Abstract
Medical devices implanted in subcutaneous tissue elicit wound-healing events that encapsulate the device. To investigate the foreign-body response to devices of clinically relevant size, we studied the relationship between capsule thickness, vascular density, and blood flow within the capsule. In nine canines, we implanted 40 subcutaneous devices (polysulfone n = 14, titanium n = 17, silicone-coated n = 9). Devices and surrounding tissue structures were harvested at 208 ± 142 (mean ± standard deviation) days after implantation. Tissues were processed for histological analysis with light microscopy. Foreign-body capsule (FBC) thickness and vascular density were determined. A subset of five animals was instrumented via thoracotomy for microsphere injections and reference blood sampling. In these animals, regional blood flow was determined in mL/min/g of tissue in the FBC to examine the relationship between capsule thickness and blood flow. One-way analysis of variance and linear regression analyses were performed. The FBC thickness was 0.92 ± 0.67 mm for all devices studied and was not influenced by device material. Capsule thickness correlated with vascular density for all devices (R2 = 0.85, P < 0.0001) and for each device material (polysulfone R2 = 0.85, P < 0.0001; silicone R2 = 0.95, P < 0.0001; titanium R2 = 0.87, P < 0.0001). Capsule thickness correlated modestly with blood flow for all devices studied (R2 = 0.59, P < 0.0001) and for each device material (polysulfone R2 = 0.77, P < 0.001; silicone R2 = 0.65, P = 0.10; titanium R2 = 0.52, P = 0.03). Capsule thickness, vascular density, and blood flow were similar within individual animals but variable across animals. Material type did not influence the thickness of FBCs surrounding devices of clinically relevant size. Capsule thickness correlated strongly with vascular density and modestly with blood flow within the capsule. Individual animals exhibited variable foreign-body responses.
Keywords: Subcutaneous, Histomorphometry, Vascular density, Microspheres, Blood flow, Foreign-body capsule, Foreign-body response, Surgical device, Vascular access port
The presence of a foreign object in a physiological environment elicits a cascade of inflammatory mechanisms and wound-healing events that encapsulate the object in a foreign-body capsule (FBC). The capsule serves as a structural and biological barrier between the tissue and the foreign body (1). If the foreign body is an implantable biomedical device, this process may facilitate infection, impair device function, or initiate tissue rejection of the device.
Despite these concerns, implantable devices have become a staple in clinical medicine as they often permit outpatient treatment for what would otherwise require hospitalization. Indeed, vascular access ports, implantable drug-delivery pumps, and artificial organs are increasingly common in an aging population and this trend is likely to continue. Advanced nanofabrication techniques herald a new generation of biomaterials that may interact with living tissues. Material surfaces may no longer be inert but rather mimic extracellular structures in order to minimize complications associated with chronic device implantation or even to manipulate local physiological pathways. As such, it is increasingly important to understand the pathophysiological response of living tissue to existing materials as a foundation for the development of novel biomaterials.
With this goal in mind, we have developed a chronically instrumented, conscious canine model to study the in vivo foreign-body response surrounding surgically implanted devices made from different materials (2). Previously in this model, we have demonstrated that during the subcutaneous foreign-body response, over months, blood flow decreased in the FBC and surrounding fascia, and device material influenced regional blood flow patterns within the FBC (3).
To further characterize the foreign-body response to devices of clinically relevant size, we investigated the relationships between FBC thickness, vascular density, and blood flow within the FBC. In chronically instrumented canines, we implanted commercially available polysulfone, silicone-coated, and titanium devices. After 3 months, when the FBCs had formed and matured, we quantified capsule thickness, vascular density, and blood flow.
MATERIALS AND METHODS
Surgical preparation
All animals received humane care and were handled in accordance with National Institutes of Health and Harvard Medical School animal care committee guidelines. Experimental procedures followed animal studies protocols approved by Harvard Medical School.
Nine adult female mixed-breed dogs (mean: 15.9 kg, range: 13.6–18.2 kg) were used for this study. Instrumentation surgeries were performed aseptically while animals were maintained under a surgical level of anesthesia as previously described (2,3).
In all animals, vascular access ports (VAP; n = 14 polysulfone GPVu, diameter = 3.25 cm, height = 1.25 cm, weight = 5.75 g; n = 17 titanium GPVu, diameter = 2.5 cm, height = 1 cm, weight = 9 g; Access Technologies, Skokie, IL, USA) were implanted subcutaneously at the mid-back in multilayers. Additionally, a silicone-coated telemetry device (D70-PCT, diameter = 5.5 cm, height = 1. 25 cm, weight = 49 g; Data Sciences International, St. Paul, MN, USA) was implanted subcutaneously at the lateral base of the back.
A subset of animals (n = 5) was instrumented with VAP/catheter systems to receive multiple injections of fluorescent-labeled, polystyrene microspheres for repeated regional blood flow determinations in the same conscious animal as previously described (2,3). Via thoracotomy, a vascular access catheter was implanted 4 cm into the left atrial appendage so that the catheter tip resided inside of the left atrium. A second vascular access catheter was advanced 10 cm into the aorta and oriented so that the catheter tip resided downstream in the lumen of the aorta (4). Catheters were tunneled from the thoracotomy incision to the mid-back and attached to subcutaneous VAPs. VAPs served a dual purpose: (i) for delivery of microspheres into the systemic arterial circulation via the left atrial appendage; and (ii) as implanted test devices. After surgery, animals recovered for a minimum of 3 weeks before participating in experimentation.
Microsphere protocols
Before sacrifice, the left atrial VAP was accessed for injection of 15 μm microspheres into the systemic arterial circulation. Simultaneously, the aortic VAP was accessed, and a reference blood sample was withdrawn at a known rate from within the aorta as previously described (2,3).
Quantification of fluorescent microspheres
After euthanasia, devices and surrounding tissue structures were harvested. From each fibrous capsule, two sections of tissue in direct contact with the base of the device were studied. Tissue and reference blood samples were sent to IMT/Stason Laboratories (Irvine, CA, USA) for automated digestion and counting of fluorescent microspheres with flow cytometry. Regional blood flows were calculated in mL/min/g of capsule tissue.
Histomorphometric analysis
Two sections were taken from each subcutaneous fibrous capsule. Sections were fixed in 10% buffered formalin (Fisher Scientific, Pittsburgh, PA, USA), processed routinely for paraffin histology, sectioned transmurally perpendicular to the capsule wall at 4 μm thickness to allow measurement of the cross-sectional dimensions of the capsule (Fig. 1, bottom panels), and stained with hematoxylin and eosin. Each slide was analyzed by light microscopy independently and blinded to the type of device material in contact with the FBC. To measure capsule thickness, the transmural section was aligned with a stage micrometer. The slide was then moved randomly to four different locations along the axis of the transmural section of tissue and measured at each location at a magnification of 10×. To measure capsule vascular density, each slide was randomly photographed twice at a magnification of 40×. Computerized planimetry was used to manually threshold vascular structures and calculate a total percentage of vascular density within each FBC.
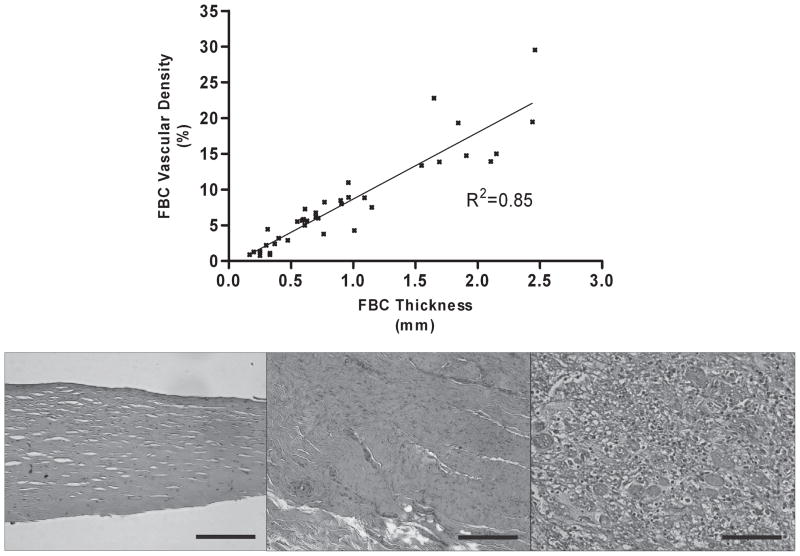
FIG. 1.
Top panel: Foreign-body capsule (FBC) thickness correlated with vascular density for all devices studied (P < 0.0001). Bottom panels: Examples of hematoxylin and eosin histology of three capsules with different thicknesses are shown. 20×, black bars = 100 μm. Left: The capsule exhibits a thin, well-organized fibrous matrix with intermittent fibroblasts and few vascular structures. Middle: The capsule exhibits a thicker, more dense, and less-organized fibrous matrix. Vascular structures are prominent in cross-section as well as weaving through the plane of the tissue. Right: The capsule exhibits loose, disorganized tissue with a multitude of inflammatory cells. Erythrocytes stained red are visible in multiple open vascular channels. Of note, this capsule was taken from an animal with a confirmed myocardial infarction in which systemic inflammation related to chronic myocardial remodeling may have also influenced chronic FBC remodeling.
Statistical analysis
Statistical analyses were performed with Graph-Pad Prism (version 4.00, San Diego, CA, USA). FBC thickness, vascular density, and blood flow for each material were analyzed by one-way analysis of variance with Tukey posthoc test to compare between material groups. Linear regression was used to characterize the relationship between capsule thickness, vascular density, and blood flow. All analyses were two-tailed, and a P value less than 0.05 (95% confidence) was considered statistically significant.
RESULTS
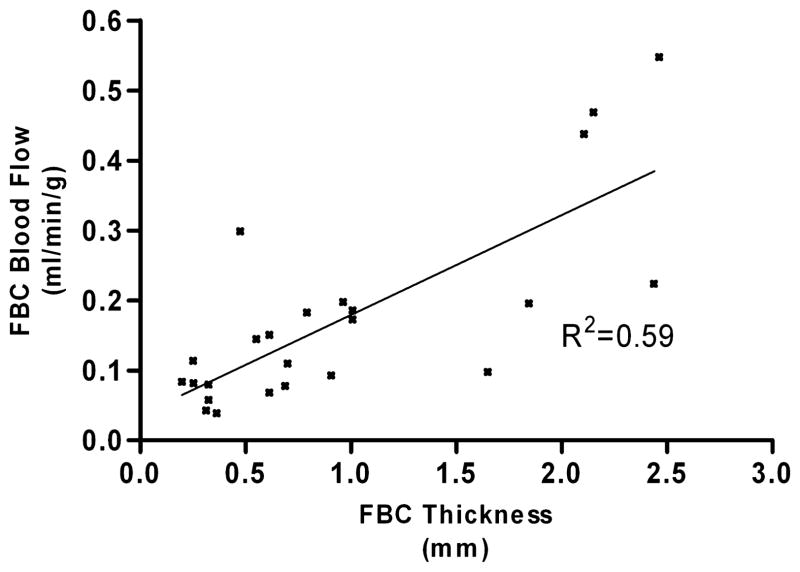
Forty subcutaneously implanted devices (14 polysulfone, 17 titanium, 9 silicone-coated) were studied in nine canines. Devices were explanted and studied at 208 ± 142 (mean ± standard deviation) postoperative days. Capsule thicknesses surrounding each of the three device materials were not statistically different and were in the range of 1 ± 0.7 mm. However, Figure 1 demonstrates that FBC thickness correlated with vascular density for all devices studied (R2 = 0.85, P < 0.0001) as well as for each material type studied (polysulfone R2 = 0.85, P < 0.0001; silicone R2 = 0.95, P < 0.0001; titanium R2 = 0.87, P < 0.0001). Figure 2 demonstrates that blood flow within FBCs exhibited a modest correlation with capsule thickness for all devices (R2 = 0.59, P < 0.0001) as well as for each material type (polysulfone R2 = 0.77, P < 0.001; silicone R2 = 0.65, P = 0.10; titanium R2 = 0.52, P = 0.03).
FIG. 2.
Foreign-body capsule (FBC) thickness correlated with blood flow for all devices studied (P < 0.0001).
Animal-to-animal differences in FBC thickness were noted. In general, capsule thicknesses within each animal were similar and independent of material type. This variable and animal-specific response was also noted with regard to vascular density and blood flow within FBCs.
DISCUSSION
The goal of this study was to evaluate the relationship between thickness, vascular density, and blood flow in FBCs surrounding devices of clinically relevant size implanted in subcutaneous tissue. Our results demonstrate that: (i) the device material type did not influence the thickness of the FBC; (ii) the thickness of the FBC correlated with vascular density within the capsule; (iii) the thickness of the FBC correlated modestly with capsule blood flow; and (iv) individual animals exhibited consistent FBC thicknesses, vascular density, and blood flows that were variable across animals.
The presence of a foreign body in a physiological environment elicits a cascade of acute and chronic inflammatory mechanisms and wound-healing events that encapsulate the device in granulomatous tissue. Within 7–14 days of implantation, tissue begins to reorganize around the device and acts as a structural and biological barrier. Over a period of months, tissue in the FBC accumulates, remodels, and condenses (1). We have previously demonstrated that during the construction of the FBC, and over a period of 8–10 weeks, significantly elevated blood flow in the FBC decreased toward chronically elevated values slightly above normal subcutaneous blood flow. During this process, the material from which the device was constructed influenced the amount of blood flow to tissues within and surrounding the FBC (3).
The results of the current study expand our understanding of the gross foreign-body response and suggest that although material type may influence blood flow within the FBC (3), the dimensions of the capsule may be independent of the material from which the device is constructed. As such, these findings may be useful for the development of novel biomaterials and implantable devices. For example, the architecture of the FBC has important implications for devices with sensory or delivery capabilities that function via diffusion (5) such as insulin pumps (6). FBCs are largely composed of dense extracellular matrix. The thickness and vascularity of the capsule influence the overall mass-transfer resistance of the tissue. Thicker or more dense capsules with minimal vascular density may impede the diffusion of analytes to and from the device. Indeed, knowledge of the thickness and vascular density of FBCs is a critical parameter for the development of subcutaneous drug-delivery devices.
It is not unexpected that FBC thickness correlated with vascular density and blood flow. Likely, as a thicker fibrous capsule develops, the diffusion of metabolites across the dense capsule wall becomes impaired. To ensure adequate oxygenation and nutrition, tissues elaborate a larger and more extensive vascular network within the FBC. However, a causal relationship between FBC thickness, vascular density, and blood flow remains undetermined. Further investigation into this relationship is needed to understand why material type influenced blood flow within the FBC (3) but not thickness despite thickness correlating with vascular density and blood flow.
Of similar interest, it is not clear why animals exhibited large variability in FBC thickness and blood flow. The robustness of an individual’s foreign-body response is likely influenced by multiple factors that include genetics, immunologic parameters, metabolism, environmental factors such as nutrition and activity level, and preexisting pathology. For example, at necropsy, an anterolateral left ventricular myocardial infarction was confirmed in one animal. On gross examination, FBCs in this animal contained nonpurulent, serous fluid and were notably thicker and more loosely organized than FBCs examined from other animals. Histologically, this animal exhibited the thickest FBCs in which the largest vascular density and greatest blood flow values in this study were observed. It is possible that the systemic inflammatory response and pathophysiological processes that accompanied myocardial infarction in this animal may have influenced the construction and maintenance of the FBCs. This observation raises two important questions: to what extent do local and systemic pathology influence the foreign-body response, and can pharmaceutical intervention or biomaterial engineering prevent pathological FBC development in high-risk patients in which devices are routinely implanted?
Additional studies are necessary to answer these questions and to further understand the construction and maintenance-remodeling of the FBC. Next-generation biomaterials may activate or suppress these responses as designed.
Limitations
Implanted devices were different in shape, size, and weight. It has been shown that device dimensions may affect the nature of the FBC (7) and may have influenced blood flow values within the FBCs. Similarly, the weight of a device may influence FBC dimensions and physiology.
During the course of this study, these chronically instrumented animals underwent additional and unrelated experimental protocols in which the implanted devices were used for data acquisition related to the study of inhaled air pollution (data not shown). Although we do not believe that these exposures influenced the present study, we acknowledge this as a limitation.
Notwithstanding these limitations, the current study contains several strengths that include the use of a clinically relevant, conscious, large animal model and multiple commercially available implantable devices.
CONCLUSIONS
The thickness of FBCs surrounding subcutaneously implanted devices of clinically relevant size is not affected by the device material type. During the foreign-body response, a standard capsule thickness is constructed. FBC thickness correlates with vascular density and blood flow in the capsule. However, a causal relationship between FBC thickness, vascular density, and blood flow remains undetermined and further studies are needed to characterize this association.
Acknowledgments
The authors acknowledge and thank Richard Verrier, PhD, Gregory Wellenius, ScD, Ichiro Akiyama, MD, Brent Coull, PhD, Edgar Diaz, MD, Kazunori Okabe, MD, Joy Lawrence, PhD, Sandra Verrier, Lani Lee, Tracy Katz, Jeffrey Pettit, Mark Long, and the Harvard veterinary staff for their support and assistance. Joe Carlos of IMT Laboratories was instrumental in the processing of micro-sphere tissue samples.
FUNDING SOURCES
This study was supported by grants RD831917, R827353, and R832416 from the US Environmental Protection Agency, and by grants R01 ES12972 and ES00002 from the National Institute of Environmental Health Sciences.
References
- 1.Thomson HG. The fate of the pseudosheath pocket around silicone implants. Plast Reconstr Surg. 1973;51:667–71. doi: 10.1097/00006534-197306000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Bartoli CR, Okabe K, Akiyama I, Coull B, Godleski JJ. Repeat microsphere delivery for serial measurement of regional blood perfusion in the chronically instrumented, conscious canine. J Surg Res. 2008;145:135–41. doi: 10.1016/j.jss.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartoli CR, Godleski JJ. Blood flow in the foreign-body capsules surrounding surgically implanted subcutaneous devices. J Surg Res. 2010;158:147–54. doi: 10.1016/j.jss.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartoli CR, Okabe K, Akiyama I, Verrier RL, Godleski JJ. Technique for implantation of chronic indwelling aortic access catheters. J Invest Surg. 2006;19:397–405. doi: 10.1080/08941930600985751. [DOI] [PubMed] [Google Scholar]
- 5.Anderson JM, Niven H, Pelagalli J, Olanoff LS, Jones RD. The role of the fibrous capsule in the function of implanted drug-polymer sustained release systems. J Biomed Mater Res. 1981;15:889–902. doi: 10.1002/jbm.820150613. [DOI] [PubMed] [Google Scholar]
- 6.Clausen TS, Kaastrup P, Stallknecht B. Effect of insulin catheter wear-time on subcutaneous adipose tissue blood flow and insulin absorption in humans. Diabetes Technol Ther. 2009;11:575–80. doi: 10.1089/dia.2009.0058. [DOI] [PubMed] [Google Scholar]
- 7.Matlaga BF, Yasenchak LP, Salthouse TN. Tissue response to implanted polymers: the significance of sample shape. J Biomed Mater Res. 1976;10:391–7. doi: 10.1002/jbm.820100308. [DOI] [PubMed] [Google Scholar]