Abstract
Mitogen-activated protein kinase kinases (MAPKK) mediate a variety of stress responses in plants. So far little is known on the functional role of MAPKKs in cotton. In the present study, Gossypium hirsutum MKK1 (GhMKK1) function was investigated. GhMKK1 protein may activate its specific targets in both the nucleus and cytoplasm. Treatments with salt, drought, and H2O2 induced the expression of GhMKK1 and increased the activity of GhMKK1, while overexpression of GhMKK1 in Nicotiana benthamiana enhanced its tolerance to salt and drought stresses as determined by many physiological data. Additionally, GhMKK1 activity was found to up-regulate pathogen-associated biotic stress, and overexpression of GhMKK1 increased the susceptibility of the transgenic plants to the pathogen Ralstonia solanacearum by reducing the expression of PR genes. Moreover, GhMKK1-overexpressing plants also exhibited an enhanced reactive oxygen species scavenging capability and markedly elevated activities of several antioxidant enzymes. These results indicate that GhMKK1 is involved in plants defence responses and provide new data to further analyze the function of plant MAPK pathways.
Introduction
Plants face various harsh terrestrial environmental conditions, including high salinity, drought, and microbial pathogens, through their lifetime. To adapt to these environmental stresses, plants have developed sophisticated signaling machineries associated with regulation of their cellular metabolism. Protein phosphorylation is one of the major mechanisms the cell uses to translate external stimuli into cellular responses, and the mitogen-activated protein kinase (MAPK) cascades are involved in many of these processes.
Generally, MAPK cascades are composed of three kinases: MAPKs are phosphorylated by MAPK kinases (MAPKKs) on the threonine residues located in the activation loop (A-loop) between subdomains VII and VIII of the kinase catalytic domain, and the MAPKKs are activated by another group of serine/threonine protein kinases, the MAPK kinase kinases (MAPKKKs), at a conserved S/TXXXXXS/T motif. Compared to our current knowledge of the 20 identified plant MAPKs and the >60 putative MAPKKKs, there is a distinct lack of knowledge about the functions of the 10 MAPKKs found in Arabidopsis [1]. These facts suggest that the cross-talk in the MAPK cascades occurs at the level of MAPKKs, and one MAPKK is likely to be involved in multiple MAPK cascades, carrying out diverse biological functions [2].
A growing body of evidence has shown that MAPKKs display important functions in response to abiotic stressors. In Arabidopsis, the activation of MAPKK9 induces ethylene and camalexin biosynthesis and enhances the plant's sensitivity to salt stress [3]. In tobacco plants, the dual-specificity protein kinase SIMKK mediates the salt-induced activation of SIMK [4]. During the generation period, AtMEK1 immunoprecipitated from seedlings subjected to wounding, cold, drought, and high salt stimuli shows an elevated kinase activity towards the kinase-negative AtMPK4. This indicates that AtMEK1 becomes active through phosphorylation and then activates its downstream target AtMPK4 during stress responses in Arabidopsis [5]. Kong et al. reported that ZmMKK4 is a positive regulator of salt and cold tolerance in maize [6]. In Nicotiana attenuate, MKK1 and MKK2 are involved in wound healing and in a specialized response to the lepidopteran herbivore Manduca sexta [7]. These reports provide us with insight into the functions of the MAPKKs in plants in response to abiotic stress, but the exact function of these kinases needs to be further explored.
Although a number of studies have focused on how MAPKKs function in response to abiotic stress, the role of MAPKK signaling following biotic stress is also an issue of great interest. Emerging evidence has indicated that MAPKKs may be involved in plant defence following biotic stress. In tobacco, the NtMEK2-SIPK/WIPK cascade appears to control the expression of 3-hydroxy-3-methylglutaryl CoA reductase (HMGR) and L-phenylalanine ammonia lyase (PAL), which are defence genes that encode key enzymes in the phytoalexin and salicylicacid biosynthesis pathways. The NtMEK2-SIPK/WIPK cascade pathway plays a positive role in N gene-mediated resistance, possibly through regulating HR cell death [8], [9]. In the Arabidopsis leaf cell system, when the early defence genes induced by flagellin, a complete plant MAPK cascade involving MEKK1-MKK4/MKK5-MPK3/MPK6 and the WRKY22/WRKY29 transcription factors has been shown to be stimulated. All of these proteins functioned downstreams of the flagellin receptor FLS2, which is a leucine-rich-repeat (LRR) receptor kinase [10]. In addition, AtMKK3 positively regulates PR gene expression and plays a role in the defence against Pst DC3000 [11]. Recently, Gao et al. suggested that GhNDR1 and GhMKK2 are required for Verticillium resistance based on gene-silencing assays performed in cotton [12]. The cross-talk between the MAPK cascades is very complicated, and many functions of the MAPKKs in response to biotic stress remain largely unknown.
Under various stresses, plants produce reactive oxygen species (ROS), including hydrogen peroxide (H2O2), superoxide anions (O2 −), and hydroxyl radicals [13]. When plants are under stress, the rate of ROS production is dramatically elevated compared to the low levels observed under optimal growth conditions. An imbalance in ROS concentrations can result in oxidative stress and cause irreversible damage. Previous studies have suggested that the MAPK pathway plays an important role in ROS homeostasis. In Arabidopsis, overexpression of MKK1 enhances the activity of the MAPK cascade, which is also activated by ROS [14], [15]. Xing et al. found that overexpression of MKK1 decreases stress-associated ROS levels and increases salt and drought tolerance, conversely, MKK1 deficiency results in elevated ROS production as well as increased stress sensitivity [16]. As previously described, AtMKK1 and AtMKK4 have both been implicates in ROS signaling [17]. Additionally, AtMKK3, a calmodulin-dependent activator of MPK8, participates in ROS homeostasis [18]. How MAPKKs participate in ROS signaling is a question that needs to be further pursued in the future.
Cotton (Gossypium hirsutum), which an important crop around the world, has been cultivated for its fiber. It also serves as a significant source of feed, foodstuffs, oil, and biofuel. Various abiotic and biotic factors have been shown to inhibit the growth and yield of this species [12], [19]. MAPKKs can be divided into at least four groups, designated A–D. Group A MAPKKs may be involved in various pathways and exhibit multiple biological functions [20]. Although the roles of MAPKKs in the response to environmental stresses have been well studied in plants, functional information on the MAPKKs present in cotton is scarce. Given that MAPK cascades are prone to cross-talk, we exploited molecular and genetic approaches to study a novel group A MAPKK gene from cotton, GhMKK1, to gain a deeper understanding of the functions of the MAPKKs in cotton. We report that GhMKK1 is a crucial regulator of the response to environmental stresses, and GhMKK1 expression markedly enhances tolerance to salt and drought stresses in N. benthamiana. In addition, GhMKK1-overexpression in plants can either inhibit ROS production or lead to efficient scavenges of the excess ROS produced due to external stresses. Overexpression of GhMKK1 also elevates the susceptibility of plants to pathogens. All of these results indicate that GhMKK1 plays a functional role in multiple mechanisms through which plants respond to abiotic and biotic stresses. These findings further broaden our knowledge of the role of GhMKK1 in signal transduction.
Experimental Procedures
Biological materials, growth conditions, and treatments
Cotton (Gossypium hirsutum L. cv. lumian 22) seeds were placed in wet carbasus to accelerate germination, and the seedlings were then transplanted to controlled environmental conditions and grown at 25±1°C with a 16 h light/8 h dark cycle (relative humidity of 60–75% and fluorescent light intensity of ∼200 µmol m−2 per second). Additionally, Nicotiana benthamiana seeds were surface-sterilized and germinated on Murashige-Skoog (MS) medium under greenhouse conditions. Two- or three-leaf stage N. benthamiana seedlings were then transplanted into soil and further maintained under greenhouse conditions. For the various treatments described hereafter, seven-day old cotton seedlings were used. NaCl, wounding, H2O2, and salicylic acid (SA) treatments were performed as described previously [21]. For temperature treatments, uniformly developed cotton seedlings were transferred to 4°C for given time periods. For the other treatments, developed seedlings were sprayed with solutions containing the indicated concentrations of polyethylene glycol (PEG), abscisic acid (ABA), salicylic acid (SA), H2O2, methyl jasmonate (MeJA), ethephon (ET), or Ralstonia solanacearum for the given time periods. The treated cotyledons were collected for RNA extraction, and the roots, stems, and leaves were harvested at the appropriate time points and frozen in liquid nitrogen. Each treatment was repeated at least twice.
GhMKK1 cloning, vector construction, and plant transformation
Under the control of the Cauliflower mosaic virus (CaMV) 35S promoter, the GhMKK1 cDNA sequence (GenBank accession number: HQ828075) was inserted into the binary pBI121 vector via the XbaI and SalI sites. The recombinant plasmid was then introduced into Agrobacterium tumefaciens (strain LBA4404), and transformantion of N. benthamiana was performed using the leaf disc method [22]. The transgenic seedlings were selected on MS agar medium containing 100 mg/L of kanamycin, then transferred to soil and grown in a greenhouse. The seeds of the transgenic lines were harvested from inbred lines. The transgenic T3 lines were used in the experiments, and plants transformed with the empty pBI121 vector were employed as controls. All of the primers used in this study are listed in Table 1.
Table 1. Oligonucleotide primers used in this study.
| Primer | Primer sequence (5′→3′) |
| The cloning of the full-length cDNA | |
| Internal degenerate primers | (D = A, G, or T; N = A, C, G, or T; R = A or G; V = A, C, or G; Y = C or T) |
| M1 | GGVACDTTYAARGATGGDGATC |
| M2 | CCCAARCTCCADATRTCRC |
| 5′-RACE primers | |
| 5P1 | CTGATGGCTTAATGGGCGG |
| 5P2 | GCACAATTCCACCATTACCC |
| 5P3 | GGACACTGAGAGGACTGG |
| AAP | GGCCACGCGTCGACTAGTACG(G)14 |
| AUAP | GGCCACGCGTCGACTAGTACG |
| 3′-RACE primers | |
| 3P1 | CCTCAGGGCTAGCAAACAC |
| 3P2 | CCCGAACCCTATCTTGCTG |
| 3P3 | CCAGTCCTCTCAGTGTCC |
| B26 | GACTCGAGTCGACATCGA(T)18 |
| B25 | GACTCGAGTCGACATCGA |
| The full-length cDNA primers | |
| Q1 | GAAGAAGAAGCAAAACCTCAGATG |
| Q2 | GTCATCACTACAGCCGCTC |
| The cloning of the genomic sequence | |
| Q1 | GAAGAAGAAGCAAAACCTCAGATG |
| Q2 | GTCATCACTACAGCCGCTC |
| Primers used in expressin vector | |
| Z1 | TCTAGAGATGAAGAAGGGAAAGGG |
| Z2 | GTCGACGTCATCACTACAGC |
| 35SF | CCAAGAAGGTTAAAGATGCAG |
| 35SR | GAAGACGTGGTTTTAACG |
| β-actin F | TGGACTCTGGTGATGGTGTC |
| β-actin R | CCTCCAATCCAAACACTGTA |
RNA extraction and semi-quantitative RT-PCR analyses
Total RNA was extracted from the cotton seedlings and N. benthamiana leaves using the CTAB method and the TRIzol Reagent (Invitrogen, Carlsbad, CA, USA), respectively. The CTAB extraction buffer and the protocol were modified from Wang and Stegemann [23]. Samples from cotton seedlings were ground into a fine powder, then transferred to tubes filled with 650 µL of pre-warmed CTAB extraction buffer and vortexed for 1 min at 25°C. An equal volume of phenol-chloroform-isoamyl alcohol (25∶24∶1) was next added to the tubes, which were then mixed well and centrifuged for 15 min at 12,500 rpm at 4°C. After centrifugation, the supernatant was mixed with LiCl and stored at 4°C overnight. The next day, the samples were centrifuged, and the obtained pellet was dissolved in 50 µL of RNase-free water. Subsequently, 5 µL of DNase I Buffer, 2 µL of DNase I, and 1 µL of PRI (TaKaRa, Dalian, China) were added to digest the DNA at 37°C for 30 min. RNase-free water (150 µL) and an equal volume of phenol-chloroform-isoamyl alcohol were added, followed by centrifugation. NaAc and ethanol were then added to the supernatants, and they were held at −80°C for 1 h. After washing with 75% ethanol, the pellets were dissolved in 50 µL of RNase-free water, and the obtained RNA was stored at −80°C. Total RNA was extracted from N. benthamiana leaves using the TRIzol Reagent according to the manufacturer's instructions. This RNA was employed for first-strand cDNA synthesis using a reverse transcriptase system (TransGen Biotech, Beijing, China). Amplifications were performed using the following thermocyling program: 94°C for 5 min, followed by 26–32 cycles of 94°C for 40 s, 50–55°C for 40 s, and 72°C for 40 s. The obtained PCR products were separated in a 1.8% agarose gel and visualized via ethidium bromide staining. The 18S rRNA gene (GenBank accession number: U42827.1) or the N. benthamiana β-actin gene (GenBank accession number: JQ256516.1) was used as a loading control in each reaction. All gene information in RT-PCR are listed in Table S1 and Table S2.
Subcellular localization of the 35S-GhMKK1::GFP fusion protein
The open reading frame (ORF) of GhMKK1 was amplified using the specific primers Q1/Q2. The resultant fragment was inserted into the binary vector pBI121-GFP, which fused the C-terminaus of the GhMKK1 gene with the N-terminus of the green fluorescence protein (GFP) gene; this fusion protein was controlled by the Cauliflower mosaic virus (CaMV) 35S promoter. For transient expression, the plasmid DNA was transformed into onion inner epidermal cells using a previously described particle bombardment method [24]. After particle bombardment, the tissues were incubated on MS agar medium under dark conditions at 25°C for 10–12 h, and the 35S-GFP plasmid was used as a control. Each construct was analyzed at least three times. The obtained fluorescence pattern was observed using a confocal laser scanning microscope (LSM 510 META, ZEISS, Germany).
3,3′-Diaminobenzidine (DAB), Nitro Blue tetrazolium (NBT), and trypan blue staining assays
For DAB staining, the N. benthamiana leaves were incubated in DAB solution (1 mg/mL, pH 3.8) for 24 h at 25°C in the dark. After staining, the leaves were soaked in 95% ethanol overnight to remove chlorophyll [25]. Superoxide anion radicals were detected via NBT staining, according to Jabs et al. [26]. In the NBT assays, collected leaves were incubated in NBT solution (0.1 mg/mL) for 24 h at 25°C in the dark. After staining, the leaves were soaked in 95% ethanol overnight to remove chlorophyll. Seedlings treated with water were used as controls. For trypan blue staining, leaves were removed after treatment and stained with lactophenol-trypan blue (10 mL of lactic acid, 10 mL of glycerol, 10 g of phenol, and 10 mg of trypan blue, all dissolved in 10 mL of distilled water), then mixed with ethanol in a 1∶1 proportion (v/v). Whole leaves were boiled for approximately 5 min in the staining solution and soaked in chloral hydrate (2.5 g of chloral hydrate dissolved in 1 mL of distilled water) for 12 h to remove chlorophyll [27].
Oxidative stress experiments
For oxidative damage tests, leaf disks 1.3 cm in diameter were excised from healthy, fully expanded leaves from 2-month-old transgenic plants. The disks were floated in 10 mL of solutions with different concentrations of Methyl Viologen (MV) (0, 200, and 400 µM) for 24 h, the disks were then immersed in 95% ethanol for 24 h to extract chlorophyll. Chlorophyll a and b contents were determined through spectrophotometric measurements. Determination of O2 − production in the leaves of both treated and control transgenic N. benthamiana plants was performed according to the method described by Xing et al. [28]. Malondialdehyde (MDA) concentrations were used as a biomarker for oxidative stress. The measurements were carried out as described previously [29]. Proline accumulation following drought stress was monitored at the indicated times, as described by Shan et al. [30]. These experiments were repeated at least three times.
Determination of total protein contents and antioxidant enzyme activities
After 8 days without water, 0.5 g samples of the transgenic seedling leaves were collected to perform CAT, APX, POD, and SOD measurements, using the method described by Leclercq et al. [31]. Total protein concentrations were determined according to the Bradford method [32].
Pathogen infection
The bacterial pathogen Ralstonia solanacearum (R. solanacearum) was cultured overnight at 37°C in Luria–Bertani (LB) broth, then harvested via centrifugation, and resuspended in sterile tap water. The fungal pathogen Rhizoctonia solani (R. solani) was cultured on potato dextrose agar (PDA) medium at 28°C for 2 weeks, and the spores were then suspended in 1% glucose. To perform the disease resistance analysis, an R. solanacearum bacterial suspension (OD600 = 0.6–0.8) or R. solani spore suspension (105 spores/mL) was infiltrated into leaves detached from 8-week-old T3 GhMKK1-overexpressing (OE) or wild-type (WT) plants using a needleless syringe. At least three independent experiments were performed for all pathogen inoculations.
Results
Isolation and sequence characterization of GhMKK1
Based on the conserved kinase subdomain of the known MKKs, a pair of degenerate primers (M1/M2) was designed to isolate a cDNA fragment from cotton cotyledons. Then, three sets of specific primers, 5P1/5P2/5P3, 3P1/3P2/3P3, and Q1/Q2, were used for 5′ rapid amplification of cDNA ends (RACE), 3′ RACE-PCR, and identification of the full-length cDNA sequence, respectively. The deduced full-length sequence of the MKK cDNA fragment consisted of 1,234 nucleotides, including a 21 bp 5′-untranslated region (UTR), a 127 bp 3′-UTR, and 1,086 bp open reading frame (ORF). This ORF encodes a polypeptide of 361 amino acid with a calculated molecular mass of 39.945 kDa and an isoelectric point of 5.08. According to the nomenclature for plant MAPKs [1] and because this clone exhibited a high level of sequence similarity to Arabidopsis AtMKK1, we designated this gene GhMKK1 (GenBank accession number: HQ828075).
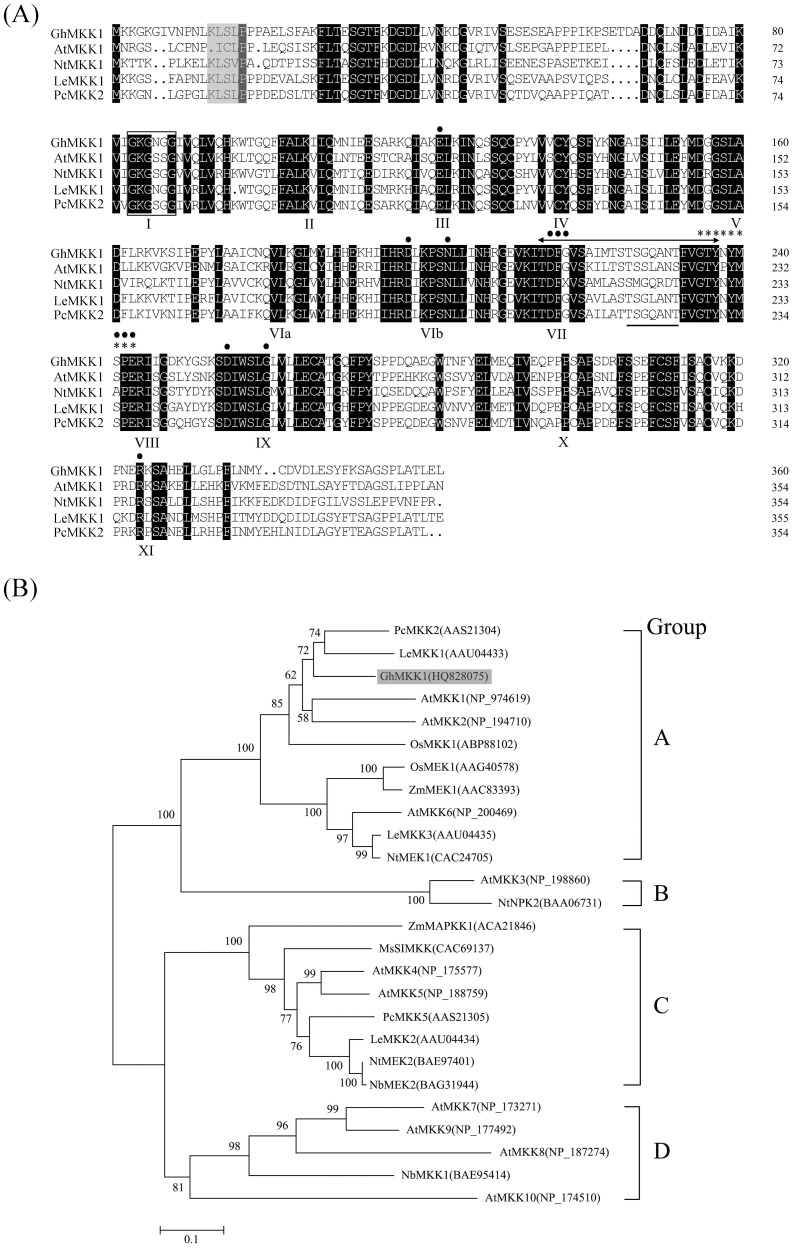
Multiple protein sequence alignment against MAPKKs from various plants showed that GhMKK1 shared high homology with AtMKK1. Comparision with other plant MAPKKs showed that the GhMKK1 protein possessed fully canonical motif structures (Fig. 1A), including 11 conserved sub-domains that are important for its catalytic function, the consensus sequence of S/TXXXXXS/T between the VII and VIII domains, a catalytic loop (activation loop), and a docking domain (D-domain). D-domains (also referred to as D box or DEJL motifs) bind MAPKs or phosphatases [33].
Figure 1. Characterization and sequence analysis of GhMKK1.
(A) Multiple amino acid sequence alignment of GhMKK1 (HQ828075) with AtMKK1 (NP_974619), NtMEK1 (CAC24705), LeMKK1 (AAU04433), and PcMKK2 (AAS21304). Identical and similar amino acids are shaded in black, and the docking domain is shaded in grey. The Roman numbers (I-XI) on the bottom indicate conserved subdomains, and a double-head arrow marks the active site motif. The serine and/or threonine residues in the conserved consensus motif, S/TXXXXXS/T, between subdomains VII and VIII of the MAPKKs are underlined, and the conserved consensus motif GXGXXG is boxed. The dots above the sequences represent activating sites, and asterisks indicate substrate specificity. (B) The phylogenetic relationships between GhMKK1 and other plant MAPKK proteins. The neighbor-joining phylogenetic tree was created using Clustal W and MEGA 4.0 software. The numbers above or below the branches indicate the bootstrap values (>50%) from 500 replicates. GhMKK1 is highlighted in grey. Each gene name is followed by its protein ID. The species of origin is indicated by the abbreviation before the gene names: Pc, Petroselinum crispum; Le, Lycopersicon esculentum; Gh, Gossypium hirsutum; At, Arabidopsis thaliana; Os, Oryza sativa; Zm, Zea mays; Nt, Nicotiana tabacum; Ms, Medicago sativa; and Nb, Nicotiana benthamiana. A, B, C, and D indicate the MAPKK group.
Plant MKK proteins are classified into four groups, designated A–D [20]. To further investigate the evolutionary relationship of the cloned MKK with the other known plant MKKs, a phylogenetic analysis was performed with the obtained amino acid sequences using the Neighbor-Joining method with MEGA version 4.0 software. The analysis showed that GhMKK1 exhibited a high similarity to members of the group A MKKs, such as PcMKK2, LeMKK1, AtMKK1, and AtMKK2 (Fig. 1B).
To investigate the genomic structure of GhMKK1, a 2,720 bp genomic fragment of GhMKK1 (GenBank accession number: JX534501) was isolated from cotton genomic DNA using the specific primers Q1/Q2. Sequence comparison revealed that GhMKK1 displayed seven introns, similar to the group A MKKs (Table 2). The obtained phylogenetic tree further indicated that GhMKK1 was a member of the MKKs, clustered within group A.
Table 2. The lengths of the exons and introns in GhMKK1 and AtMKK1.
| Gene | Exon | Length (bp) | Intron | Length |
| GhMKK1 | 1 | 83 | 1 | 100 |
| 2 | 85 | 2 | 182 | |
| 3 | 147 | 3 | 578 | |
| 4 | 225 | 4 | 83 | |
| 5 | 183 | 5 | 123 | |
| 6 | 224 | 6 | 264 | |
| 7 | 138 | 7 | 156 | |
| 8 | 1 | |||
| AtMKK1 | 1 | 71 | 1 | 109 |
| 2 | 85 | 2 | 279 | |
| 3 | 138 | 3 | 86 | |
| 4 | 224 | 4 | 86 | |
| 5 | 181 | 5 | 99 | |
| 6 | 224 | 6 | 108 | |
| 7 | 47 | 7 | 83 | |
| 8 | 95 | 8 | 93 |
Subcellular localization of GhMKK1
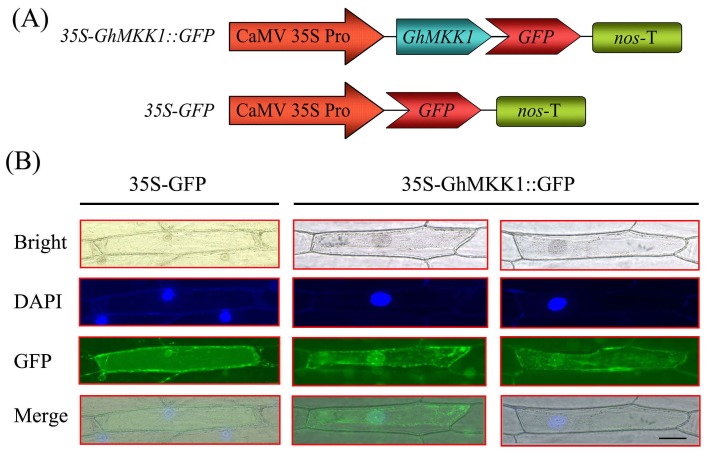
In silico analysis using the Plant-mPLoc program predicted that the GhMKK1 protein would localize to the nucleus, whereas, the PSORT program predicted that GhMKK1 would localize to the cytoplasm. To determine which of these predictions was correct, the 35S-GhMKK1::GFP fusion protein and a 35S-GFP fusion protein (as a control) were constructed to investigate the subcellular localization of GhMKK1 (Fig. 2A). These two constructs were individually introduced into onion epidermal cells through particle bombardment. The fluorescence of both 35S-GhMKK1::GFP and 35S-GFP was detected in both the nucleus and the cytoplasm (Fig. 2B). These results revealed that the GhMKK1 protein is found in the nucleus and in the cytoplasm, which might provide a structural basis for the proposed function of the protein.
Figure 2. Subcellular localization of the GhMKK1 protein transiently expressed in onion epidermal cells.
(A) Schematic diagram of the 35S-GhMKK1::GFP fusion construct and the control 35S-GFP construct. (B) Transient expression of the 35S-GhMKK1::GFP and 35S-GFP constructs in onion epidermal cells. Green fluorescence was observed using a confocal microscope 12 h after particle bombardment. The nuclei of the onion cells were visualized via DAPI staining. Bar = 200 µm.
Differential expression patterns of GhMKK1 in different organs and under different stresses
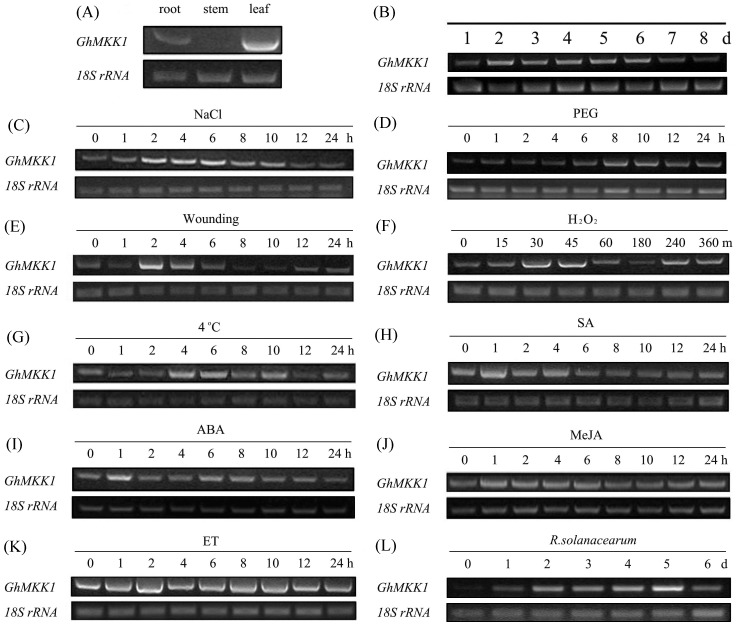
To identify the organ-specific expression patterns of GhMKK1, RT-PCR was carried out using 7-day-old cotton seedlings obtained from hydroponic cultures. As shown in Fig. 3A, the majority of GhMKK1 expression was found in the cotyledon leaves, followed by the roots and stems, which showed that GhMKK1 displayed an organ-specific expression pattern. At the same time, the expression of GhMKK1 in the seedling stage was found to be negligible (Fig. 3B).
Figure 3. Expression patterns of GhMKK1 in different tissues, developmental stages and under different stress conditions.
(A) The tissue-specific expression of GhMKK1 was analysed via RT-PCR using total RNA extracted from the roots, stems, and cotyledon leaves of 7-day-old cotton seedlings. (B) The expression profiles of GhMKK1 were measured in the cotyledon leaves of 7-day-old cotton seedlings. For the stress treatments, 7-day-old cotton seedlings were obtained from a hydroponic culture and were subjected to treatment with 100 mM NaCl (C), 15% PEG (D), wounding (E), 100 µM H2O2 (F), low temperature (4°C) (G), 2 mM SA (H), 100 µM ABA (I), 100 µM MeJA (J), 100 µM ET (K), release from ethephon, and R. solanacearum infection (L). Total RNA was isolated at the indicated times following the initiation of treatments and was subjected to RT-PCR analysis. The obtained PCR products were visualized via agarose gel electrophoresis, followed by ethidium bromide staining. The 18S rRNA gene was employed as an internal control. This experiment was repeated at least twice.
To determine the expression pattern of GhMKK1 following various environmental stresses, the cotton seedlings were exposed to abiotic stresses such as NaCl, PEG, wounding, H2O2, and 4°C chilling treatments. As shown in Fig. 3C–G, the NaCl, wounding, and H2O2 treatments significantly induced the expression of GhMKK1, and the NaCl treatment resulted in a relatively persistent effect from 2 to 10 h. The expression of GhMKK1 was rapidly and transiently enhanced after the seedlings were wounded, and there was no change in GhMKK1 transcript levels detected during the 6 to 24 h series. Following PEG and 4°C treatment, there was a mild accumulation of GhMKK1 transcripts. In addition, we measured the expression of GhMKK1 in cotton seedlings under biotic stresses, such as infection with R. solanacearu. This type of stress led to a significant induction of GhMKK1 on day 2, which peaked on day 5 of the time course (Fig. 3L). These data suggest that GhMKK1 transcript levels are responsive to environmental stresses and might play pivotal roles in the plant stress response.
SA, ABA, MeJA, and ET, which are involved in diverse signaling pathways, were also examined to explore the GhMKK1 signal transduction mechanism that underlies the response to environmental stresses (Fig. 3H–K). The transcription of GhMKK1 peaked 1 h after ABA treatment, then declined back to its initial level. There was a slight negative effect on the accumulation of GhMKK1 observed upon SA treatment. In addition, MeJA and ET had no clear effect on the transcription of GhMKK1. Taken together, these results indicate that GhMKK1 may be responsible for mediating defence-related signal molecules and may play a role in multiple plant defence transduction pathways.
Overexpression of GhMKK1 increases the salt tolerance of transgenic plants
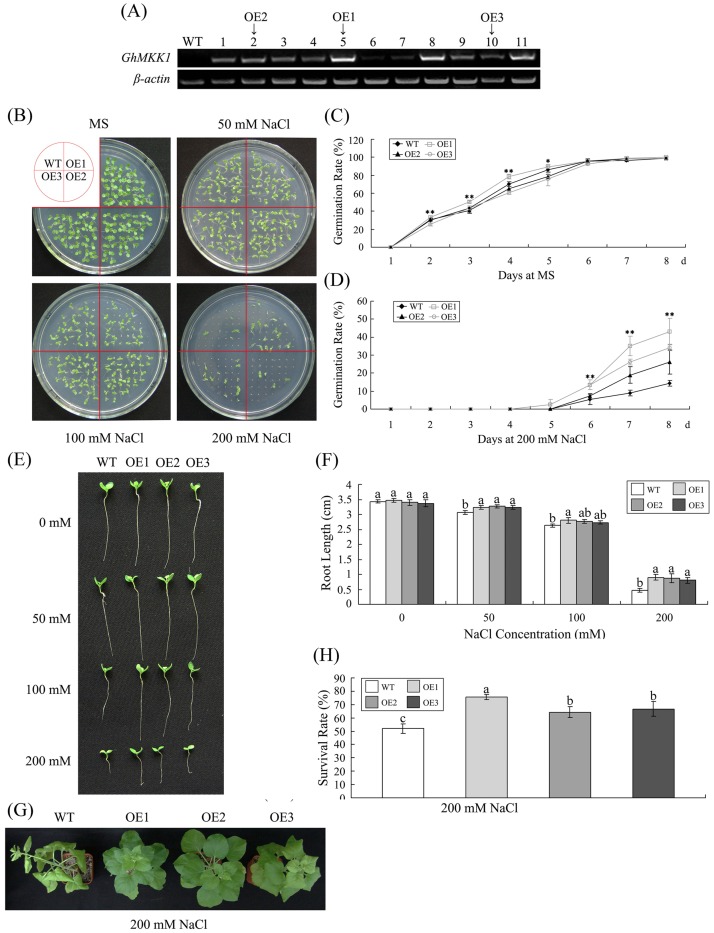
To investigate the function of GhMKK1 in response to environmental stresses, GhMKK1 was overexpressed in transgenic N. benthamiana plants. Through kanamycin resistance selection, a total of 20 independent transgenic lines were obtained; these lines were confirmed using PCR. Subsequently, eleven lines of transgenic T1 plants were selected to detect the levels of GhMKK1 expression in leaf tissue (Fig. 4A). Compared to the control, no visible alternation in morphology was observed in the wild-type (WT) plants (data not shown). However, three representative lines overexpressing GhMKK1, OE1 (5#), OE2 (2#), and OE3 (10#) exhibited different levels of GhMKK1 expression. The T3 progeny of these transgenic plants were randomly selected for further investigation.
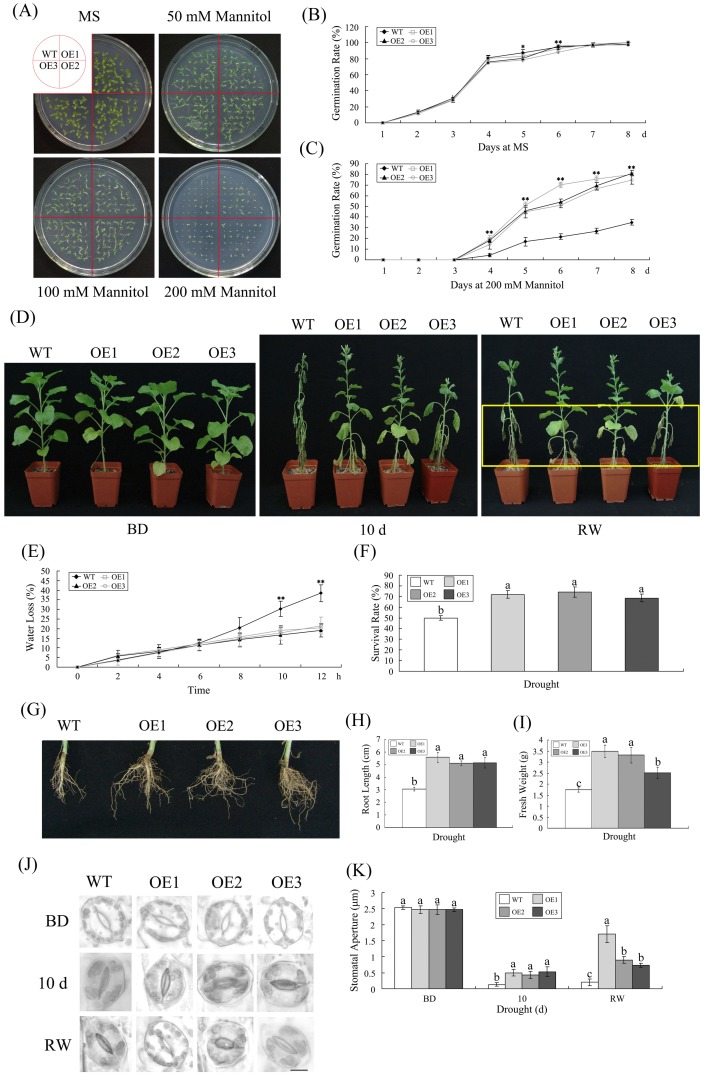
Figure 4. Salt tolerance test comparing the wild-type and GhMKK1-overexpressing N. benthamiana plants.
(A) Analysis of GhMKK1 expression in wild-type (WT) and T1 OE plants. (B) Seed germination on MS medium containing different concentrations of NaCl. (C–D) Germination rates of the WT and OE lines under normal and NaCl treatment conditions. Based on daily scoring, the results obtained using MS medium containing 200 mM NaCl are presented. The presented data are the means ±SE of three independent experiments (n = 3). Asterisks (* or **) above the lines indicate significant differences (*P<0.05; **P<0.01) according to Duncan's multiple range test performed in SAS version 9.1 software. (E) Post-germination seedling development of the WT and the OE lines on MS supplemented with different concentrations of NaCl. The seeds sown on MS medium that showed radicle emergence after 3 d were transferred to MS medium containing different concentrations of NaCl. The plates were oriented vertically, with seedlings kept upside down, and a photograph was taken 14 d after transfer. (F) Primary root lengths of the seedlings 14 d after germination in the presence of different NaCl concentrations. The presented data are the means ±SE of three independent experiments (n = 6). Different letters above the columns indicate significant differences (P<0.05) according to Duncan's multiple range test performed using SAS version 9.1 software. (G) Photograph of representative 10-week-old WT and OE plants grown in soil containing 200 mM NaCl for 14 d. (H) Survival rates of 10-week-old plants treated with 200 mM NaCl for 14 d. The presented data are the means ±SE of three independent experiments (n = 6). Different letters above the columns indicate significant differences (P<0.01) according to Duncan's multiple range test performed using SAS version 9.1 software.
To explore whether constitutive expression of GhMKK1 in transgenic plants effects their salt tolerance, we first studied the transgenic plants' salt tolerance during the germination and seedling stages of development. Seeds from the wild-type plants and the three homozygous transgenic lines (OE1, OE2, and OE3) were surface sterilized and germinated on MS agar medium containing 0, 50, 100, or 200 mM NaCl. As shown in Fig. 4B and 4C, there was no visible difference between the wild-type and transgenic lines following treatment with 0, 50 and 100 mM NaCl. When the NaCl concentration was increased to 200 mM, ∼14% of the wide-type seeds and ∼26–43% of the transgenic seeds germinated after 8 days (Fig. 4D). To further confirm the transgenic phenotypes, the seeds of the wild-type and the transgenic plants were germinated on MS agar medium for 3 days, and were then transferred to medium supplemented with various NaCl concentrations (0, 50, 100, or 200 mM). The data indicated the root length of each plant under the established conditions. There was no significant difference between the wild-type and transgenic lines treated when they were with 0 or 50 mM NaCl. However, the root growth was much greater in the transgenic lines than in the wild-type plants when grown on 100 or 200 mM NaCl (Fig. 4E and 4F).
To verify that GhMKK1 enhanced the salt tolerance of the transgenic plants, the wild-type and T3 transgenic plants were irrigated with salt water (200 mM NaCl) and grown in soil in a greenhouse. After two weeks, severe growth retardation was observed in the wild-type plants, whereas the inhibition of growth was relatively less evident in the transgenic plants exposed to 200 mM NaCl. The leaves of the wild-type plants became wilted and curled (Fig. 4G), and we found that only ∼50% of the wild-type plants survived, and the survival rate of the wild-type plants was less than that of the transgenic plants, with almost all of the transgenic plants surviving (Fig. 4H). These data show that expression of GhMKK1 alleviated the positive effects imposed by salt stress in the transgenic plants during both seed germination and vegetative growth.
GhMKK1-overexpressing plants display increased drought tolerance
Because salt stress results in osmotic stress in plants cells, we performed drought tolerance tests in both the wild-type and transgenic plants to gain a better understanding of GhMKK1 under drought conditions. First, seeds were germinated to capacity on MS agar medium containing different concentrations of exogenous mannitol (0, 50, 100, or 200 mM) to mimic drought stress. No obvious morphological differences were visible in the seedlings when they were treated with 0 to 100 mM NaCl. However, following treatment with 200 mM mannitol, the germination of both the wild-type and transgenic lines was inhibited, and wild-type seeds were more strongly suppressed than transgenic seeds, as only ∼35% of the wild-type seeds germinated compared to ∼80% of the transgenic seeds 8 days after sowing (Fig. 5A–C). Next, wild-type and transgenic plants were examined to confirm their drought tolerance at the vegetable growth stage. The wild-type and T3 transgenic plants were grown in the same pot without water for 10 days. After 10 days of drought treatment, the wild-type plants were completely wilted, whrease the transgenic plants were less affected (Fig. 5D). The transgenic plants recovered more rapidly than the wild-type plants when they were watered for 2 days following the drought treatment, though several of their leaves did not recover completely. Additionally, the rate of water loss from the detached leaves of the wild-type plants was ∼38%, which was nearly twice the amount of water loss observed in the transgenic plants (∼20%). Furthermore, the survival rate of the transgenic plants was ∼20% higher than that of the wild-type plants (Fig. 5E and 5F).
Figure 5. Drought tolerance test comparing the wild-type and the GhMKK1-overexpressing N. benthamiana plants.
(A) Seed germination on MS medium with 0, 50, 100, or 200 mM mannitol. (B–C) Germination rates of the WT and OE lines under normal and mannitol treatment conditions. Based on daily scoring, the results obtained on the MS medium containing 200 mM mannitol are presented. The presented data are the means ±SE of three independent experiments (n = 3). Asterisks (* or **) above the lines indicate (highly) significant differences (*P<0.05; **P<0.01) according to Duncan's multiple range test performed using SAS version 9.1 software. (D) Photograph of representative 10-week-old WT and OE plants grown in soil under drought conditions for 10 d, then watered for 2 d to allow them to recover. BD, before drought treatment; RW, rewatering. (E) The water loss from the detached leaves of WT and OE plants at the indicated times. The rate of water loss was calculated by the loss of fresh weight in the samples. The presented data are the means ±SE of three independent experiments (n = 6). Asterisks (**) above the lines indicate (highly) significant differences (P<0.01) according to Duncan's multiple range test performed using SAS version 9.1 software. (F) Survival rates of WT and OE plants under drought stress. The presented data are the means ±SE of three independent experiments (n≥50). Different letters above the columns indicate significant differences (P<0.0001) according to Duncan's multiple range test performed using SAS version 9.1 software. (G–I) Phenotype of roots subjected to drought stress for WT and OE plants, together with additional root lengths and fresh weights. The presented data are the means ±SE of three independent experiments (n = 6). Different letters above the columns indicate significant differences (P<0.0001) according to Duncan's multiple range test performed using SAS version 9.1 software. (J–K) Stomatal changes observed with a microscope before and after drought treatment. The stomatal aperture is displayed. BD, before drought treatment; RW, rewatering. Bar = 200 µm. The presented data are the means ±SE of three independent experiments (n = 6). Different letters above the columns indicate significant differences (P<0.01) according to Duncan's multiple range test performed using SAS version 9.1 software.
Plants control water loss through well-developed root systems and by regulating stomatal closure during drought stress. As shown in Fig. 5G–I, the transgenic plants exhibited longer and thicker roots than the wild-type plants as well as a higher fresh weight. However, there was no significant difference observed in the stomatal aperture between the transgenic and the wild-type lines under normal conditions, neverthless, almost all of the stomata of the wild-type plants closed after drought stress, and the stomatal apertures in the transgenic lines were more open than those of the wild-type plants (Fig. 5J and 5K). After 2 days of watering and recovery, the stomatal apertures of the transgenic plants were more than 5 times wider, on average, than those of the wild-type plants. In summary, these results suggest that overexpression of GhMKK1 can enhance the drought tolerance of the transgenic plants at both the seedling and the vegetable growth stages.
GhMKK1 mediates the accumulation of ROS in transgenic plants
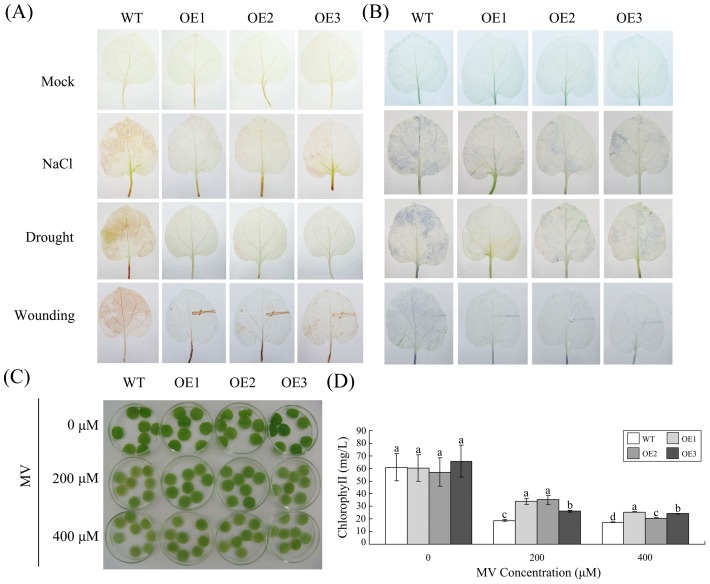
Cellular ROS can be induced by many types of environmental stresses, such as salt and drought, among other types of stress [34], [35]. To investigate whether GhMKK1 expression conferred an elevated tolerance to oxidative stress in the transgenic plants, detached leaves from the wild-type and the transgenic plants subjected to NaCl, drought, and wounding stresses were used to measure the accumulation of H2O2 and O2 − using the 3,3′-diaminobenzidine (DAB) and Nitro Blue tetrazolium (NBT) staining methods, respectively. Leaves detached from untreated wild-type and the transgenic plants were used as mock controls. Under normal growth conditions (Fig. 6A and 6B), the H2O2 contents of the wild-type and the transgenic plants were similar. However, following treatment with NaCl, drought and wounding, the DAB staining was found to be much lighter in the transgenic plants, indicating that the level of H2O2 was considerably lower in the transgenic plants compared to the mock-treated plants. Similar to what was observed for DAB staining, NBT staining revealed that there was less O2 − accumulation in the leaves from the transgenic plants.
Figure 6. Analysis of ROS accumulation in WT and OE plants in response to abiotic stresses.
(A–B) Abiotic stress-induced H2O2 and O2 − accumulation detected via DAB staining and NBT staining, respectively. (C) Leaf disks from WT and OE plants were incubated in different concentrations of MV (0, 200, or 400 µM) under greenhouse conditions. (D) Relative chlorophyll contents were determined in the leaf disks of WT and OE plants following MV treatments. Disks floated in water were used as a control. The presented data are the means ±SE of three independent experiments (n = 6). Different letters above the columns indicate significant differences (P<0.0001) according to Duncan's multiple range test performed using SAS version 9.1 software.
To illustrate how GhMKK1 overexpression leads to oxidative resistance, leaf disks from the wild-type and the transgenic plants were exposed to increasing concentrations of MV (0, 200, or 400 µM) for 48 h to stimulate oxidative stress. The results presented in Fig. 6C and 6D show that the transgenic plants exhibited intense oxidative resistance. Leaf disks from the wild-type plants changed from green to almost yellow in color following oxidative stress treatment, while the disks from the transgenic plants displayed oxidative tolerance to the MV-induced damage. All of these data indicate that the transgenic plants produce lower levels of ROS, suggesting that overexpression of GhMKK1 either inhibits ROS production or results in the efficient scavenging of excess ROS.
GhMKK1 overexpression enhances oxidative stress tolerance in plants
To ascerain the possible mechanisms underlying the enhanced antioxidant defence ability observed in the transgenic plants, we examined several physiological indexes and measured the enzymatic activity of genes that encoded ROS-scavenging enzymes in both the transgenic plants and the control plants under drought stress.
Excess ROS produces superoxide radicals, including superoxide anions (O2 −), which are harmful to plants. Lipid hydroperoxidation contributes to and serves as an indicator of cellular oxidative damage [36]. Proline accumulation in response to osmotic stress has also been well documented in both prokaryotic and eukaryotic organisms. By acting as a hydroxyl radical scavenger, proline contributes to osmotic adjustment and protects macromolecules during dehydration [29]. As shown in Table 3, while no remarkable difference was observable between the untreated wide-type and the untreated transgenic plants, the O2 − and MDA contents in the wide-type plants were dramatically increased compared to the transgenic plants under drought stress. However, there was little proline detected in any of the untreated plant lines. While the proline concentration dramatically increased with drought stress, there was a discrepancy in the results obtained between the transgenic lines.
Table 3. Several physiological indexes in WT and OE plants under drought stress.
| WT | OE1 | OE2 | OE3 | ||
| O2 −(nmol/g FW) | No treatment | 247.143±21.666 | 149.038±3.364 | 200.666±9.664 | 212.373±14.225 |
| drought | 848.929±23.690 | 479.591±13.591 | 569.981±14.069 | 697.965±14.075 | |
| MDA (µmol/g FW) | No treatment | 1.727±0.015 | 1.936±0.022 | 1.921±0.079 | 1.457±0.075 |
| drought | 6.598±0.503 | 2.492±0.347 | 3.058±0.267 | 3.957±0.219 | |
| Proline (µg/g FW) | No treatment | 71.332±14.736 | 64.810±10.171 | 55.219±5.857 | 43.569±8.959 |
| drought | 548.860±10.386 | 721.036±36.276 | 645.841±29.351 | 775.031±7.453 | |
| CAT (U/g FW) | No treatment | 58.104±2.236 | 41.830±3.947 | 62.409±1.794 | 51.751±15.923 |
| drought | 59.163±6.120 | 109.491±0.692 | 76.576±3.078 | 89.929±17.494 | |
| APX (U/g FW) | No treatment | 115.562±0.645 | 92.032±1.739 | 120.749±2.713 | 86.125±1.883 |
| drought | 98.606±1.360 | 139.470±3.539 | 111.506±5.498 | 122.658±3.175 | |
| POD (U/g FW) | No treatment | 162.225±2.332 | 157.520±0.733 | 167.722±1.106 | 164.832±1.141 |
| drought | 144.315±10.475 | 238.443±2.906 | 189.655±2.235 | 163.729±3.568 | |
| SOD (U/g FW) | No treatment | 85.413±5.885 | 110.790±6.223 | 105.345±12.074 | 135.244±8.130 |
| drought | 145.426±6.863 | 108.173±9.321 | 113.548±10.780 | 122.367±7.507 |
To determine the nature of the antioxidant response to drought stress caused by GhMKK1 overexpression, the enzymatic activities of catalase (CAT), ascorbate peroxidase (APX), peroxidase (POD) and superoxide dismutase (SOD) were monitored under both normal and drought conditions (Table 3). Prior to drought stress, there were no notable differences in POD activity between any of the lines. However, the transgenic lines displayed a significant increase in POD activity when maintained under drought conditions. Likewise, the activities of CAT and SOD were greatly increased in the transgenic lines compared to the wild-type plants under drought stress. Drought treatment also markedly increased APX activity in the transgenic plants, with the exception of the OE2 line, which displayed a higher APX activity than the wild-type plants. Briefly, these results indicate that GhMKK1-overexpression enhanced the antioxidant ability of multiple antioxidant enzymes in the transgenic plants, suggesting that GhMKK1 might play a role in the regulation of the ROS network pathway.
GhMKK1-overexpressing plants exhibit enhanced pathogen susceptibility
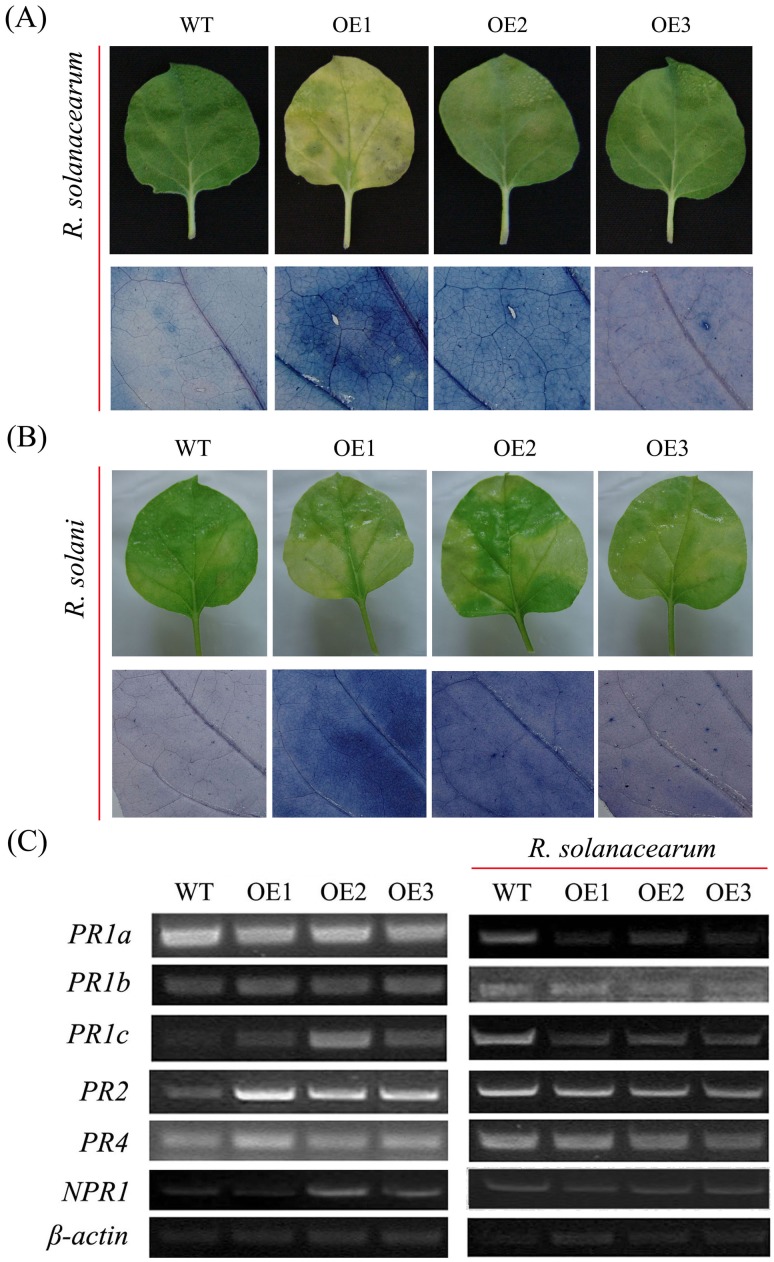
To confirm the response of the GhMKK1-overexpressing plants to pathogens, 10 µL of an R. solanacearum or R. solani culture suspension (OD600 = 0.6–0.8) was infiltrated into both sides of the main veins of detached N. benthamiana leaves using a needleless syringe. Six days after R. solanacearum inoculation, the transgenic plants displayed chlorotic signs and enlarged water-soaked lesions on their leaves. However, the wild-type plants showed only mild signs of disease and remained predominantly green. Trypan blue staining of the inoculated leaves revealed relatively more staining in the leaves of the transgenic plants than those of the wild-type plants, indicating that overexpression of GhMKK1 rendered the plants more susceptible to R. solanacearum, including to cell death induced by this organism. Similarly, in the transgenic plants leaves, R. solani infection caused larger spots and rapidly spreading chlorosis, and the leaves of these plants exhibited severe wilting and yellowing. In contrast, the spots and chlorosis did not spread in the wild-type plants, resulting in little damage to the leaves. Trypan blue staining resulted in more staining in the leaves of the transgenic plants than in those of the wild-type plants, indicating that overexpression of GhMKK1 enhanced the plant's susceptibility to R. solani, leading to increased levels of R. solani-induced cell death in the transgenic plants (Fig. 7A). These results showed that overexpression of GhMKK1 increased the pathogen susceptibility of the transgenic plants.
Figure 7. Enhanced susceptibility to R. solanacearum and R. solani in GhMKK1-overexpressing plants.
(A) Disease signs on the detached leaves from WT and OE plants 6 d after inoculation with R. solanacearum and the relative trypan blue staining, indicating pathogen infection. (B) Disease signs on the detached leaves from WT and OE plants 6 d after inoculation with R. solani and the relative trypan blue staining, indicating pathogen infection. (C) RT-PCR analysis of PR genes expression in WT and OE plants under normal and R. solanacearum inoculation conditions.
To examine the possible mechanisms underlying the enhanced pathogen susceptibility observed in the transgenic plants, we evaluated the expression levels of several disease-responsive genes in N. benthamiana including PR1a, PR1b, PR1c, PR2 (β-1, 3-glucanase), PR4, and NPR1. As shown in Fig. 7C, the expression of PR1c, PR2, and NPR1 was constitutively elevated in the transgenic plants compared to the wild-type plants when they were not subjected to any stress treatment. However there was no clear change in the expression levels of PR1a, PR1b, and PR4 when no stress was applied to the leaves. Folowing injection with R. solanacearum, the expression of PR1a, PR1b, PR1c, and PR2 decreased in both the wild-type and transgenic plants. In addition, the expression of PR4 was lower in the transgenic plants than in the wild-type plants following injection (Fig. 7B). The enhanced pathogen susceptibility of the transgenic plants might be due to the reduced expression of these PR genes.
Discussion
The defence mechanisms in plants are sophisticated, including MAPK cascades which transfer singal from sensors to incite cellular responses. As the nodal point of MAPK cascades, the significant role of group A MAPKKs in response to abiotic and biotic stresses have begun to emerge [5], [7], [12], [37]. In the present study, a novel cotton group A MAPKK gene, designated GhMKK1, was characterised. Our results revealed that overexpression of GhMKK1 enhanced salt and drought tolerance and increased the pathogen susceptibility in the transgenic N. benthamiana. Notably, GhMKK1 could either lead to inhibition of ROS production or efficient scavenging of excess ROS. These results not only increase our knowledge of the biological functions of the group A MAPKKs, but also provide new evidence of the role of GhMKK1 in the regulation of the plant defence responses.
Extracellular signals lead to the activation of MAPK in the cytoplasm, following by its translocation into the nucleus. Accordingly, the definition of MAPKK proteins subcellular localization is so important for exploring the roles of MAPK cascades in signal transduction. Many of the MAPKKs contain a nuclear localization signal peptide (NLS, sequence PKKKRKV) and are localized in the nucleus [38]. Takahashi et al. have reported that GFP fluorescence could constitutively observed in the nucleus following the expression of a group D MAPKK in N. benthamiana [39]. Similarly, Kong et al. suggested that a novel group C MAPKK in maize (Zea mays), ZmMKK4, was also localized in the nucleus [6]. In contrast, in the present study, there was no obvious NLS identified in the GhMKK1 amino acid sequence, but the active GhMKK1-GFP was constitutively localized in both the nucleus and the cytoplasm (Fig. 3). Previous studies have suggested that functionally important proteins lacking NLSs are also present in the nucleus [40]. Moreover, several lines of evidence have indicated that MAPKKs may be localized not only in the nucleus, but also in the cytoplasm. For example, AtMKK4 was localized in both of these cellular compartments [41]. Recently, Zhang et al. have also reported that a group B MAPKK, ZmMKK3, whose ZmMKK3-GFP fusion protein showed signals throughout the cell, indicating that ZmMKK3 might be localized in both the cytoplasm and the nucleus [42]. These results highlight the importance of controlling MAPK activity at a subcellular level. Therefore, we speculated that GhMKK1 might act as a transcriptional activator in the nucleus, by inducing the expression of a number of target genes and as a signal transmitter in the cytoplasm. The mechanisms underlying the nuclear localization of GhMKK1 and its regulation of downstream targets should be addressed in future work.
Plant cultivation, especially in irrigated lands, is severely affected by salinity stress in many parts of the world. Additionly, drought is also a major constraint to increasing the yields of crop plants. Based on the presence of general and specific abiotic stress tolerance mechanisms, it is reasonable to anticipate that plants exhibit multiple stress perception and signal transduction pathways, which may interact at various steps in their progression. MAPK signalling pathway mediates many stress responses through regulating the expression of various stress-responsive genes [43]. In this study, GhMKK1 may be involved in the GhMKK1-related positive response to abiotic stresses. Firstly, RT-PCR analysis revealed that the expression of GhMKK1 was strongly induced by various abiotic stresses (Fig. 3). Secondly, the overexpression of GhMKK1 in N. benthamiana enhanced its tolerance to salt and drought stresses, as determined by statistical analyses of germination rates, lateral root length, plant survival rates, and leaf water loss (Fig. 4, 5). In line with these results, the expression and kinase activity of the alfalfa p44MKK4 gene are activated under cold and drought conditions [44]. SbMPKK gene in Salicornia brachiate can also possesses a high salt tolerance [45]. Recently, it was reported that OsMKK1, OsMKK4, and OsMKK6 are involved in the salinity and drought stress response of Oryza. sativa [46]. However, Arabidopsis AtMKK9 functions as a negative regulator to participate in the response to salt stress [3], which suggests that the regulatory mechanisms of the response to abiotic stress involving GhMKK1 significantly vary from those of AtMKK9. Even in the same species, MAPKKs functions may be different. Zhang et al. reported that GhMKK5 could induced by salt and dought stress, but transgenic plants overexpressing GhMKK5 showed reduced salt and drought tolerance [47]. Therefore, elucidating the potential functions of GhMKK1 is necessary to understand how plants respond to major environmental stressors.
Interestingly, compared with abiotic stress, overexpression of GhMKK1 increased the pathogen susceptibility of the transgenic plants, and this susceptibility could be determined by the reduced expression of PR genes (Fig. 7). These results indicated that GhMKK1 might be involved in the negative defence responses. Several recent studies have highlighted the importance of MAPKK signaling in the overlapping plant responses to pathogen challenge [48], [49]. But, this defence mechanism may be different. Similar to our result, Brader et al. have also reported that overexpression of constitutively active AtMKK2 increased sensitivity to the fungal necrotroph Alternaria brassicicola in OE plant [50]. In contrast, AtMKK3 can be activated by pathogen and decreased the growth of virulent Pst DC3000 in MKK3-overexpressing plants. This response is most likely mediated through the ability of MKK3 to interact with MPK7, in which MPK7-mediated signaling can activate pathogenesis-related genes in Arabidopsis [11]. These results suggest that plant MAPKKs have complex functions, including roles in regulating defence response either positively or negatively.
Environment stress, such as Salinity and drought, disrupt plants photosynthesis and alter the normal homeostasis of cells, resulting in ROS production [12]. Low ROS concentrations can act as secondary messengers regulating cell growth, while high ROS concentrations induce cellular senescence and lead to oxidative damage to special molecules, consequentially injuring tissues [51]. In this study, under drought stress, the overexpression of GhMKK1 resulted in the decreased accumulation of O2 − and MDA in transgenic plants compared to wide-type plants. Moreover, there are higher proline concentration and higher antioxidant enzymes activities (CAT, APX, POD and SOD) after drought treatment (Table 3). Consistent with this result, the overexpression of active AtMKK1 or AtMKK2 regulates ROS metabolism by controlling the catalase genes that encode the H2O2-scavenging enzymes [52]. Recently, it was reported that Ca2+/CaMs-MKK3-MPK8 pathways are simultaneously involved in the perception of mechanical wounding and in ensuring an optimal balance in the toxic accumulation of ROS in Arabidopsis [18]. Therefore, these results indicate that GhMKK1 might take part in the regulation of the ROS network pathway. Further biochemical and molecular experiments are necessary to elucidate the antioxidant mechanism of GhMKK1.
In conclusion, our results strongly suggest that GhMKK1 is responsive to a variety of stress and that overexpression of GhMKK1 in N. benthamiana plants enhanced their salt and drought tolerance while also increased the transgenic plants' pathogen sensitivity. Furthermore, GhMKK1-overexpressing plants exhibited higher antioxidant activity. Although the complex regulatory mechanisms involving GhMKK1 are still unclear, our results contribute greatly to our understanding of how plants have evolved to cope with environmental stresses. We believe that understanding the specific and shared signal transduction pathways could lead to strategies involving the manipulation of key signaling molecules to achieve broad-spectrum resistance to abiotic stresses and diseases.
Supporting Information
Gene information in RT-PCR.
(DOC)
RT-PCR amplification conditions.
(DOC)
Funding Statement
This work was financially supported by the Genetically Modified Organisms Breeding Major Projects of China (2009ZX08009-092B) and the National Science Foundation of China (grant no. 31171837; 30970225). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. MAPK Group (2002) Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trends in Plant Science 7: 301–308. [DOI] [PubMed] [Google Scholar]
- 2. Andreasson E, Ellis B (2010) Convergence and specificity in the Arabidopsis MAPK nexus. Trends Plant Sci 15(2): 106–113. [DOI] [PubMed] [Google Scholar]
- 3. Xu J, Li Y, Wang Y, Liu H, Lei L, et al. (2008) Activation of MAPK kinase 9 induces ethylene and camalexin biosynthesis and enhances sensitivity to salt stress in Arabidopsis . J. Biol. Chem 283(40): 26996–27006. [DOI] [PubMed] [Google Scholar]
- 4. Kiegerl S, Cardinale F, Siligan C, Gross A, Baudouin E, et al. (2000) SIMKK, a mitogen-activated protein kinase (MAPK) kinase, is a specific activator of the salt stress-induced MAPK, SIMK. Plant Cell 12(11): 2247–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Matsuoka D, Nanmori T, Sato K, Fukami Y, Kikkawa U, et al. (2002) Activation of AtMEK1, an Arabidopsis mitogen-activated protein kinase kinase, in vitro and in vivo: analysis of active mutants expressed in E. coli and generation of the active form in stress response in seedlings. Plant J 29(5): 637–647. [DOI] [PubMed] [Google Scholar]
- 6. Kong X, Pan J, Zhang M, Xing X, Zhou Y, et al. (2011) ZmMKK4, a novel group C mitogen-activated protein kinase kinase in maize (Zea mays), confers salt and cold tolerance in transgenic Arabidopsis . Plant Cell Environ 34(8): 1291–1303. [DOI] [PubMed] [Google Scholar]
- 7. Heinrich M, Baldwin IT, Wu J (2011) Two mitogen-activated protein kinase kinase, MKK1 and MKK2, are involved in wounding- and specialist lepidopteran herbivore Manduca sexta-induced responses in Nicotana attenuate. J. Exp. Bot 62(12): 4355–4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang KY, Liu Y, Zhang S (2001) Activation of a mitogen-activated protein kinase pathway is involved in disease resistance in tobacco. Proc. Natl. Acad. Sci. U. S. A 98(2): 741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jin H, Liu Y, Yang KY, Kim CY, Baker B, et al. (2003) Function of a mitogen-activated protein kinase pathway in N gene-mediated resistance in tobacco. Plant J 33(4): 719–731. [DOI] [PubMed] [Google Scholar]
- 10. Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, et al. (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415(6875): 977–983. [DOI] [PubMed] [Google Scholar]
- 11. Dóczi R, Brader G, Pettkó-Szandtner A, Rajh I, Djamei A, et al. (2007) The Arabidopsis mitogen-activated protein kinase kinase MKK3 is upstream of group C mitogen-activated protein kinases and participates in pathogen signaling. Plant Cell 19(10): 3266–3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gao X, Wheeler T, Li Z, Kenerley CM, He P, et al. (2011) Silencing GhNDR1 and GhMKK2 compromises cotton resistance to Verticillium wilt. Plant J 66(2): 293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33(4): 453–467. [DOI] [PubMed] [Google Scholar]
- 14. Yuasa T, Ichimura K, Mizoguchi T, Shinozaki K (2001) Oxidative stress activates ATMPK6, an Arabidopsis homologue of MAP kinase. Plant Cell Physiol 42(9): 1012–1016. [DOI] [PubMed] [Google Scholar]
- 15. Teige M, Scheikl E, Eulgem T, Dóczi R, Ichimura K, et al. (2004) The MKK2 pathway mediates cols and salt stress signaling in Arabidopsis . Mol. Cell 15(1): 141–152. [DOI] [PubMed] [Google Scholar]
- 16. Xing Y, Jia W, Zhang J (2008) AtMKK1 mediates ABA-induced CAT1 expression and H2O2 production via AtMPK6-coupled signaling in Arabidopsis . Plant J 54(3): 440–451. [DOI] [PubMed] [Google Scholar]
- 17. Colcombet J, Hirt H (2008) Arabidopsis MAPKs: a complex signalling network involved in multiple biological processes. Biochem. J 413(2): 217–226. [DOI] [PubMed] [Google Scholar]
- 18. Takahashi F, Mizoguchi T, Yoshida R, Ichimura K, Shinozaki K (2011) Calmodulin-dependent activation of MAP kinase for ROS homeostasis in Arabidopsis . Mol. Cell 41(6): 649–660. [DOI] [PubMed] [Google Scholar]
- 19. Sunilkumar G, Campbell LM, Puckhaber L, Stipanovic RD, Rathore KS (2006) Engineering cottonseed for use in human nutrition by tissue-specific reduction of toxic gossypol. Proc. Natl. Acad. Sci. U. S. A 103(48): 18054–18059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hamel LP, Nicole MC, Sritubtim S, Morency MJ, Ellis M, et al. (2006) Ancient signals: comparative genomics of plant MAPK and MAPKK gene families. Trends Plant Sci 11(4): 192–198. [DOI] [PubMed] [Google Scholar]
- 21. Wang H, Ngwenyama N, Liu Y, Walker JC, Zhang S (2007a) Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis . Plant Cell 19: 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Horsch RB, Fry JE, HoVmann NL, Eichholtz D, Rogers SG, et al. (1985) A simple and general method for transferring genes into plants. Science 227: 1229–1231. [DOI] [PubMed] [Google Scholar]
- 23. Wang L, Stegemann JP (2010) Extraction of high quality RNA from polysaccharide matrices using cetyltrimethylammonium bromide. Biomaterials 31(7): 1612–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shi J, An HL, Zhang L, Gao Z, Guo XQ (2010) GhMPK7, a novel multiple stress-responsive cotton group C MAPK gene, has a role in broad spectrum disease resistance and plant development. Plant Mol. Biol 74: 1–17. [DOI] [PubMed] [Google Scholar]
- 25. Thordal-Christensen H, Zhang ZG, Wei YD, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. The Plant Journal 11(6): 1187–1194. [Google Scholar]
- 26. Jabs T, Dietrich RA, Dangl JL (1996) Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science 273(5283): 1853–1856. [DOI] [PubMed] [Google Scholar]
- 27. Koch E, Slusarenko A (1990) Arabidopsis is susceptible to infection by a downy mildew fungus. The Plant Cell 2: 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xing Y, Jia W, Zhang J (2007) AtMEK1 mediates stress-induced gene expression of CAT1 catalase by triggering H2O2 production in Arabidopsis . J. Exp. Bot 58(11): 2969–2981. [DOI] [PubMed] [Google Scholar]
- 29. Yang L, Tang R, Zhu J, Liu H, Mueller-Roeber B, et al. (2008) Enhancement of stress tolerance in transgenic tobacco plants constitutively expressing AtIpk2β, an inositol polyphosphate 6-/3-kinase from Arabidopsis thaliana . Plant Mol. Biol 66(4): 329–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shan DP, Huang JG, Yang YT, Guo YH, Wu CA, et al. (2007) Cotton GhDREB1 increases plant tolerance to low temperature and is negatively regulated by gibberellic acid. New Phytol 176: 70–81. [DOI] [PubMed] [Google Scholar]
- 31. Leclercq J, Martin F, Sanier C, Clément-Vidal A, Fabre D, et al. (2012) Overexpression of a cytosolic isoform of the HbCuZnSOD gene in Hevea brasiliensis changes its response to a water deficit. Plant Mol. Biol 80(3): 255–272. [DOI] [PubMed] [Google Scholar]
- 32. Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of people dye binding. Anal. Biochem 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 33. Hanks SK, Quinn AM, Hunter T (1988) The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science 241: 42–52. [DOI] [PubMed] [Google Scholar]
- 34. Bowler C, Marc Van M, Inzé D (1992) Superoxide dismutase and stress tolerance. Annu Rev Plant Physiol. Plant Mol. Biol 43: 83–116. [Google Scholar]
- 35. Allen RD (1995) Dissection of oxidative stress tolerance using transgenic plants. Plant Physiol 107: 1049–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yoshimura K, Miyao K, Gaber A, Takeda T, Kanaboshi H, et al. (2004) Enhancement of stress tolerance in transgenic tobacco plants overexpressing Chlamydomonas glutathione peroxidase in chloroplasts or cytosol. Plant J 37(1): 21–33. [DOI] [PubMed] [Google Scholar]
- 37. Nakagami H, Pitzschke A, Hirt H (2005) Emerging MAP kinase pathways in plant stress signalling. Trends Plant Sci 10(7): 339–346. [DOI] [PubMed] [Google Scholar]
- 38. Sánchez-Mir L, Franco A, Madrid M, Vicente-Soler J, Villar-Tajadura MA, et al. (2012) Biological significance of nuclear localization of mitogen-activated protein kinase Pmk1 in fission yeast. J. Biol. Chem 287(31): 26038–26051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Takahashi Y, Nasir KH, Ito A, Kanzaki H, Matsumura H, et al. (2007) A high-throughput screen of cell-death-inducing factors in Nicotiana benthamiana identifies a novel MAPKK that mediates INF1-induced cell death signaling and non-host resistance to Pseudomonas cichorii . Plant J 49(6): 1030–1040. [DOI] [PubMed] [Google Scholar]
- 40. Dahan J, Wendehenne D, Ranjeva R, Pugin A, Bourque S (2010) Nuclear protein kinases: still enigmatic components in plant cell signalling. New Phytol 185(2): 355–368. [DOI] [PubMed] [Google Scholar]
- 41. Yoo SD, Cho YH, Tena G, Xiong Y, Sheen J (2008) Dual control of nuclear EIN3 by bifurcate MAPK cascades in C2H4 signalling. Nature 451: 789–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang M, Pan J, Kong X, Zhou Y, Liu Y, et al. (2012) ZmMKK3, a novel maize group B mitogen-activated protein kinase kinase gene, mediates osmotic stress and ABA signal responses. J Plant Physiol 169(15): 1501–1510. [DOI] [PubMed] [Google Scholar]
- 43. Sinha AK, Jaggi M, Raghuram B, Tuteja N (2011) Mitogen-activated protein kinase signaling in plants under abiotic stress. Plant Signal. Behav 6(2): 196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jonak C, Kiegerl S, Ligterink W, Barker PJ, Huskisson NS, et al. (1996) Stress signaling in plants: a mitogen-activated protein kinase pathway is activated by cold and drought. Proc. Natl. Acad. Sci. U. S. A 93(20): 11274–11279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Agarwal PK, Gupta K, Jha B (2010) Molecular characterization of the Salicornia brachiata SbMAPKK gene and its expression by abiotic stress. Mol. Biol. Rep 37(2): 981–986. [DOI] [PubMed] [Google Scholar]
- 46. Kumar K, Rao KP, Sharma P, Sinha AK (2008) Differential regulation of rice mitogen activated protein kinase kinase (MKK) by aviotic stress. Plant Physiol. Biochem 46(10): 891–897. [DOI] [PubMed] [Google Scholar]
- 47. Zhang L, Li YZ, Lu WJ, Meng F, Wu CA, et al. (2012) Cotton GhMKK5 affects disease resistance, induces HR-like cell death, and reduces the tolerance to salt and drought stress in transgenic Nicotiana benthamiana . J. Exp. Bot 63(10): 3935–3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brader G, Djamei A, Teige M, Palva ET, Hirt H (2007) The MAP kinase kinase MKK2 affects disease resistance in Arabidopsis . Mol. Plant Microbe Interact 20(5): 589–596. [DOI] [PubMed] [Google Scholar]
- 49. Qiu JL, Zhou L, Yun BW, Nielsen HB, Fiil BK, et al. (2008) Arabidopsis mitogen-activated protein kinase kinases MKK1 and MKK2 have overlapping functions in defence signaling mediated by MEKK1, MPK4, and MKS1. Plant Physiol 148(1): 212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brader G, Djamei A, Teige M, Palva ET, Hirt H (2007) The MAP kinase kinase MKK2 affects disease resistance in Arabidopsis . Mol. Plant Microbe Interact 20(5): 589–596. [DOI] [PubMed] [Google Scholar]
- 51. Poli G, Leonarduzzi G, Biasi F, Chiarpotto E (2004) Oxidative stress and cell signalling. Curr. Med. Chem 11(9): 1163–1182. [DOI] [PubMed] [Google Scholar]
- 52. Pitzschke A, Djamei A, Bitton F, Hirt H (2009) A major role of the MEKK1-MKK1/2-MPK4 pathway in ROS signaling. Mol. Plant 2(1): 120–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gene information in RT-PCR.
(DOC)
RT-PCR amplification conditions.
(DOC)