Abstract
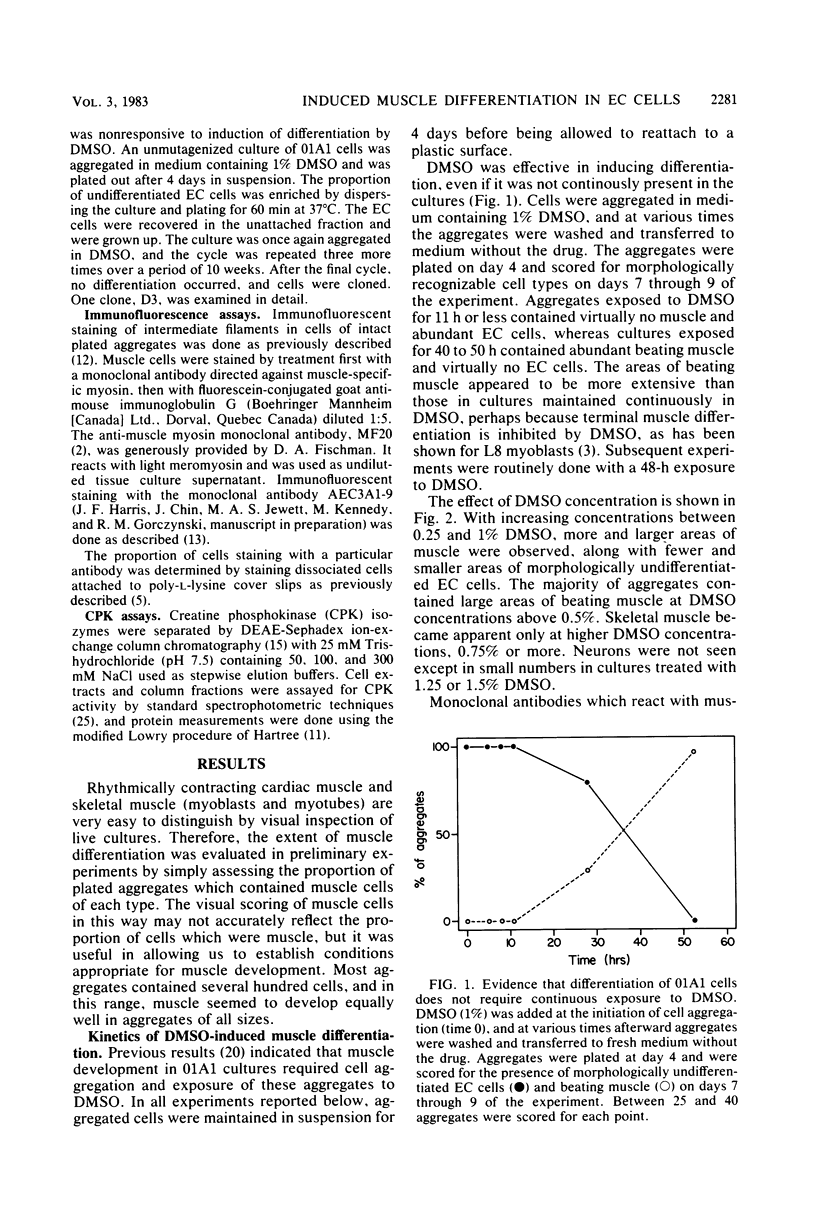
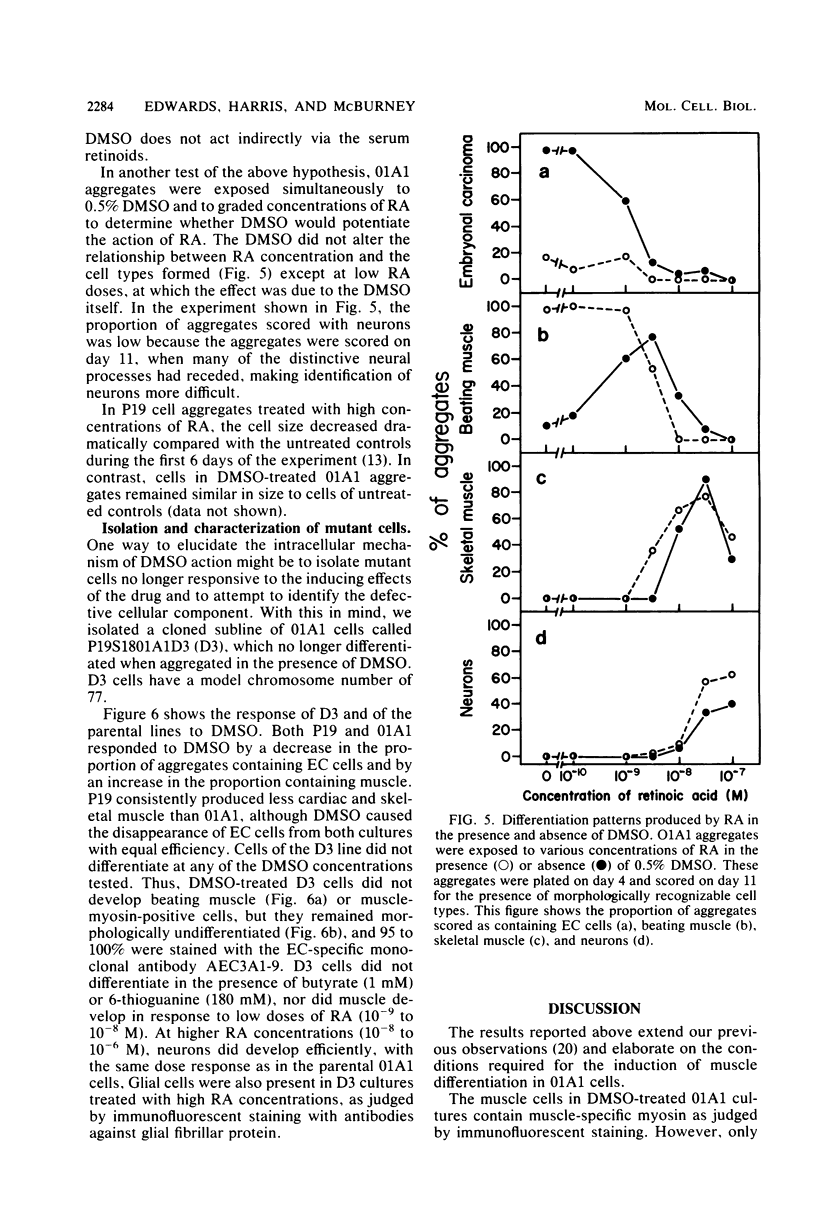
Cells of the teratocarcinoma-derived line P19S1801A1 (01A1) are pluripotent embryonal carcinoma cells and can be induced to differentiate when aggregated and exposed to dimethyl sulfoxide. Many nonneural cell types appear in dimethyl sulfoxide-treated cultures, cardiac and skeletal muscle being the most easily identified. We have used immunofluorescence procedures with monoclonal antibodies directed against muscle myosin to confirm and quantitate the number of muscle cells formed. A monoclonal antibody reactive with an embryonal carcinoma-specific surface antigen was used to confirm the disappearance of undifferentiated cells after dimethyl sulfoxide treatment. Cardiac muscle cells developed within 4 to 5 days of drug exposure, but skeletal muscle cells did not become evident until 7 to 8 days. We have isolated a mutant cell line (D3) which appears to be incapable of muscle development but which does form neurons and glial cells when exposed to high retinoic acid concentrations. We propose that this system will be useful for investigation of the means by which pluripotent cells become committed to development along the striated muscle lineages.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson E. D. Isoenzyme transitions of creatine phosphokinase, aldolase and phosphoglycerate mutase in differentiating mouse cells. J Embryol Exp Morphol. 1976 Apr;35(2):355–367. [PubMed] [Google Scholar]
- Bader D., Masaki T., Fischman D. A. Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J Cell Biol. 1982 Dec;95(3):763–770. doi: 10.1083/jcb.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau H. M., Epstein C. J. Manipulation of myogenesis in vitro: reversible inhibition by DMSO. Cell. 1979 May;17(1):95–108. doi: 10.1016/0092-8674(79)90298-8. [DOI] [PubMed] [Google Scholar]
- Bridges K., Levenson R., Housman D., Cantley L. Calcium regulates the commitment of murine erythroleukemia cells to terminal erythroid differentiation. J Cell Biol. 1981 Aug;90(2):542–544. doi: 10.1083/jcb.90.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M. K., McBurney M. W. The concentration of retinoic acid determines the differentiated cell types formed by a teratocarcinoma cell line. Dev Biol. 1983 Jul;98(1):187–191. doi: 10.1016/0012-1606(83)90348-2. [DOI] [PubMed] [Google Scholar]
- Friend C., Freedman H. A. Effects and possible mechanism of action of dimethylsulfoxide on Friend cell differentiation. Biochem Pharmacol. 1978 May 1;27(9):1309–1313. doi: 10.1016/0006-2952(78)90112-0. [DOI] [PubMed] [Google Scholar]
- Friend C., Scher W., Holland J. G., Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971 Feb;68(2):378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E., Green H. Regulation of terminal differentiation of cultured human keratinocytes by vitamin A. Cell. 1981 Sep;25(3):617–625. doi: 10.1016/0092-8674(81)90169-0. [DOI] [PubMed] [Google Scholar]
- Gusella J. F., Housman D. Induction of erythroid differentiation in vitro by purines and purine analogues. Cell. 1976 Jun;8(2):263–269. doi: 10.1016/0092-8674(76)90010-6. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Jones-Villeneuve E. M., McBurney M. W., Rogers K. A., Kalnins V. I. Retinoic acid induces embryonal carcinoma cells to differentiate into neurons and glial cells. J Cell Biol. 1982 Aug;94(2):253–262. doi: 10.1083/jcb.94.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Villeneuve E. M., Rudnicki M. A., Harris J. F., McBurney M. W. Retinoic acid-induced neural differentiation of embryonal carcinoma cells. Mol Cell Biol. 1983 Dec;3(12):2271–2279. doi: 10.1128/mcb.3.12.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson R., Housman D., Cantley L. Amiloride inhibits murine erythroleukemia cell differentiation: evidence for a Ca2+ requirement for commitment. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5948–5952. doi: 10.1073/pnas.77.10.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lough J., Bischoff R. Differentiation of creatine phosphokinase during myogenesis: quantitative fractionation of isozymes. Dev Biol. 1977 Jun;57(2):330–344. doi: 10.1016/0012-1606(77)90219-6. [DOI] [PubMed] [Google Scholar]
- Marks P. A., Rifkind R. A. Erythroleukemic differentiation. Annu Rev Biochem. 1978;47:419–448. doi: 10.1146/annurev.bi.47.070178.002223. [DOI] [PubMed] [Google Scholar]
- Martin G. R., Evans M. J. Differentiation of clonal lines of teratocarcinoma cells: formation of embryoid bodies in vitro. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1441–1445. doi: 10.1073/pnas.72.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. R. Teratocarcinomas and mammalian embryogenesis. Science. 1980 Aug 15;209(4458):768–776. doi: 10.1126/science.6250214. [DOI] [PubMed] [Google Scholar]
- Martin G. R. Teratocarcinomas as a model system for the study of embryogenesis and neoplasia. Cell. 1975 Jul;5(3):229–243. doi: 10.1016/0092-8674(75)90098-7. [DOI] [PubMed] [Google Scholar]
- McBurney M. W., Jones-Villeneuve E. M., Edwards M. K., Anderson P. J. Control of muscle and neuronal differentiation in a cultured embryonal carcinoma cell line. Nature. 1982 Sep 9;299(5879):165–167. doi: 10.1038/299165a0. [DOI] [PubMed] [Google Scholar]
- McBurney M. W., Rogers B. J. Isolation of male embryonal carcinoma cells and their chromosome replication patterns. Dev Biol. 1982 Feb;89(2):503–508. doi: 10.1016/0012-1606(82)90338-4. [DOI] [PubMed] [Google Scholar]
- Papaioannou V. E., McBurney M. W., Gardner R. L., Evans M. J. Fate of teratocarcinoma cells injected into early mouse embryos. Nature. 1975 Nov 6;258(5530):70–73. doi: 10.1038/258070a0. [DOI] [PubMed] [Google Scholar]
- Rossant J., McBurney M. W. The developmental potential of a euploid male teratocarcinoma cell line after blastocyst injection. J Embryol Exp Morphol. 1982 Aug;70:99–112. [PubMed] [Google Scholar]
- Rothblat G. H., Arbogast L. Y., Ouellette L., Howard B. V. Preparation of delipidized serum protein for use in cell culture systems. In Vitro. 1976 Aug;12(8):554–557. doi: 10.1007/BF02797438. [DOI] [PubMed] [Google Scholar]
- STOUGHTON R. B., FRITSCH W. INFLUENCE OF DIMETHYLSULFOXIDE (DMSO) ON HUMAN PERCUTANEOUS ABSORPTION. Arch Dermatol. 1964 Nov;90:512–517. doi: 10.1001/archderm.1964.01600050060012. [DOI] [PubMed] [Google Scholar]
- Shainberg A., Yagil G., Yaffe D. Alterations of enzymatic activities during muscle differentiation in vitro. Dev Biol. 1971 May;25(1):1–29. doi: 10.1016/0012-1606(71)90017-0. [DOI] [PubMed] [Google Scholar]
- Stanners C. P., Eliceiri G. L., Green H. Two types of ribosome in mouse-hamster hybrid cells. Nat New Biol. 1971 Mar 10;230(10):52–54. doi: 10.1038/newbio230052a0. [DOI] [PubMed] [Google Scholar]
- Stewart T. A., Mintz B. Successive generations of mice produced from an established culture line of euploid teratocarcinoma cells. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6314–6318. doi: 10.1073/pnas.78.10.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiftsoglou A. S., Mitrani A. A., Housman D. E. Procaine inhibits the erythroid differentiation of MEL cells by blocking commitment: possible involvement of calcium metabolism. J Cell Physiol. 1981 Sep;108(3):327–335. doi: 10.1002/jcp.1041080306. [DOI] [PubMed] [Google Scholar]
- Ziter F. A. Creatine kinase in developing skeletal and cardiac muscle of the rat. Exp Neurol. 1974 Jun;43(3):539–546. doi: 10.1016/0014-4886(74)90193-9. [DOI] [PubMed] [Google Scholar]




