scholar]
- 6.WHO/ICO Information Centre on HPV and Cervical Cancer (HPV Information Centre) Human Papillomavirus and Related Cancers in World. Summary Report. 2010. [Accessed Nov 15, 2012]. Available at: http://www.who.int/hpvcentre.
- 7.Bergstrom R, Sparen P, Adami HO. Trends in cancer of the cervix uteri in sweden following cytological screening. Br J Cancer. 1999;81(1):159–66. doi: 10.1038/sj.bjc.6690666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sitas F, Parkin M, Chirenje Z, et al. Cancers. In: Jamison DT, Feachem RG, Makgoba MW, et al., editors. Disease and Mortality in Sub-Saharan Africa. 2nd ed. Chapter 20 Washington, DC: World Bank; 2006. [Google Scholar]
- 9.Anorlu RI. Cervical cancer: the sub-saharan African perspective. Reprod Health Matters. 2008;16(32):41–9. doi: 10.1016/S0968-8080(08)32415-X. [DOI] [PubMed] [Google Scholar]
- 10.Ibrahim A, Rasch V, Pukkala E, Aro AR. Predictors of cervical cancer being at an advanced stage at diagnosis in Sudan. Int J Womens Health. 2011;3:385–9. doi: 10.2147/IJWH.S21063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikechebelu JI, Onyiaorah IV, Ugboaja JO, Anyiam DC, Eleje GU. Clinicopathological analysis of cervical cancer seen in a tertiary health facility in Nnewi, south-east Nigeria. J Obstet Gynaecol. 2010;30(3):299–301. doi: 10.3109/01443610903531394. [DOI] [PubMed] [Google Scholar]
- 12.Uzoigwe SA, Seleye-Fubara D. Cancers of the uterine cervix in Port Harcourt, Rivers State-a 13-year clinico-pathological review. Niger J Med. 2004;13(2):110–3. [PubMed] [Google Scholar]
- 13.Ijaiya MA, Aboyeji PA, Buhari MO. Cancer of the cervix in Ilorin, Nigeria. West Afr J Med. 2004;23(4):319–22. doi: 10.4314/wajm.v23i4.28148. [DOI] [PubMed] [Google Scholar]
- 14.Oguntayo OA, Zayan M, Kolawole AOD, et al. Cancer of the cervix in Zaria, Northern Nigeria. Ecancermedicinalscience. 2011;5:219. doi: 10.3332/ecancer.2011.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olatunji AO, Sule-Odu AO. Cancer of the cervix. Niger Postgrad Med J. 2005;12(4):308–11. [PubMed] [Google Scholar]
- 16.Umezulike AC, Tabansi SN, Ewunonu HA, Nwana EJ. Epidemiological characteristics of carcinoma of the cervix in the Federal capital Territory of Nigeria. Niger J Clin Pract. 2007;10(2):143–6. [PubMed] [Google Scholar]
- 17.Ketiku KK, Ola ER, Ekanem EE. Cancer of the cervix in Nigeria: A case-control study of some epidemiological factors. NQJHM. 2004;14(2):161–5. [Google Scholar]
- 18.Ijaiya MA, Aboyeji PA, Olatinwo AW, Buhari MO. Cancer of the cervix in Ilorin, Nigeria. Nigerian J Surgical Res. 2002;4(3–4):89–93. [Google Scholar]
- 19.Anorlu RI, Orakwue CO, Oyeneyin L, Abudu OO. Late presentation of patients with cervical cancer to a tertiary hospital in Lagos: what is responsible? Eur J Gynaecol Oncol. 2004;25(6):729–32. [PubMed] [Google Scholar]
- 20.Kyari O, Nggada H, Mairiga A. Malignant tumours of female genital tract in North Eastern Nigeria. East Afr Med J. 2004;81(3):142–5. doi: 10.4314/eamj.v81i3.9144. [DOI] [PubMed] [Google Scholar]
- 21.Yakasai IA, Ugwa EA, Otubu J. Gynaecological malignancies in Aminu Kano Teaching Hospital Kano: a 3 year review. Niger J Clin Pract. 2013;16(1):63–6. doi: 10.4103/1119-3077.106768. [DOI] [PubMed] [Google Scholar]
- 22.Ngoan LT, Yoshimura T. Parity and Illiteracy as Risk Factors of Cervical Cancers in Viet Nam. Asian Pac J Cancer Prev. 2001;2(3):203–6. [PubMed] [Google Scholar]
- 23.Malik AI. The role of human papilloma virus (HPV) in the aetiology of cervical cancer. JPMA. 2005;55(12):553–8. [PubMed] [Google Scholar]
- 24.Okolo C, Franceschi S, Adewole I, et al. Human papillomavirus infection in women with and without cervical cancer in Ibadan, Nigeria. Infect Agent Cancer. 2010;5(1):24. doi: 10.1186/1750-9378-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barron BA, Richart RM. An epidemiologic study of cervical neoplastic disease, based on a self-selected sample of 7,000 women in Barbados, West Indies. Cancer. 1971;27(4):978–86. doi: 10.1002/1097-0142(197104)27:4<978::aid-cncr2820270433>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 26.Rosenblatt C, Lucon AM, Pereyra EA, Pinotti JA, Arap S, Ruiz CA. HPV prevalence among partners of women with cervical intraepithelial neoplasia. Int J Gynecol Obstet. 2004;84(2):156–61. doi: 10.1016/j.ijgo.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Eze JN, Umeora OU, Obuna JA, Egwuatu VE, Ejikeme BN. Cervical cancer awareness and cervical screening uptake at the Mater Misericordiae Hospital, Afikpo, Southeast Nigeria. Ann Afr Med. 2012;11(4):238–43. doi: 10.4103/1596-3519.102856. [DOI] [PubMed] [Google Scholar]
- 28.Umeora OU, Onuh SO, Umeora MC. Socio-cultural barriers to voluntary blood donation for obstetric use in a rural Nigerian village. Afr J Reprod Health. 2005;9(3):72–6. [PubMed] [Google Scholar]
- 29.Adewuyi SA, Shittu SO, Rafindadi AH. Sociodemographic and clinicopathologic characterization of cervical cancers in northern Nigeria. Eur J Gynaecol Oncol. 2008;29(1):61–4. [PubMed] [Google Scholar]
- 30.Thulaseedharam JV, Malila N, Hakama M, et al. Socio demography and reproductive risk factors for cervical cancer—a large prospective cohort study from rural India. Asian Pac J Cancer Prev. 2012;13(6):2991–5. doi: 10.7314/apjcp.2012.13.6.2991. [DOI] [PubMed] [Google Scholar]
- 31.Singh GK, Azuine RE, Siahpush M. Global inequalities in cervical cancer incidence and mortality are linked to deprivation, low socioeconomic status, and human development. IJMA. 2012;1(1):17–30. doi: 10.21106/ijma.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolawole AO. Cervical cancer prevention in Nigeria: issues arising. Int J Gynecol Obstet. 2012;16(1) [Google Scholar]