Abstract
Context
Advancing paternal age has been linked to autism.
Objective
To further expand knowledge about the relation between paternal age and autism by studying the effect of grandfathers’ age on childhood autism.
Design
Population-based multigenerational case-control study.
Setting
Nationwide Multi-Generation and Patient registers in Sweden.
Participants
We conducted a study of individuals born in Sweden since 1932. Parental age at birth was obtained for over 90% of the cohort. Grandparental age at the time of birth of the parent was obtained for a smaller subset (5,936 cases and 30,923 controls).
Main Outcome Measures
International Classification of Diseases (ICD) diagnosis of childhood autism in the Patient Registry.
Results
There was a statistically significant monotonic association between advancing grandpaternal age at the time of birth of the parent and risk of autism in grandchildren. Men who had a daughter when they were 50 or older were 1.79 times (95% confidence interval: 1.35-2.37, p<0.001) more likely to have a grandchild with autism, and men who had a son when they were 50 or older were 1.67 times (95% confidence interval: 1.35-2.37, p<0.001) more likely to have a grandchild with autism, compared to men who had children when they were 20-24, after controlling for birth year, sex, age of the spouse, family history of psychiatric disorders, highest family education and residential county. There was also a statistically significant monotonic association between advancing paternal age and risk of autism in the offspring. Sensitivity analyses indicated that these findings were not the result of bias due to missing data on grandparental age.
Conclusion
Advanced grandparental age was associated with increased risk of autism, suggesting that risk for autism could develop over generations. The results are consistent with mutations and/or epigenetic alterations associated with advancing paternal age.
Introduction
Autism is a neurodevelopmental disorder characterized by social deficiencies, language impairments and repetitive behavior patterns.1 The disorder onsets early in life, has a high heritability, and is associated with a marked reduction in birth rates.2
During the last decade, evidence has accumulated suggesting that the offspring of older fathers have an increased risk of developing autism.3-6 A recent meta-analysis found that fathers aged 50 years and older were 2.2 times more likely to have a child diagnosed with autism compared to fathers younger than 30 years.5 Advanced paternal age has also been associated with other mental disorders such as schizophrenia,7-9 bipolar disorder,10 and general neurocognitive development in children.11 The mechanism behind the paternal age effect on adverse neuropsychiatric outcomes is unknown. It has been suggested that de novo mutations occurring in the male germ cell line underlie the association.12 In men, spermatogonial cells replicate every sixteenth day, resulting in approximately 200 divisions by the age of 20 years and 660 divisions by the age of 40.13 Each time the cell divides, the replication of the genome introduces the possibility of copy error mutations. In humans, it has been confirmed that sperm from older men have significantly more mutations.12, 14, 15 Levels of DNA proof-reading and repair enzymes decline as a function of advancing paternal age 16, 17 and DNA fragmentation increases,18 further compromising the integrity of gene replication. Experiments based on mouse model related to advanced paternal age have confirmed that the offspring of older sires have a significantly increased risk of de novo copy number variants and several of these mutations involved genes previously linked to autism.19
Recently, several studies have reported that de novo mutations in autism pedigrees are predominantly paternal in origin and are significantly associated with advancing paternal age.20-23 An Icelandic study on individuals with sporadic schizophrenia or autism even showed that the rate of new mutations in relation to paternal age is two new mutations per year.24 In addition, commentators have noted that the genetic architecture of neurodevelopmental disorders such as autism and schizophrenia is characterized by locus heterogeneity, variable expressivity of the same mutations and a cumulative impact on common biological pathways.25, 26 Thus, it is feasible the some paternal age-related de novo mutations may not result in adverse health outcomes in the offspring, but still contribute the overall burden of mutations inherited by subsequent generations. Thus, it would be predicted that both paternal and grandpaternal age could contribute to a cumulative ‘threshold’ of mutation that emerges in an increased risk of neurodevelopmental disorders such as schizophrenia and autism. By using the unique Swedish national registers we can test if the older the grandfather is when the parent is born, the greater the risk for autism in the grandchild and thus, further explore the paternal age effect.
Method
Data source
By linking population-based Swedish longitudinal registers we compared the ages of parents and grandparents at offspring birth among individuals with or without childhood autism diagnosis. The unique personal identification number assigned to each Swedish citizen at birth or upon arrival to the country (immigrants) enables linkage of national registers. The Patient Register includes practically all psychiatric inpatient discharge diagnoses in Sweden since 1973 recorded according to the International Classification of Diseases (ICD).27-29 The Patient Register also includes outpatient care in Sweden since 2001. The Swedish Multi-Generation Register contains information about biological parents of an index person and their birth dates.30 A prerequisite for being included in the register is that the index person was born after January 1st 1932, and ever registered as living in Sweden after 1960. Ethical approval was given by the research ethics committee at Karolinska Institutet, Stockholm, Sweden.
Analytic cohort
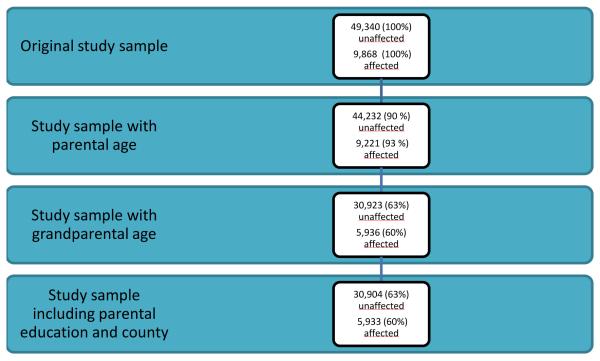
We identified individuals diagnosed with childhood autism in the patient register (ICD-9 code 299,0 and ICD-10 code F84.0). We included diagnoses given at discharge from inpatient care since 1987 when the specific diagnostic code for childhood autism was first introduced and diagnoses given during outpatient care since 2001. Medical records are computerized and contain notations from psychiatrists, psychologists, neurologists, social workers, and nurses for inpatient and outpatient treatment. High validity of ICD diagnoses recorded in the Swedish patient register has been found by comparing diagnostic register code with medical records. The positive predictive value for most somatic and psychiatric diagnoses is about 85-95% 31 and a medical record review substantiated the presence of DSM-IV autism in 94.3% of cases (83 of 88).The patient register was followed until December 31st 2009. Individuals that did not meet our criteria for autism were considered unaffected. Five unaffected individuals for each affected subject were frequency matched on gender and exact year of birth. Age data for parents and grandparents were linked to the study subjects. Ages of parents were defined as the parent’s age at the time of the index person’s birth. Ages of grandparents were defined as the grandparent’s age at the time of the parent’s birth. Birth dates were obtained from the Multi-Generation Register. We identified 9,868 individuals affected with childhood autism and 49,340 unaffected individuals. After linking ages of parents and grandparents, the final study sample consisted of 5,936 (60% of the initial sample) affected individuals and 30,923 (63 % of the initial sample) unaffected individuals with complete data on both maternal and paternal grandparents. A description of the study sample is outlined in Figure 1.
Figure 1.
Study sample
Covariates
A family history of psychiatric diagnosis was defined as having a parent or a grandparent with a diagnosis of schizophrenia, bipolar disorder or autism in the patient register, defined by ICD-8 and ICD 9 codes 295-299 (except 296,2 and 296B) and ICD-10 codes F21-29, F30-31 and F84. The selected diagnoses are possible confounders since they have been associated with paternal age in earlier studies. For the analyses without grandparental information, only information of parental history of psychiatric diagnosis were used. As a proxy measure of the socio-economic home environment of the grandchild, we examined parental education defined as highest achieved education level within each parental pair. Information about education level was retrieved from the longitudinal integration database for health insurance and labour market studies. Since coverage of outpatient care might vary across counties and place of residence might be a potential confounder, we also collected information about the probands’ residential county from the Total Population Register. The distribution of covariates in the final data set is described in eTable 1. This table also shows the distribution of birth year divided into 10-year categories as well as the gender distribution where boys represented 72 % of our sample and 28 % of our study subjects were girls.
Statistical analysis
We estimated the relative risk of autism in offspring comparing different categories of parental and grandparental age by calculating the odds ratio (OR) and associated two-sided 95% confidence intervals (CI) using logistic regression. Ages were categorized into 5-year intervals with 20-24 years as the reference category. The analyses were performed in four steps. First, we adjusted only for birth year and sex (Model 1), followed by an analysis also adjusted for the age of each parent’s or grandparent’s partner/spouse (Model 2). We did not control for age of the maternal grandparents when analyzing paternal grandparents and vice versa, since we do not consider these ages to be directly correlated in the same manner as age of spouses. In Model 3, we added family history of psychiatric disorders and lastly, we included parental education as well as residential county (Model 4). Logistic regression analyses were performed in SAS (version 9.2; SAS Institute Inc, Cary, North Carolina), using Proc logistic. Statistical hypothesis testing was based on the 2-sided 5% level of significance. The models were evaluated for goodness-of-fit by visual inspections of the model residuals. We calculated variance inflation factors to check for collinearity between parental and grandparental age covariates and found no signs of such problems. Using a paternal age cutoff at 40 years we calculated the attributable risk (AR) by P(E∣D)*(RR-1)/RR. In other words, we obtain estimates on the proportion of autistic children that could be avoided if fathers and grandfathers at ages 40 or older had their child before 40 years, assuming a causal effect.
Sensitivity analyses
Diagnoses given during outpatient care have not been included in the Swedish Patient register until 2001. We therefore did sensitivity analyses including only inpatient data to examine potential differences between patients treated in inpatient care and those treated in outpatient care. These analyses included 1,845 cases (Model 1-3) and 1,843 cases (Model 4), respectively, with autism diagnoses assigned only during inpatient care.
We performed additional analyses on grandparental ages adjusting for ages of the parents to explore if this affected the results. We also wanted to investigate effects of potential truncation of parental ages after linkage of grandparental age data and did this by analyzing parental ages before the linkage and comparing the results with data from the main analysis (sample described in Figure 1). We could identify 9,221 affected and 44,232 unaffected individuals with parental age data, corresponding to 93% and 90% of the original samples. To address the issue of potential bias due to the different probabilities of being selected for the different sets of analysis, with and without requirements of valid grandparental data, we applied inverse probability weighting to the regression models with robust standard errors.
Results
Grandparental age and autism
The logistic regression analyses on grandpaternal ages are presented in Table 1. Analyses adjusted for birth year and sex showed a statistically significant association between older grandfathers and autism on both the maternal and the paternal sides. The risk of autism increased monotonically with advancing grandpaternal age. When adjusting for age of the spouse the effect was statistically significant across all age categories including maternal grandfathers 30 years and or older paternal grandfathers 25 years or older. The results remained after controlling for family history of psychiatric disorders and parental education. The highest risk was found in the oldest age categories in all 4 models. In the fully adjusted model, maternal grandfathers 50 years or older had an OR of 1.79 (CI= 1.35-2.37) compared to grandfathers aged 20-24 years, and paternal grandfathers 50 years or older had an OR of 1.67 (CI= 1.25-2.24). We found no trend indicating an increased risk for autism in grandchildren of women who gave birth at older ages (eTable 2).
Table 1.
Results from logistic regression analyses on grandpaternal ages and autism risk
| No of controls (%) Model 1-3 | No of cases (%) Model 1-3 | OR | CI | OR | CI | OR | CI | OR | CI | |
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |||||||
| Maternal grandfather age, years | ||||||||||
| <20 | 675 (2.18) | 122 (2.06) | 0.96 | 0.79-1.18 | 0.91 | 0.74-1.12 | 0.91 | 0.73-1.12 | 0.90 | 0.73-1.11 |
| 20-24 | 6721 (21.73) | 1253 (21.11) | 1.00 | 1.00 | 1.00 | |||||
| 25-29 | 9801 (31.69) | 1787 (30.10) | 0.98 | 0.91-1.06 | 1.07 | 0.98-1.17 | 1.07 | 0.98-1.17 | 1.08 | 0.99-1.18 |
| 30-34 | 7082 (22.90) | 1334 (22.47) | 1.01 | 0.93-1.10 | 1.18 | 1.06-1.31 | 1.18 | 1.06-1.31 | 1.19 | 1.07-1.32 |
| 35-39 | 3868 (12.51) | 808 (13.61) | 1.10 | 1.00-1.22 | 1.33 | 1.17-1.50 | 1.32 | 1.16-1.50 | 1.31 | 1.15-1.49 |
| 40-44 | 1843 (5.96) | 393 (6.62) | 1.12 | 0.99-1.27 | 1.32 | 1.13-1.55 | 1.31 | 1.11-1.53 | 1.32 | 1.12-1.54 |
| 45-49 | 666 (2.15) | 154 (2.59) | 1.22 | 1.01-1.46 | 1.39 | 1.12-1.73 | 1.37 | 1.10-1.71 | 1.34 | 1.07-1.67 |
| ≥ 50 | 267 (0.86) | 85 (1.43) | 1.67 | 1.30-2.15 | 1.90 | 1.44-2.51 | 1.87 | 1.42-2.48 | 1.79 | 1.35-2.37 |
| Paternal grandfather age, years | ||||||||||
| <20 | 702 (2.27) | 123 (2.07) | 0.96 | 0.79-1.18 | 0.88 | 0.72-1.09 | 0.88 | 0.72-1.09 | 0.91 | 0.73-1.12 |
| 20-24 | 6293 (20.35) | 1139 (19.19) | 1.00 | 1.00 | 1.00 | |||||
| 25-29 | 9694 (31.35) | 1793 (30.21) | 1.02 | 0.94-1.11 | 1.09 | 1.00-1.19 | 1.09 | 0.99-1.19 | 1.10 | 1.00-1.20 |
| 30-34 | 7046 (22.79) | 1387 (23.37) | 1.08 | 0.99-1.17 | 1.18 | 1.06-1.31 | 1.17 | 1.05-1.30 | 1.17 | 1.05-1.30 |
| 35-39 | 4277 (13.83) | 831 (14.00) | 1.05 | 0.95- 1.16 | 1.17 | 1.04-1.33 | 1.17 | 1.03-1.32 | 1.15 | 1.02-1.31 |
| 40-44 | 1971 (6.37) | 405 (6.82) | 1.11 | 0.98-1.26 | 1.26 | 1.08-1.47 | 1.24 | 1.06-1.46 | 1.23 | 1.05-1.44 |
| 45-49 | 672 (2.17) | 180 (3.03) | 1.45 | 1.22-1.74 | 1.64 | 1.34 -2.02 | 1.62 | 1.32-1.99 | 1.60 | 1.30-1.97 |
| ≥ 50 | 268 (0.87) | 78 (1.31) | 1.56 | 1.20-2.02 | 1.76 | 1.32-2.35 | 1.72 | 1.29-2.30 | 1.67 | 1.25-2.24 |
OR:Odds ratios
CI: 95% Confidence Intervals
Model 1: Adj for birth year, sex
Model 2: Adj for birth year, sex, age of spouse
Model 3: Adj for birth year, sex, age of spouse, family history
Model 4: Adj for birth year, sex, family history, highest education, county
Model 1-3: controls=30923 cases=5936
Model 4: controls=30904 cases=5933
Parental age and autism
Logistic regression analyses on parental ages (Table 2) suggested a statistically significantly risk increase of childhood autism in all paternal age categories 35 years or older in Model 1, again with a monotonic increase in risk with increasing age. After adjustments for age of the mother, the paternal age effect was found for fathers 30 years or older. Further adjustments did not have any evident effect on the point estimates or the confidence intervals. In the fully adjusted model (Model 4), the highest risk was found in men 50 years or older (OR= 2.26; 95% CI= 1.61-3.18). We found no evident trend for an increased risk of autism in offspring of older mothers after adjusting for father’s age and the other covariates, with the exception of an excess of mothers aged 40 years and older in cases. The AR for grandfathers above the age 40 was estimated to between 3 % for both maternal and paternal grandfathers. For fathers, the corresponding proportion was 6 %.”
Table 2.
Results from logistic regression analyses on parental ages and autism risk (sample with grandparental ages)
| No of controls (%) Model 1-3 | No of cases (%) Model 1-3 | OR | CI | OR | CI | OR | CI | OR | CI | |
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |||||||
| Paternal age, years | ||||||||||
| <20 | 244 (0.79) | 49 (0.83) | 1.11 | 0.81-1.53 | 0.98 | 0.70-1.37 | 0.97 | 0.69-1.36 | 1.00 | 0.71-1.40 |
| 20-24 | 3452 (11.16) | 650 (10.95) | 1.00 | 1.00 | 1.00 | |||||
| 25-29 | 9685 (31.32) | 1716 (28.91) | 0.96 | 0.87-1.06 | 1.04 | 0.94-1.16 | 1.05 | 0.94-1.17 | 1.06 | 0.95-1.19 |
| 30-34 | 9989 (32.30) | 1836 (30.93) | 1.02 | 0.92-1.12 | 1.14 | 1.02-1.29 | 1.15 | 1.02-1.30 | 1.18 | 1.04-1.33 |
| 35-39 | 5236 (16.93) | 1051 (17.71) | 1.13 | 1.01-1.26 | 1.22 | 1.07-1.40 | 1.23 | 1.08-1.41 | 1.24 | 1.08-1.42 |
| 40-44 | 1711 (5.53) | 429 (7.23) | 1.41 | 1.23-1.62 | 1.47 | 1.24 -1.73 | 1.47 | 1.24 -1.73 | 1.45 | 1.23-1.71 |
| 45-49 | 460 (1.49) | 149 (2.51) | 1.82 | 1.49- 2.24 | 1.87 | 1.49-2.34 | 1.85 | 1.47-2.31 | 1.83 | 1.46-2.30 |
| ≥ 50 | 146 (0.47) | 56 (0.94) | 2.23 | 1.61-3.07 | 2.25 | 1.61-3.15 | 2.23 | 1.59-3.12 | 2.26 | 1.61-3.18 |
| Maternal age, years | ||||||||||
| <20 | 826 (2.67) | 191 (3.22) | 1.19 | 1.01-1.42 | 1.23 | 1.02-1.48 | 1.22 | 1.02-1.47 | 1.15 | 0.96-1.39 |
| 20-24 | 6255 (20.23) | 1216 (20.49) | 1.00 | 1.00 | 1.00 | |||||
| 25-29 | 11256 (36.40) | 2009 (33.84) | 0.93 | 0.86-1.01 | 0.89 | 0.81-0.97 | 0.89 | 0.82-0.97 | 0.93 | 0.85-1.01 |
| 30-34 | 8726 (28.22) | 1586 (26.72) | 0.98 | 0.90-1.06 | 0.86 | 0.78- 0.95 | 0.87 | 0.78-0.96 | 0.92 | 0.83-1.02 |
| 35-39 | 3305 (10.69) | 772 (13.01) | 1.27 | 1.15-1.41 | 1.02 | 0.90-1.16 | 1.02 | 0.90-1.16 | 1.11 | 0.97-1.26 |
| ≥ 40 | 555 (1.79) | 162 (2.73) | 1.59 | 1.32-1.92 | 1.13 | 0.92-1.40 | 1.13 | 0.92-1.40 | 1.26 | 1.02-1.56 |
OR:Odds ratios
CI: 95% Confidence Intervals
Model 1: Adj for birth year, sex
Model 2: Adj for birth year, sex, age of spouse
Model 3: Adj for birth year, sex, age of spouse, family history
Model 4: Adj for birth year, sex, family history, highest education, county
Model 1-3: controls=30923 cases=5936
Model 4: controls=30904 cases=5933
Sensitivity analyses
The analyses conducted separately on inpatients showed similar age effects as in the main analysis (eTable 3). Although the confidence intervals were expectedly broader than in the main analyses, we identified a trend of increasing autism risk in grandchildren of older maternal and paternal grandfathers. Again, no such effects of advanced age were found for grandmothers. Similarly, the association between paternal age and autism was also evident in these analyses. Again, we could see an association between mothers aged 40 years or more, but we could not detect any overall trend between maternal age and autism.
Adjustments for parental ages did not have any major effect on the risk estimates in comparison to the main analyses (eTable 4); the statistically significantly increased risk for autism in the grandchildren of older grandfathers remained.
The analyses on all individuals with parental age data (eTable 5) showed similar associations between parental ages and autism as compared to the sample that required present grandparental age (Table 2). In addition, estimated odds ratios, associated confidence intervals and P-values were close to identical to our main results when using the inverse probability weighting procedure.
Discussion
We can for the first time report that grandfather’s age is associated with risk of childhood autism, independent of paternal or maternal age. We also confirm a statistically significant association between advanced paternal age and an increased offspring risk of autism. Associations between paternal age and autism has been reported in previous studies,3-6 including one using a 10-year Swedish birth cohort.5 We could also see some evidence of an association between maternal age and autism, in congruent with a novel meta-analysis.32
A recent study reported an association between grandpaternal age and schizophrenia.33 This association was exclusive for maternal grandfathers. There are, however, no reports of an association between paternal age and autism being transmitted to further generations. The only study that has, to our knowledge, looked at grandparents’ ages in association to autism found no evidence of an effect of grandfathers’ age but instead an association between age of maternal grandmothers and ASD. This study did, however, included 86 individuals with ASD as compared to the 5,936 individuals with the specific diagnosis childhood autism included in the present study.34
Since autism is characterized by lower birth rates and high heritability,2 it is puzzling that the disorder still exists and may even be increasing in prevalence.35, 36 The strong negative selection pressure should remove genes associated with this disorder from the gene pool promptly. One possible explanation to this paradox is that genetic variants increasing the risk for autism constantly arise as de novo mutations.37 This hypothesis is supported by recent findings of de novo mutations in autism pedigrees being predominantly paternal in origin and significantly associated with advancing paternal age.20-24Mendelian inheritance laws indicate that offspring who acquire a de novo autosomal mutation from their father’s sperm should pass (on average) this mutation to half of their offspring. Age-related mutations in the male germ line could accumulate over several generations, and only influence the offspring’s health after a certain “mutational threshold” has been breached 12, 38. Thus, paternal age and grandpaternal (both maternal and paternal grandfathers) may contribute to an offspring’s mutational load resulting in an increased risk of disorders in the offspring. If this is true, autism should not only be associated with paternal age but also with grandpaternal age. In this study, we show for the first time that paternal age not only has an impact on autism risk in offspring but also affects subsequent generations.
Although the most favored hypothesis behind the paternal age effect suggests that the associations is caused by an increased rate of mutations in sperm of older men it has also been suggested that the paternal age effect is explained by men with mental or personality disorders being more likely to become fathers at older ages.39, 40 Although the coverage of family history of mental disorders might be incomplete and questionable in quality, the paternal age effect has been consistent in studies controlling for mental disorders in the parents. Also, within-family analysis of discordant siblings showed that the siblings affected with autism had older paternal age,5 supporting the notion of a causal association between paternal age and autism in the offspring. Petersen et al. showed that paternal age effect on schizophrenia was not present in later born children when father’s age at the first born child was accounted for, opposing the hypothesis of de novo mutations.40 By contrast, a Swedish study testing if the paternal age effect in autism only pertains to first-born children showed that the risk of autism increased in later born children irrespectively of when the father had his first child.5
Autism risk increased with advancing paternal and grandpaternal age. The risk estimates were very similar for maternal and paternal grandfathers. Although confidence intervals overlapped, the grandpaternal age risk estimates were lower than the risk estimates for paternal age which is consistent with the hypothesis of genetically mediated causes since the strength of a genetically mediated effect should reflect the genetic relatedness. On average, you inherit 50% of your genes from your parent and only 25 % from your grandparent so the overall mutational load would thus be higher if your father is old as compared to if your grandfather is old. Similarly, if the de novo mutation hypothesis is true the risk for autism would be greater in offspring of old fathers than in grandchildren of older men.
The coverage of data on parental age for cases and controls was very high (~90%). After linking grandparental ages approximately 60% of the initial sample remained. To collate a sample consisting of three generations, parents had to be born after 1932. Older parents are therefore more likely to be excluded than younger parents. Grandparental ages are not truncated but since parental and grandparental ages are correlated this should only result in an underestimation of the grandpaternal age effect as a result of parental age truncation. However the analyses conducted on the sample without requiring grandparental age data linkage suggested a similar paternal age effect as compared to the sample used for the main analyses. We therefore conclude that there was no major truncation of parental ages, which adds to the validity of our findings.
Although the proportion of girls with autism is relatively high in Sweden an extensive review including 29 studies showed the male/female sex ratio varied from 1.33 to 16.0.41 Since the sex ratio in the present study is well within this interval we do not consider the sex ratio to be atypical in the present study.
The main strength of this study is the inclusion of a large number of individuals diagnosed with childhood autism and available birth date information across three generations. The consistency in results between the main analyses and the analyses restricted to inpatients further strengthen our findings and the generalizability of the results.
Our findings have added salience in light of the recent evidence that autism is associated with de novo and inherited mutations.20-24, 42-44 Considering the association between advanced paternal age and de novo copy number variants in an animal model,45 we speculate that paternal age-related mutagenesis is associated with an increased risk of autism via two mechanisms. The offspring of older fathers may be at increased risk of acquiring de novo mutations, as previously speculated.46 Considering our finding linking grandpaternal age and risk of schizophrenia, we propose that a proportion of age-related de novo mutations are phenotypically silent in the offspring, but can still influence risk of autism in subsequent generations, perhaps via the interaction with other susceptibility factors. This indirect mechanism is consistent with the evidence that some mutations associated with neurodevelopmental disorders can occur in apparently healthy individual.47, 48
Age of parenthood is increasing in many societies and thus it is feasible that the incidence of paternal age-related disorders will increase over time. Our findings provide new information about the paternal age effect and its impact on future generations. Older men should not be discouraged to have children based on these findings but the results may be important in understanding the mechanism behind childhood autism and other psychiatric and neurodevelopmental disorders.
Supplementary Material
Acknowledgement
This study was supported by Swedish Research Council (CM Hultman), Swedish Council for Working Life and Social Research (guest research fellowship A Reichenberg) and Karolinska Institutet.
Footnotes
None of the authors have any financial interest relevant to the submitted manuscript.
Referenses
- 1.American Psychiatric Association. American Psychiatric Association . Diagnostic and statistical manual of mental disorders : DSM-IV. 4th ed American Psychiatric Association; Washington, DC: 1994. Task Force on DSM-IV. [Google Scholar]
- 2.Uher R. The role of genetic variation in the causation of mental illness: an evolution- informed framework. Molecular psychiatry. 2009 Dec;14(12):1072–1082. doi: 10.1038/mp.2009.85. [DOI] [PubMed] [Google Scholar]
- 3.Croen LA, Najjar DV, Fireman B, Grether JK. Maternal and paternal age and risk of autism spectrum disorders. Arch Pediatr Adolesc Med. 2007 Apr;161(4):334–340. doi: 10.1001/archpedi.161.4.334. [DOI] [PubMed] [Google Scholar]
- 4.Durkin MS, Maenner MJ, Newschaffer CJ, Lee LC, Cunniff CM, Daniels JL, Kirby RS, Leavitt L, Miller L, Zahorodny W, Schieve LA. Advanced parental age and the risk of autism spectrum disorder. Am J Epidemiol. 2008 Dec 1;168(11):1268–1276. doi: 10.1093/aje/kwn250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hultman CM, Sandin S, Levine SZ, Lichtenstein P, Reichenberg A. Advancing paternal age and risk of autism: new evidence from a population-based study and a meta-analysis of epidemiological studies. Mol Psychiatry. 2011 Dec;16(12):1203–1212. doi: 10.1038/mp.2010.121. [DOI] [PubMed] [Google Scholar]
- 6.Reichenberg A, Gross R, Weiser M, Bresnahan M, Silverman J, Harlap S, Rabinowitz J, Shulman C, Malaspina D, Lubin G, Knobler HY, Davidson M, Susser E. Advancing paternal age and autism. Arch Gen Psychiatry. 2006 Sep;63(9):1026–1032. doi: 10.1001/archpsyc.63.9.1026. [DOI] [PubMed] [Google Scholar]
- 7.Byrne M, Agerbo E, Ewald H, Eaton WW, Mortensen PB. Parental age and risk of schizophrenia: a case-control study. Arch Gen Psychiatry. 2003 Jul;60(7):673–678. doi: 10.1001/archpsyc.60.7.673. [DOI] [PubMed] [Google Scholar]
- 8.Malaspina D, Harlap S, Fennig S, Heiman D, Nahon D, Feldman D, Susser ES. Advancing paternal age and the risk of schizophrenia. Arch Gen Psychiatry. 2001 Apr;58(4):361–367. doi: 10.1001/archpsyc.58.4.361. [DOI] [PubMed] [Google Scholar]
- 9.Sipos A, Rasmussen F, Harrison G, Tynelius P, Lewis G, Leon DA, Gunnell D. Paternal age and schizophrenia: a population based cohort study. BMJ. 2004 Nov 6;329(7474):1070. doi: 10.1136/bmj.38243.672396.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frans EM, Sandin S, Reichenberg A, Lichtenstein P, Langstrom N, Hultman CM. Advancing paternal age and bipolar disorder. Arch Gen Psychiatry. 2008 Sep;65(9):1034–1040. doi: 10.1001/archpsyc.65.9.1034. [DOI] [PubMed] [Google Scholar]
- 11.Saha S, Barnett AG, Foldi C, Burne TH, Eyles DW, Buka SL, McGrath JJ. Advanced paternal age is associated with impaired neurocognitive outcomes during infancy and childhood. PLoS medicine. 2009 Mar 10;6(3):e40. doi: 10.1371/journal.pmed.1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crow JF. The origins, patterns and implications of human spontaneous mutation. Nat Rev Genet. 2000 Oct;1(1):40–47. doi: 10.1038/35049558. [DOI] [PubMed] [Google Scholar]
- 13.Drake JW, Charlesworth B, Charlesworth D, Crow JF. Rates of spontaneous mutation. Genetics. 1998 Apr;148(4):1667–1686. doi: 10.1093/genetics/148.4.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bosch M, Rajmil O, Egozcue J, Templado C. Linear increase of structural and numerical chromosome 9 abnormalities in human sperm regarding age. Eur J Hum Genet. 2003 Oct;11(10):754–759. doi: 10.1038/sj.ejhg.5201049. [DOI] [PubMed] [Google Scholar]
- 15.Glaser RL, Broman KW, Schulman RL, Eskenazi B, Wyrobek AJ, Jabs EW. The paternal-age effect in Apert syndrome is due, in part, to the increased frequency of mutations in sperm. Am J Hum Genet. 2003 Oct;73(4):939–947. doi: 10.1086/378419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malaspina D. Paternal factors and schizophrenia risk: de novo mutations and imprinting. Schizophr Bull. 2001;27(3):379–393. doi: 10.1093/oxfordjournals.schbul.a006882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tarin JJ, Brines J, Cano A. Long-term effects of delayed parenthood. Hum Reprod. 1998 Sep;13(9):2371–2376. doi: 10.1093/humrep/13.9.2371. [DOI] [PubMed] [Google Scholar]
- 18.Wyrobek AJ, Eskenazi B, Young S, Arnheim N, Tiemann-Boege I, Jabs EW, Glaser RL, Pearson FS, Evenson D. Advancing age has differential effects on DNA damage, chromatin integrity, gene mutations, and aneuploidies in sperm. Proc Natl Acad Sci U S A. 2006 Jun 20;103(25):9601–9606. doi: 10.1073/pnas.0506468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flatscher-Bader T, Foldi CJ, Chong S, Whitelaw E, Moser RJ, Burne THJ, Eyles W, McGrath JJ. Increased de novo copy number variants in the offspring of older males. 2011;(1):e34. doi: 10.1038/tp.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iossifov I, Ronemus M, Levy D, Wang Z, Hakker I, Rosenbaum J, Yamrom B, Lee YH, Narzisi G, Leotta A, Kendall J, Grabowska E, Ma B, Marks S, Rodgers L, Stepansky A, Troge J, Andrews P, Bekritsky M, Pradhan K, Ghiban E, Kramer M, Parla J, Demeter R, Fulton LL, Fulton RS, Magrini VJ, Ye K, Darnell JC, Darnell RB, Mardis ER, Wilson RK, Schatz MC, McCombie WR, Wigler M. De novo gene disruptions in children on the autistic spectrum. Neuron. 2012 Apr 26;74(2):285–299. doi: 10.1016/j.neuron.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neale BM, Kou Y, Liu L, Ma’ayan A, Samocha KE, Sabo A, Lin CF, Stevens C, Wang LS, Makarov V, Polak P, Yoon S, Maguire J, Crawford EL, Campbell NG, Geller ET, Valladares O, Schafer C, Liu H, Zhao T, Cai G, Lihm J, Dannenfelser R, Jabado O, Peralta Z, Nagaswamy U, Muzny D, Reid JG, Newsham I, Wu Y, Lewis L, Han Y, Voight BF, Lim E, Rossin E, Kirby A, Flannick J, Fromer M, Shakir K, Fennell T, Garimella K, Banks E, Poplin R, Gabriel S, DePristo M, Wimbish JR, Boone BE, Levy SE, Betancur C, Sunyaev S, Boerwinkle E, Buxbaum JD, Cook EH, Jr., Devlin B, Gibbs RA, Roeder K, Schellenberg GD, Sutcliffe JS, Daly MJ. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature. 2012 May 10;485(7397):242–245. doi: 10.1038/nature11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP, Levy R, Ko A, Lee C, Smith JD, Turner EH, Stanaway IB, Vernot B, Malig M, Baker C, Reilly B, Akey JM, Borenstein E, Rieder MJ, Nickerson DA, Bernier R, Shendure J, Eichler EE. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012 May 10;485(7397):246–250. doi: 10.1038/nature10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanders SJ, Murtha MT, Gupta AR, Murdoch JD, Raubeson MJ, Willsey AJ, Ercan-Sencicek AG, DiLullo NM, Parikshak NN, Stein JL, Walker MF, Ober GT, Teran NA, Song Y, El-Fishawy P, Murtha RC, Choi M, Overton JD, Bjornson RD, Carriero NJ, Meyer KA, Bilguvar K, Mane SM, Sestan N, Lifton RP, Gunel M, Roeder K, Geschwind DH, Devlin B, State MW. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012 May 10;485(7397):237–241. doi: 10.1038/nature10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong A, Frigge ML, Masson G, Besenbacher S, Sulem P, Magnusson G, Gudjonsson SA, Sigurdsson A, Jonasdottir A, Wong WS, Sigurdsson G, Walters GB, Steinberg S, Helgason H, Thorleifsson G, Gudbjartsson DF, Helgason A, Magnusson OT, Thorsteinsdottir U, Stefansson K. Rate of de novo mutations and the importance of father’s age to disease risk. Nature. 2012 Aug 23;488(7412):471–475. doi: 10.1038/nature11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coe BP, Girirajan S, Eichler EE. The genetic variability and commonality of neurodevelopmental disease. American journal of medical genetics. Part C, Seminars in medical genetics. 2012 May 15;160C(2):118–129. doi: 10.1002/ajmg.c.31327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mowry BJ, Gratten J. The emerging spectrum of allelic variation in schizophrenia: current evidence and strategies for the identification and functional characterization of common and rare variants. Molecular psychiatry. 2012 May 1; doi: 10.1038/mp.2012.34. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organisation . International Classification of Diseases (8th Revision) World Health Organisation; Geneva: 1967. [Google Scholar]
- 28.World Health Organisation . International Classification of Diseases (9th Revision) Geneva: 1977. [Google Scholar]
- 29.World Health Organisation . International Classification of Diseases (10th Revision) Geneva: 1992. [Google Scholar]
- 30.Statistics Sweden Multi-Generation Register 2004 - A description of contents and quality. 2005 [Google Scholar]
- 31.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO. External review and validation of the Swedish national inpatient register. BMC public health. 2011;11:450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sandin S, Hultman CM, Kolevzon A, Gross R, MacCabe JH, Reichenberg A. Advancing maternal age is associated with increasing risk for autism: a review and meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry. 2012 May;51(5):477–486. doi: 10.1016/j.jaac.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 33.Frans EM, McGrath JJ, Sandin S, Lichtenstein P, Reichenberg A, Langstrom N, Hultman CM. Advanced paternal and grandpaternal age and schizophrenia: A three- generation perspective. Schizophr Res. 2011 Oct 13; doi: 10.1016/j.schres.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Golding J, Steer C, Pembrey M. Parental and grandparental ages in the autistic spectrum disorders: a birth cohort study. PLoS One. 2010;5(4):e9939. doi: 10.1371/journal.pone.0009939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fombonne E. Epidemiology of pervasive developmental disorders. Pediatric research. 2009 Jun;65(6):591–598. doi: 10.1203/PDR.0b013e31819e7203. [DOI] [PubMed] [Google Scholar]
- 36.Newschaffer CJ, Croen LA, Daniels J, Giarelli E, Grether JK, Levy SE, Mandell DS, Miller LA, Pinto-Martin J, Reaven J, Reynolds AM, Rice CE, Schendel D, Windham GC. The epidemiology of autism spectrum disorders. Annual review of public health. 2007;28:235–258. doi: 10.1146/annurev.publhealth.28.021406.144007. [DOI] [PubMed] [Google Scholar]
- 37.Keller MC, Miller G. Resolving the paradox of common, harmful, heritable mental disorders: which evolutionary genetic models work best? The Behavioral and brain sciences. 2006 Aug;29(4):385–404. doi: 10.1017/S0140525X06009095. discussion 405-352. [DOI] [PubMed] [Google Scholar]
- 38.McGrath JJ. The romance of balancing selection versus the sober alternatives: let the data rule (Commentary on Keller and Miller) Behavioural and Brain Sciences. 2006;29(4):417–418. [Google Scholar]
- 39.Granville-Grossman KL. Parental age and schizophrenia. Br J Psychiatry. 1966 Sep;112(490):899–905. doi: 10.1192/bjp.112.490.899. [DOI] [PubMed] [Google Scholar]
- 40.Petersen L, Mortensen PB, Pedersen CB. Paternal age at birth of first child and risk of schizophrenia. Am J Psychiatry. 2011 Jan;168(1):82–88. doi: 10.1176/appi.ajp.2010.10020252. [DOI] [PubMed] [Google Scholar]
- 41.Fombonne E. Epidemiological surveys of autism and other pervasive developmental disorders: an update. Journal of autism and developmental disorders. 2003 Aug;33(4):365–382. doi: 10.1023/a:1025054610557. [DOI] [PubMed] [Google Scholar]
- 42.O’Roak BJ, Deriziotis P, Lee C, Vives L, Schwartz JJ, Girirajan S, Karakoc E, Mackenzie AP, Ng SB, Baker C, Rieder MJ, Nickerson DA, Bernier R, Fisher SE, Shendure J, Eichler EE. Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nat Genet. 2011 Jun;43(6):585–589. doi: 10.1038/ng.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanders SJ, Ercan-Sencicek AG, Hus V, Luo R, Murtha MT, Moreno-De-Luca D, Chu SH, Moreau MP, Gupta AR, Thomson SA, Mason CE, Bilguvar K, Celestino-Soper PB, Choi M, Crawford EL, Davis L, Wright NR, Dhodapkar RM, DiCola M, DiLullo NM, Fernandez TV, Fielding-Singh V, Fishman DO, Frahm S, Garagaloyan R, Goh GS, Kammela S, Klei L, Lowe JK, Lund SC, McGrew AD, Meyer KA, Moffat WJ, Murdoch JD, O’Roak BJ, Ober GT, Pottenger RS, Raubeson MJ, Song Y, Wang Q, Yaspan BL, Yu TW, Yurkiewicz IR, Beaudet AL, Cantor RM, Curland M, Grice DE, Gunel M, Lifton RP, Mane SM, Martin DM, Shaw CA, Sheldon M, Tischfield JA, Walsh CA, Morrow EM, Ledbetter DH, Fombonne E, Lord C, Martin CL, Brooks AI, Sutcliffe JS, Cook EH, Jr., Geschwind D, Roeder K, Devlin B, State MW. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron. 2011 Jun 9;70(5):863–885. doi: 10.1016/j.neuron.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levy D, Ronemus M, Yamrom B, Lee YH, Leotta A, Kendall J, Marks S, Lakshmi B, Pai D, Ye K, Buja A, Krieger A, Yoon S, Troge J, Rodgers L, Iossifov I, Wigler M. Rare de novo and transmitted copy-number variation in autistic spectrum disorders. Neuron. 2011 Jun 9;70(5):886–897. doi: 10.1016/j.neuron.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 45.Flatscher-Bader T, Foldi CJ, Chong S, Whitelaw E, Moser RJ, Burne THJ, Eyles DW, McGrath JJ. Increased de novo copy number variants in the offspring of older males. Translational Psychiatry. 2011;1(e34) doi: 10.1038/tp.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malaspina D, Corcoran C, Fahim C, Berman A, Harkavy-Friedman J, Yale S, Goetz D, Goetz R, Harlap S, Gorman J. Paternal age and sporadic schizophrenia: evidence for de novo mutations. Am J Med Genet. 2002 Apr 8;114(3):299–303. doi: 10.1002/ajmg.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cooper GM, Coe BP, Girirajan S, Rosenfeld JA, Vu TH, Baker C, Williams C, Stalker H, Hamid R, Hannig V, Abdel-Hamid H, Bader P, McCracken E, Niyazov D, Leppig K, Thiese H, Hummel M, Alexander N, Gorski J, Kussmann J, Shashi V, Johnson K, Rehder C, Ballif BC, Shaffer LG, Eichler EE. A copy number variation morbidity map of developmental delay. Nat Genet. 2011;43(9):838–846. doi: 10.1038/ng.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Donovan MC, Kirov G, Owen MJ. Phenotypic variations on the theme of CNVs. Nat Genet. 2008 Dec;40(12):1392–1393. doi: 10.1038/ng1208-1392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.