Abstract
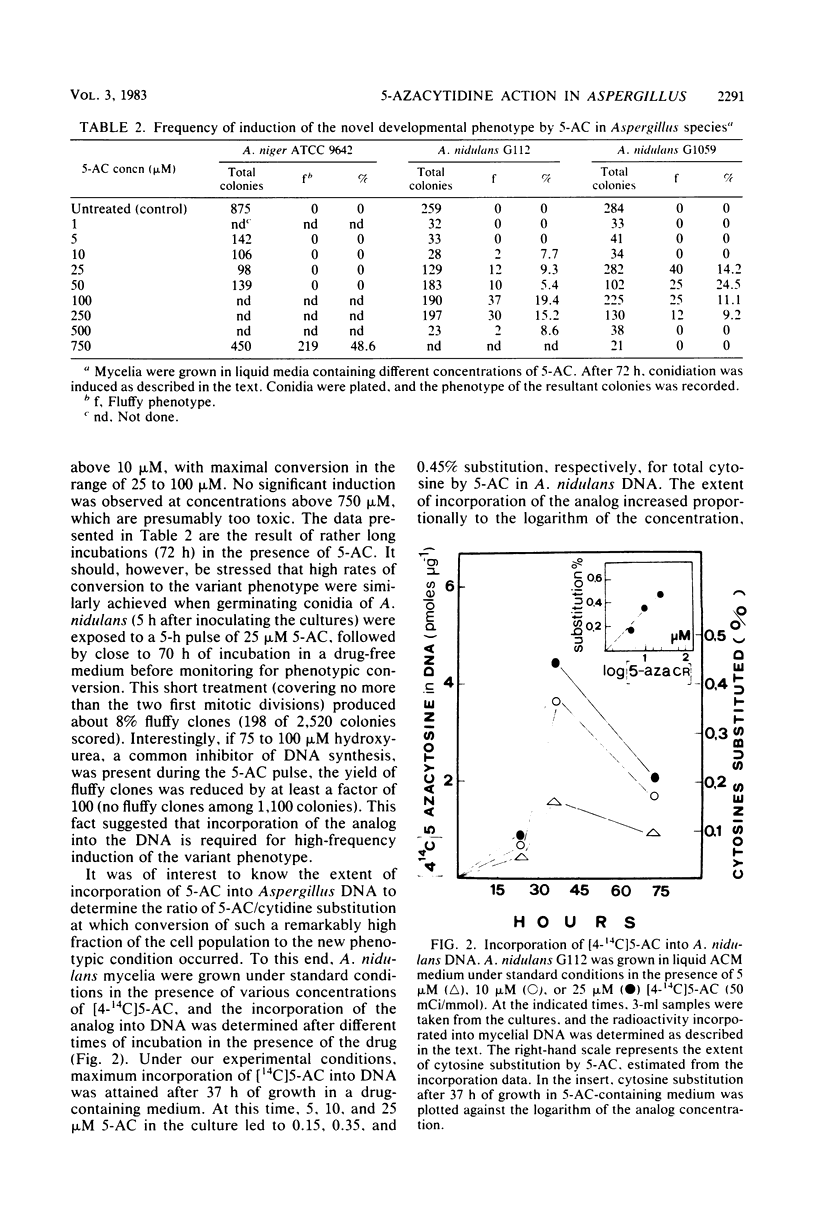
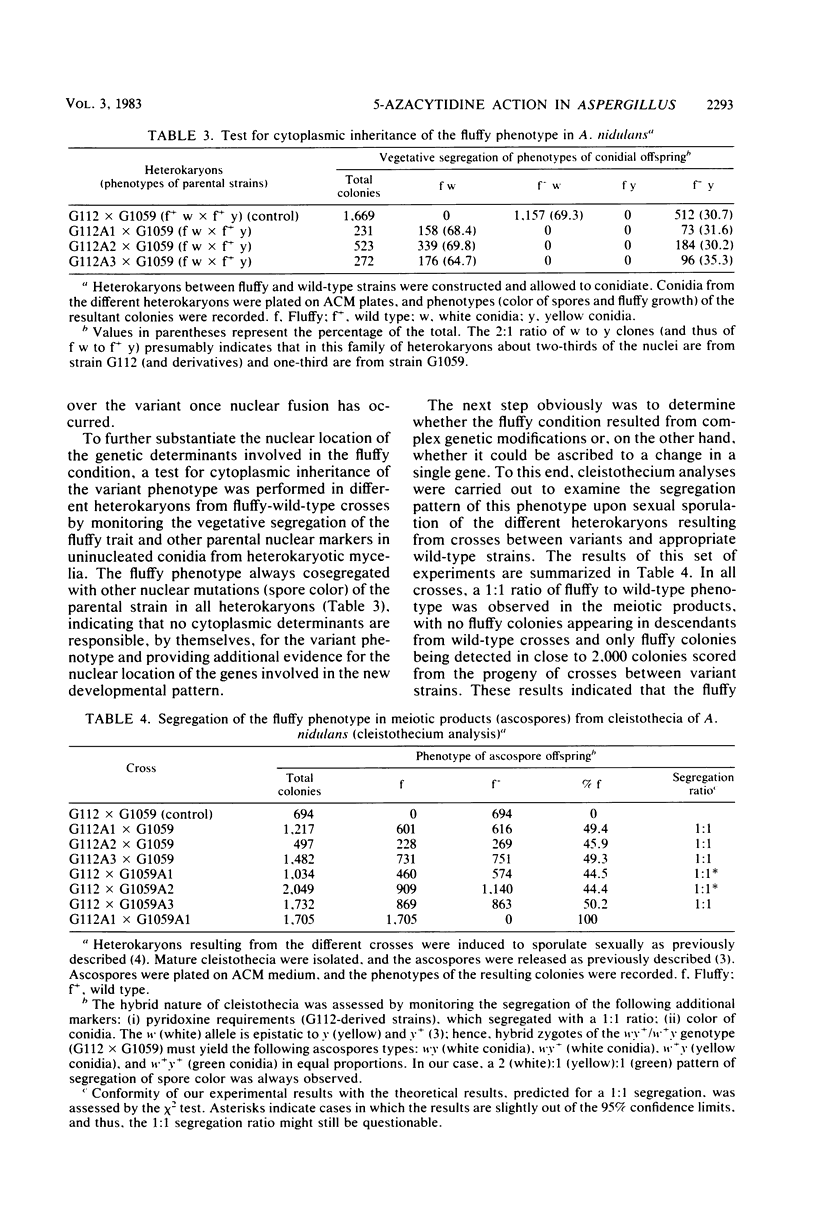
Transient exposure of mycelia from Aspergillus niger and Aspergillus nidulans to the cytidine analog 5-azacytidine, leading to no more than 0.3 to 0.5% substitution for cytosine by 5-azacytosine in A. nidulans DNA, resulted in the conversion of a high fraction of the cell population (more than 20%) to a mitotically and meiotically stable "fluffy" developmental phenotype. The phenotypic variants are characterized by the developmentally timed production of a profuse fluffy network of undifferentiated aerial hyphae that seem to escape signals governing vegetative growth. Genetic analysis with six different fluffy clones reveals that this trait is not cytoplasmically coded, is recessive in heterozygous diploids but codominant in heterokaryons, and exhibits a 1:1 Mendelian segregation pattern upon sexual sporulation of heterozygous diploids. Complementation and mitotic haploidization studies indicated that all variants are affected in the same gene, which can be tentatively located on chromosome VIII of A. nidulans. Molecular analysis to search for modified bases showed that DNA methylation is negligible in in both A. niger and A. nidulans and that no differences could be detected among DNAs from wild-type cells, fluffy clones, or mycelia exposed to 5-azacytidine. It thus appears that high-frequency conversion of fungal mycelia to a stable, variant developmental phenotype by 5-azacytidine is the result of some kind of target action on a single nuclear gene and that this conversion can occur in organisms virtually devoid of DNA methylation.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cedar H., Solage A., Glaser G., Razin A. Direct detection of methylated cytosine in DNA by use of the restriction enzyme MspI. Nucleic Acids Res. 1979;6(6):2125–2132. doi: 10.1093/nar/6.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compere S. J., Palmiter R. D. DNA methylation controls the inducibility of the mouse metallothionein-I gene lymphoid cells. Cell. 1981 Jul;25(1):233–240. doi: 10.1016/0092-8674(81)90248-8. [DOI] [PubMed] [Google Scholar]
- Creusot F., Acs G., Christman J. K. Inhibition of DNA methyltransferase and induction of Friend erythroleukemia cell differentiation by 5-azacytidine and 5-aza-2'-deoxycytidine. J Biol Chem. 1982 Feb 25;257(4):2041–2048. [PubMed] [Google Scholar]
- Dorn G. L. Genetic and morphological properties of undifferentiated and invasive variants of Aspergillus nidulans. Genetics. 1970 Oct;66(2):267–279. doi: 10.1093/genetics/66.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groudine M., Eisenman R., Weintraub H. Chromatin structure of endogenous retroviral genes and activation by an inhibitor of DNA methylation. Nature. 1981 Jul 23;292(5821):311–317. doi: 10.1038/292311a0. [DOI] [PubMed] [Google Scholar]
- Gruenbaum Y., Cedar H., Razin A. Substrate and sequence specificity of a eukaryotic DNA methylase. Nature. 1982 Feb 18;295(5850):620–622. doi: 10.1038/295620a0. [DOI] [PubMed] [Google Scholar]
- Harris M. Induction of thymidine kinase in enzyme-deficient Chinese hamster cells. Cell. 1982 Jun;29(2):483–492. doi: 10.1016/0092-8674(82)90165-9. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980 May;20(1):85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M., Mohandas T., Shapiro L. J. Cell cycle-specific reactivation of an inactive X-chromosome locus by 5-azadeoxycytidine. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1215–1219. doi: 10.1073/pnas.79.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law D. J., Timberlake W. E. Developmental regulation of laccase levels in Aspergillus nidulans. J Bacteriol. 1980 Nov;144(2):509–517. doi: 10.1128/jb.144.2.509-517.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCully K. S., Forbes E. The use of p-fluorophenylalanine with 'master strains' of Aspergillus nidulans for assigning genes to linkage groups. Genet Res. 1965 Nov;6(3):352–359. doi: 10.1017/s0016672300004249. [DOI] [PubMed] [Google Scholar]
- Miller D. A., Okamoto E., Erlanger B. F., Miller O. J. Is DNA methylation responsible for mammalian X chromosome inactivation? Cytogenet Cell Genet. 1982;33(4):345–349. doi: 10.1159/000131782. [DOI] [PubMed] [Google Scholar]
- Sager R., Kovac P. Pre-adipocyte determination either by insulin or by 5-azacytidine. Proc Natl Acad Sci U S A. 1982 Jan;79(2):480–484. doi: 10.1073/pnas.79.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specht C. A., DiRusso C. C., Novotny C. P., Ullrich R. C. A method for extracting high-molecular-weight deoxyribonucleic acid from fungi. Anal Biochem. 1982 Jan 1;119(1):158–163. doi: 10.1016/0003-2697(82)90680-7. [DOI] [PubMed] [Google Scholar]
- Sweet R. W., Chao M. V., Axel R. The structure of the thymidine kinase gene promoter: nuclease hypersensitivity correlates with expression. Cell. 1982 Dec;31(2 Pt 1):347–353. doi: 10.1016/0092-8674(82)90128-3. [DOI] [PubMed] [Google Scholar]
- Taylor S. M., Jones P. A. Multiple new phenotypes induced in 10T1/2 and 3T3 cells treated with 5-azacytidine. Cell. 1979 Aug;17(4):771–779. doi: 10.1016/0092-8674(79)90317-9. [DOI] [PubMed] [Google Scholar]
- del Rey F., Santos T., García-Acha I., Nombela C. Synthesis of 1,3-beta-glucanases in Saccharomyces cerevisiae during the mitotic cycle, mating, and sporulation. J Bacteriol. 1979 Sep;139(3):924–931. doi: 10.1128/jb.139.3.924-931.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]