Abstract
BACKGROUND
The abnormal movements seen in motor conversion disorder are affected by distraction and entrainment, similar to voluntary movement. Unlike voluntary movement, however, patients lack a sense of control for the abnormal movements, a failure of “self-agency.” The action-effect binding paradigm has been used to quantify the sense of self-agency, because subjective contraction of time between an action and its effect only occurs if the subject feels that they are the agent responsible for the action. We used this paradigm, coupled with emotional stimuli, to investigate the sense of agency with voluntary movements in patients with motor conversion disorder.
METHODS
Twenty patients with motor conversion disorder and 20 age- and gender-matched healthy volunteers used a rotating clock to judge the time of their own voluntary keypresses (action) and a subsequent auditory tone (effect), after completing conditioning blocks in which high, medium and low tones were coupled to images of happy, fearful and neutral faces.
RESULTS
The results replicate those shown previously: an effect following a voluntary action was reported as occurring earlier, and the preceding action later, compared to trials of only keypresses or tones. Patients had reduced overall binding scores relative to healthy volunteers, suggesting a reduced sense of agency. There was no effect of the emotional stimuli (faces) or other interaction effects. Healthy volunteers with subclinical depressive symptoms had higher overall binding scores.
CONCLUSIONS
We show that motor conversion disorder patients have decreased action-effect binding for normal voluntary movements compared to healthy volunteers, consistent with the greater experience of lack of control.
Keywords: agency, action-effect binding, conversion disorder, psychogenic movement disorder, forward model
INTRODUCTION
Conversion disorder (CD) refers to unexplained neurological symptoms thought to be mediated by psychological factors, for which the patient denies any sense of control or “self-agency” over the movements.1 Yet the abnormal movements in these patients often appear similar to voluntary movement in several ways. “Entrainment” is often used in diagnosing motor CD, by asking the patient to tap the same rhythm as the examiner. In patients with conversion tremor, the tremor will often assume the same frequency as the tapping.2 In this way, the abnormal movements in CD are vulnerable to interference from other motor tasks, as in voluntary movement, but patients report that their abnormal movements are completely involuntary. How the sense of voluntariness or agency could be dissociated from such movements is unclear.
The sense of agency has been studied with the paradigm of action-effect binding.3 Subjects use a rotating clock hand (based on the method developed by Libet)4 to judge either the time of their own voluntary keypress or of an auditory tone in baseline trials. They then judge either the time of their keypress that led to a tone or the time of a tone that followed their keypress in subsequent trials. When voluntary actions were followed by tones, such that the subjects’ action clearly led to an effect, they judged actions to occur later and effects to occur earlier compared to the baseline trials of only actions or only tones (Figure 1). In the case of a similar involuntary movement (passively induced by transcranial magnetic stimulation (TMS)) the reverse was found: actions were judged to occur earlier and effects to occur later when coupled compared to the baseline trials.
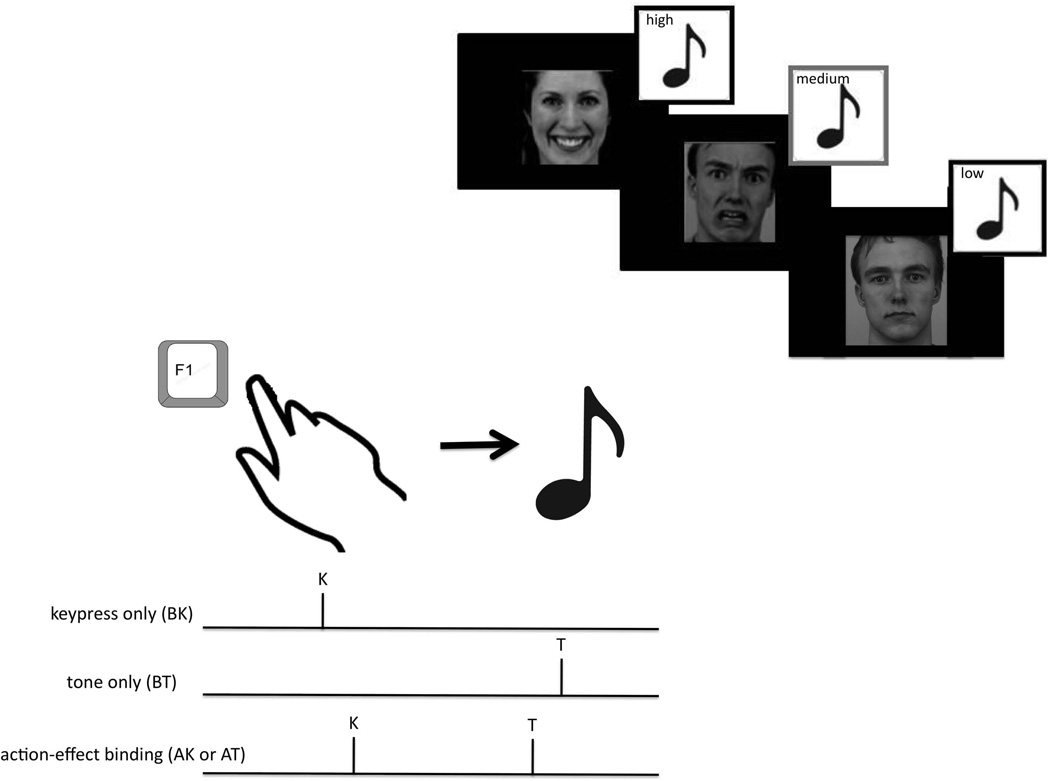
Figure 1.
Upper right: Conditioning for action-effect binding task. Subjects viewed standardized pictures of happy, fearful and neutral faces that were coupled to high, medium and low tones. The association of which faces were coupled to which tones (for example, happy faces and high tones) was randomized between subjects, and the same association was used for each patient-matched control pair. Lower left: Action-effect Binding paradigm. When subjects perceive their voluntary action to be the cause of an effect in the action keypress (AK) or action tone (AT) trials, subjects report the action as occurring later and the effect as occurring earlier than in the baseline keypress (BK) and baseline tone (BT) trials. This compression of the subjective sense of time is also termed “intentional binding” as it does not occur with involuntary or unintentional actions.
Because action-effect binding is only found for intentional, voluntary movements, and motor CD patients deny volition for their abnormal movements, we hypothesized that these patients would show reduced binding. A recent study using Libet’s original paradigm to characterize the subjective sense of when a movement was willed and when it occurred in motor CD patients showed that timing of these mental events differed significantly from healthy volunteers, even though only normal voluntary movements were studied in patients.5 Thus we hypothesized that action-effect binding could be applied to motor CD patients making normal voluntary movements and still reflect a possible abnormality in the greater sense of agency for movement.
How might psychological factors interfere with motor control, causing movement to occur without a sense of agency? It has been shown that an emotional affect associated with a motor outcome changes the sensory perceptions associated with the motor act, even in healthy subjects.6 We have previously shown that in motor CD patients, exposure to arousing stimuli such as happy and fearful faces resulted in greater functional connectivity between limbic regions such as the amygdala and supplementary motor area (SMA) compared to healthy volunteers.7 Motor planning regions such as the SMA are implicated in the sense of agency, possibly by linking voluntary actions to their effects.8 Since amygdala activity and amygdala-SMA functional connectivity is affected by emotional stimuli in CD patients,7 the sense of agency in CD may also be modulated by emotional context.
In this study, we used action-effect binding as an implicit measure of the sense of agency associated with normal voluntary movements in motor conversion patients compared to healthy volunteers. To explore the effects of emotional stimuli as a secondary outcome, three different auditory tones were paired with three emotional stimuli (positive, negative or neutral) in initial conditioning blocks.
METHODS
Subjects
Motor CD patients >18 years were recruited from the Human Motor Control clinic at the National Institute of Neurological Disorders and Stroke. Patients were included if they had “clinically definite” psychogenic movement disorder,9 as well as motor CD by DSM-IV-TR criteria: “psychological factors are judged to be associated with the symptom or deficit because the initiation or exacerbation of the symptom or deficit is preceded by conflicts or other stressors.”1 Patients were excluded if they had a serious medical or neurological illness. Age- (+/− 5 years) and gender-matched healthy volunteers were recruited from the National Institutes of Health healthy volunteer database.
The study was approved by the National Institutes of Health Institutional Review Board and all subjects signed informed consent.
Experimental design
In an initial conditioning block, high, medium and low tones were paired with happy, neutral and fearful faces (Figure 1, from the Karolinska Directed Emotional Faces (1998), 24 conditioning trials per each valence) in order to establish positive, neutral and negative associations (valence) with the different tones. To ensure attention while viewing the faces, subjects were asked to identify the gender of the faces verbally during the conditioning blocks. The order of coupling (e.g., whether high tones were coupled with happy faces) was distributed evenly between subjects. Each patient and control pair were tested with the same order.
The conditioning phase was immediately followed by the test phase. Subjects then watched a clock (diameter 3 cm, rotating 1 revolution/2560 ms, marked at conventional 5 minute intervals) on a computer screen to judge the timing of keypresses and auditory tones.4 Faces were only shown during the conditioning phase, while during the test phase subjects saw only the clock. Four blocks were performed in random order: baseline keypress (BK), baseline tone (BT), and two agency blocks in which the keypress led to a tone (AK and AT), each with 25 trials.3 In each block subjects were instructed whether they would be reporting the time of the keypress or of the tone, and did not switch between these within any block. During blocks requiring keypresses (BK, AK and AT), subjects pressed a key (1.7 cm2) with their dominant index finger, and were specifically instructed not to “aim” their action at a specific preselected position of the clock, but rather to press the key when they felt like doing so. In agency blocks (AK and AT), the keypress was followed by the high, medium or low tone 250 ms later presented in random order. Tones lasted 75 ms. The clock continued to rotate for a random interval before stopping. The subject was then asked to report verbally a number corresponding to the position of the clock when they pressed the key (BK and AK) or heard the tone (BT and AT).
Prior to the study, subjects completed the Beck Depression Inventory (BDI)10 and the State portion of the State-Trait Anxiety Inventory (STAI)11 questionnaires, to assess for depressive and anxiety symptoms.
Because patients typically had generalized abnormal movements, no attempt was made to have patients use an “unaffected” hand for keypresses. They were instructed to use their dominant hand, and to stop if they felt that they could not participate.
Data analysis
In order to calculate binding, each subject’s baseline keypress or tone times were subtracted from the agency blocks, so that each subject’s times were self-corrected. Thus individual differences or general biases in time perception and cross-modal matching against the clock are removed, and do not affect the results. To calculate action binding for each subject, we subtracted the perceived time of keypresses in the BK blocks from the perceived time of keypresses in the AK blocks. To calculate tone binding, the perceived time of the auditory tone in the BT blocks was likewise subtracted from the perceived time of the tone in the AT blocks. Overall binding is defined as the sum of action binding and tone binding multiplied by −1, with the sign change in tone binding required to account for the different directions of the binding effect for tones compared to actions. Below is an example of these calculations.
Baseline keypress (BK) = reported at mean 40 ms after keypress actually occurred, according to computer
Agency keypress (AK) = reported at mean 100 ms after keypress resulting in tone actually occurred
Action binding = AK-BK = 60 ms
Baseline tone (BT) = reported at mean 55 ms after tone actually occurred
Agency tone (AT) = reported at mean 30 ms after tone resulting from keypress actually occurred
Tone binding = AT-BT = −25 ms
Overall binding = action binding + tone binding × −1
Overall binding = 85 ms
Scores were analyzed for normality using Shapiro-Wilks test, and were inspected for outliers (> 3 SD from the mean) which were removed from the dataset. We assessed action binding, tone binding and overall binding using three separate mixed measures 2 × 3 ANOVAs with group (patient, healthy volunteer) as a between-subjects factor and valence (positive, negative, neutral) as a within-subjects factor. To rule out an effect of depressive or anxiety symptoms, BDI and STAI scores were assessed for interaction with factors in the model and included as a covariate in an ANCOVA if there were no interactions. P<0.05 was considered significant.
RESULTS
Twenty CD patients (8 men, 12 women) and age- and gender-matched healthy volunteers were tested. The motor CD patients [age: 46.36 (SD12.42); BDI: 13.0 (SD 7.71); STAI: 39.88 (SD 11.40)] scored higher on depressive and anxiety scales relative to healthy volunteers [age: 46.52 (SD 11.78); BDI: 3.11 (SD 2.91); STAI: 25.83 (SD 29.1)] (age: t=0.04, p=0.97; BDI: t=5.49 p=0.0001; STAI: t=2.01, p=0.05). Patient characteristics are described in Table 1. While several patients had frequent ongoing movements in their dominant hand, the number of trials that had to be excluded due to interruption by involuntary movements was less than 10 in each of these patients. A trial (a single report of BK, BT, AK or AT) was excluded only if the movement caused the patient to look away from the computer screen, as verified by the experimenter, and thus the patient was not able to report a time for that trial.
Table 1.
Patient characteristics.
| Patient number |
Age | Gender | Abnormal movement(s) | Medications | BDI | Duration of movement symptoms (years) |
|---|---|---|---|---|---|---|
| 1 | 57 | F | Tremor and myoclonus of both arms and trunk | None | 15 | 10 |
| 2 | 37 | F | Dystonia of both arms | None | 5 | 5 |
| 3 | 53 | M | Gait disturbance, myoclonus of both arms | None | 9 | 8 |
| 4 | 43 | M | Tremor of both arms | None | 13 | 5 |
| 5 | 66 | M | Myoclonus of both legs, trunk, and neck | None | 3 | 5 |
| 6 | 66 | M | Myoclonus of both arms and trunk | Levetiracetam | 12 | 15 |
| 7 | 51 | M | Blinking, myoclonus of neck and both arms | None | 13 | 17 |
| 8 | 21 | F | Myoclonus of trunk and all limbs | Mirtazapine | 9 | 2 |
| 9 | 34 | F | Myoclonus of trunk and both arms | Cyclobenzaprine | 0 | 2 |
| 10 | 44 | F | Blinking, grimacing, tremor and myoclonus of head, neck, and all limbs | Cyclobenzaprine | 2 | 2 |
| 11 | 51 | F | Gait difficulty, tremor of all limbs | Sertraline | 20 | 5 |
| 12 | 51 | M | Myoclonus of neck and trunk | Duloxetine, methylphenidate, zolpidem, lorazepam | 20 | 22 |
| 13 | 53 | F | Dystonia and tremor of face and neck | Diazepam, methocarbamol | 14 | 17 |
| 14 | 54 | F | Myoclonus of all limbs and trunk | Citalopram and clonazepam | 13 | 7 |
| 15 | 57 | F | Tremor of all limbs and gait difficulty | None | 9 | 1 |
| 16 | 28 | M | Tremor of both arms | Paroxetine and quetiapine | 17 | 10 |
| 17 | 29 | F | Gait disturbance | Clonazepam | 17 | 11 |
| 18 | 41 | F | Dystonia of face and neck | Venlafaxine | 22 | 18 |
| 19 | 45 | F | Dystonia and myoclonus of both arms and trunk | Sertraline, diazepam, clomipramine, trazodone | 32 | 38 |
| 20 | 40 | M | Myoclonus of face | None | 7 | 1 |
Data from three outliers with scores > 3 SD from the mean were removed (Overall binding: Patient 1: Positive=388 ms, Negative=444 ms; Patient 2: Negative=340 ms; Healthy volunteer: Neutral: −121 ms; Negative=−166 ms). All variables had a Shapiro-Wilks score > 0.5 suggesting normal distribution. Binding scores are reported in Table 2.
Table 2.
Binding scores in milliseconds. The action binding (perceived time of key press in AK – BK), tone binding (perceived time of tone in AT – BT) and overall binding (combined action and tone binding) mean scores are reported in milliseconds (SD) for motor conversion disorder (CD) patients and healthy volunteers.
| CD patient | Healthy control | ||
|---|---|---|---|
| No. subjects | 18 | 19 | |
| Action Binding | Neutral | 31 (79) | 36 (56) |
| Positive | 13 (64) | 32 (55) | |
| Negative | 16 (56) | 29 (38) | |
| Tone Binding | Neutral | −68 (74) | −132 (69) |
| Positive | −79 (80) | −130 (60) | |
| Negative | −61 (80) | −128 (73) | |
| Overall binding | Neutral | 100 (113) | 168 (78) |
| Positive | 93 (90) | 161 (69) | |
| Negative | 76 (81) | 158 (63) |
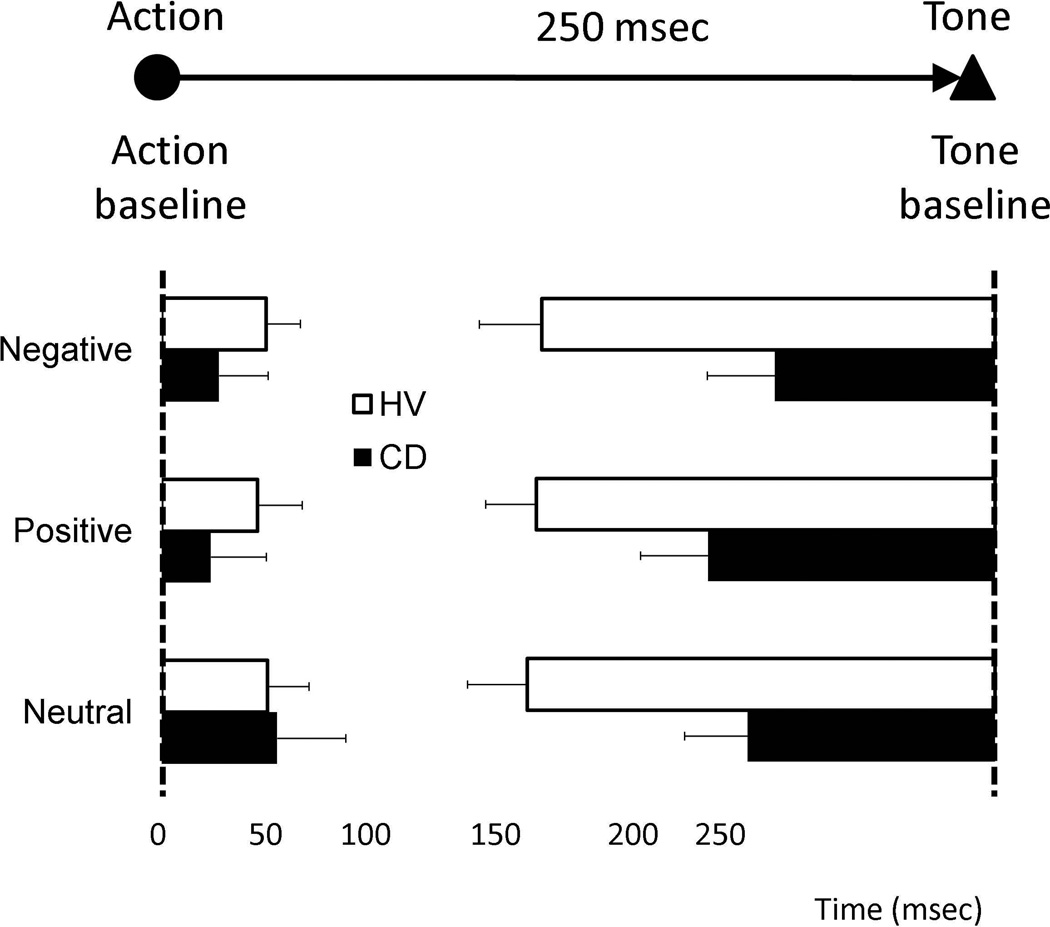
For action binding, there was no main effect of group (F(1,35)=0.59, p=0.45) or of valence (F(2,34)=0.88, p=0.43), and no interaction (F(2,34)=0.26, p=0.78) (Figure 2). For tone binding, there was a main effect of group: patients had less binding than healthy volunteers (F(1,35)=7.48, p=0.01). Tone binding has been shown in previous studies to be more robust and more statistically reliable than action binding; this greater reliability may have allowed us to see differences between the groups more easily.12, 13 There was no effect of valence (F=2,34)=0.66, p=0.53), and no interaction (F(2,34)=0.57, p=0.57). For overall binding, there was again a main effect of group: patients had overall lower binding scores compared to healthy volunteers (mean inter-group difference in overall binding: −73 ms (95%CI: −122 - −23), F(1,35)=8.83, p=0.005). The overall binding data imply that while healthy volunteers perceived the action-effect interval as significantly shorter than it really was, without judging directly the interval duration, the patients did not share this perception. There was no main effect of valence (F(2,35)=0.83, p=0.45) or interaction effect (F(2,35)=0.17, p=0.84). There were no correlations between any binding scores and symptom duration (p>0.05).
Figure 2.
Action-effect binding scores. Action binding and Tone binding for action-effect blocks (AK and AT) for conversion disorder patients (CD) and normal volunteers (NV). Error bars represent Standard Error. Group effect of mixed measures ANOVA: p=0.005
As there was an interaction between group with BDI scores for both tone binding (F(2,34)=4.63, p=0.02), and overall binding (F(2,34)=5.96, p=0.006), BDI was not entered into the ANCOVA as a covariate. The main effects of group for tone binding and overall binding remained significant with STAI scores as a covariate (p=0.01 and p=0.03 respectively).
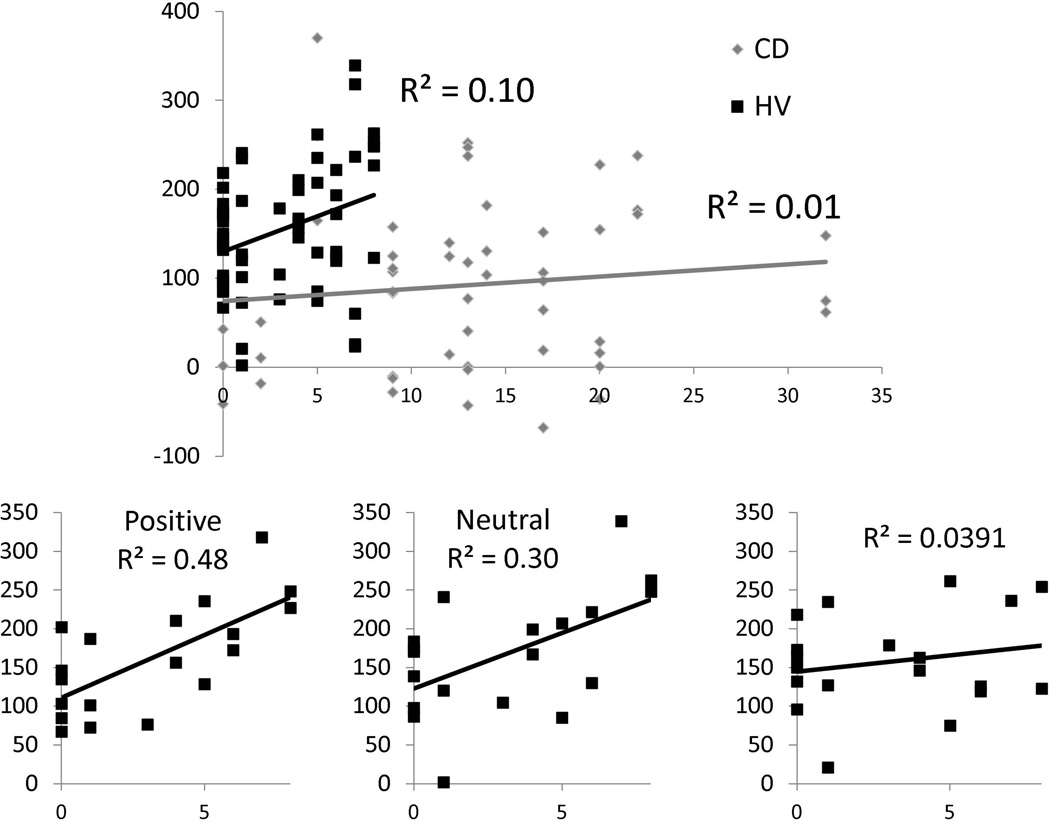
The relationship between binding and BDI scores was further assessed by regression analyses evaluating BDI as an independent variable and overall binding as a dependent variable separately in patients and healthy volunteers. Overall binding was used to correlate with clinical measures because our interest was in the greater linkage between action and effect, rather than either event individually. Higher depression scores were associated with greater overall binding in healthy volunteers (R2=0.11, p=0.01) but not in CD patients (R2=0.01, p=0.4) (Figure 3). To further assess for a relationship between depressive scores and valence, we analyzed overall binding separately in the patients and healthy volunteers using a mixed measures 2 × 3 ANOVA comparing valence (positive, negative, neutral) as a within-subjects factor and BDI as a covariate. There was a significant interaction between valence and BDI in the healthy volunteer group (F(2,16)=4.63, p=0.03) but no significant interactions in the patient group (F(2,16)=0.02, p=0.93). In the healthy volunteer group, regression analyses showed that higher BDI scores were associated with higher binding scores for positive (R2=0.48, p=0.001) and neutral (R2=0.30, p=0.015) but not with negative pictures (R2=0.009, p=0.70) (Bonferroni-corrected p-value = 0.02). There was no association between BDI and binding scores in CD patients (all p>0.05).
Figure 3.
Upper: Regression analyses of depressive symptoms and overall binding. The graph demonstrates regression analyses for the Beck Depression Inventory (BDI) as an independent factor and overall binding as a dependent factor for conversion disorder patients (CD) and healthy volunteers (HV). Lower: Regression analyses of depressive symptoms and overall binding as a function of valence in healthy volunteers. The graphs show regression analyses for BDI as an independent factor and overall binding as a dependent factor as a function of valence in healthy volunteers. The data for CD patients is not shown. The Bonferroni corrected p-value < 0.02 was considered significant.
DISCUSSION
Motor CD is characterized by abnormal movements, reported as outside of the patient’s control. We used an action-effect binding task as an implicit measure of the sense of agency. In CD patients, actions that produced a specific outcome were associated with reduced binding, relative to the binding shown by healthy volunteers. This decrease in an implicit measure of agency may be interpreted as a decreased sense of control over actions in patients with motor CD. This finding reflects other behavioral results in motor CD patients. Even in normal voluntary movements, the sense of volition associated with movement is altered in these individuals.5
Action-effect binding has been applied to schizophrenic patients who, similar to PMD, are felt to suffer from a disordered sense of self-agency. By using a probabilistic component of the task, in which the tone followed only 50% of the keypresses in some trials, and 75% of the keypresses in other trials, this study showed that action-effect binding in schizophrenic patients was more reliant on whether the effect actually occurred.14 In healthy volunteers, when the tone was likely to occur (in the 75% trials) but did not, action-effect binding was still observed, interpreted as reflecting the internal prediction that the tone was likely to occur. Thus schizophrenic patients seemed to be more dependent on external cues in making judgments regarding agency, while healthy volunteers relied on internal predictions when the likelihood of the outcome was high. Current models of how agency becomes disordered in PMD have hypothesized that unlike schizophrenic patients, patients with PMD may be too reliant on internal, pathological predictions regarding movement.15 In comparison to the increased binding seen in schizophrenic patients, our study shows reduced action-effect binding in PMD patients. Our study did not include a probabilistic component in the binding task, and therefore is unable to determine the relative contribution of internal prediction or external cues in these patients.
The pre-SMA is implicated in action-effect binding,8 and the SMA in greater voluntary action-outcome associations.16 Interruption of pre-SMA function using TMS decreases action-effect binding.8 In a previous study comparing conversion movements with voluntary movements, we inferred that motor prediction may be impaired in motor CD.17 In a follow-up study isolating the process of motor preparation, motor CD patients had hypoactivity of the SMA relative to healthy volunteers consistent with impaired binding.16 We have previously shown greater functional connectivity between the amygdala and SMA to arousing stimuli in motor CD patients compared to healthy volunteers suggesting that arousing stimuli may interfere with SMA function.7 However, our current results do not demonstrate an effect of valence on binding, in either healthy volunteers or motor CD patients. This may be related to insufficient conditioning, or to the fact that the same action was randomly and unpredictably associated with different valences, rather than a specific action being associated with a specific outcome valence. Thus, in our study, any effect of valence could only be retrospective, based on inference after the outcome has occurred, and could only be indirect, based on previous associations of outcome stimuli with affects. Designs that more directly assess affective outcomes of action18 or in which valence can be known in advance might be better able to detect any interactions between affect and control in motor CD.
Given the interaction between group and depressive scores, we further evaluated the relationship between depressive scores and binding. In healthy volunteers, greater depressive scores were associated with higher overall binding (greater sense of agency) but no relationship was observed in CD patients. These findings suggest that the higher depressive symptoms seen in CD patients as compared to healthy volunteers are unlikely to be a confounder in our primary findings. In healthy volunteers, the association between greater depressive symptoms and higher binding scores was more prominent for positive and neutral pictures but not negative pictures. This latter finding suggests that the conditioning in this task was effective, although a significant effect of valence on binding was not demonstrated for either healthy volunteers or conversion patients. Without demonstration of whether tone-valence associations were learned, we cannot definitively assess whether this differed between groups.
This paradigm has consistently shown reduced binding when subjects do not judge themselves to be the agent driving the effect. Still, the perceptual framework surrounding agency is difficult to quantify in isolation, and it is possible that action-effect binding reflects cognitive processes other than the sense of agency, or that the appearance of binding occurs spuriously. CD patients may have reported the tone closer to its actual time due to a greater amount of attention focused on outcomes or as a consequence of living with involuntary movement. While the difficulty associated with making accurate keypresses in the context of an ongoing involuntary movement disorder also could be a potential confound, very few trials were actually affected by involuntary movement and had to be excluded, likely due to the distractibility often demonstrated in CD patients. Finally, although not powered to determine the effects of any medication on action-effect binding, there may have been an effect from any of the patients’ medications or from other factors not evaluated in this study such prior exposure to drugs or previous experience with psycho- or physiotherapy.
It would be of interest to compare CD patients with different types of abnormal movements, or those with hyperkinetic motor conversion to those with conversion paralysis. Patients with different types of movements were grouped together in this study because our hypothesis was that regardless of the type of involuntary movement, these patients experience a movement without an associated sense of agency. Action-effect binding has been demonstrated to occur for volitional movements but not in involuntary or passively made movements,12, 19, 20 and thus regardless of the type of abnormal movement in PMD patients, we hypothesized that they would have reduced action-effect binding. That a group effect in overall binding could be demonstrated despite such clinical heterogeneity in terms of the patients’ movements implies that the defining characteristic in motor CD is not the type of movement, but rather a reduced or altered sense of agency for movement.
Using an implicit measure of agency, we show that motor CD patients have decreased action-effect binding when making normal voluntary movements compared to healthy volunteers. Our findings are therefore consistent with motor CD patients’ reported subjective experience of being unable to control their motor symptoms.2 The implication of these results is that there is a pervasive difference in the generation of movement in these patients such that the sense of agency is disrupted. We believe this is the first quantitative, behavioral study of sense of agency in this group.
Acknowledgments
Funding sources for study: This study was supported by the National Institute of Neurological Disorders and Stroke Intramural Program. Dr. Voon is a Wellcome Trust Intermediate Clinical Fellow.
Footnotes
Author Roles:
Dr. Kranick contributed to conception, organization and execution of the research project, design of statistical analysis, and drafting of manuscript.
Dr. Moore contributed to conception, organization, and execution of the research project, design and execution of statistical analysis, review and critique of the manuscript.
Ms. Yusuf contributed to conception, organization, and execution of the research project.
Ms. Martinez contributed to execution of the research project.
Dr. LaFaver contributed to execution of the research project.
Dr. Edwards contributed to execution of the research project.
Dr. Mehta contributed to execution of the research project.
Ms. Collins contributed to execution of the research project.
Dr. Harrison contributed to review of the manuscript.
Dr. Haggard contributed to conception of the research project and review of the manuscript.
Dr. Hallett contributed to conception and organization of the research project and review and critique of the manuscript.
Dr. Voon contributed to conception, organization, and execution of the research project, design and execution of the statistical analysis, and drafting and review of the manuscript.
Full Financial Disclosures of All Authors for the Past Year
Dr. Kranick reports no disclosures.
Dr. Moore reports no disclosures.
Ms. Yusuf reports no disclosures.
Ms. Martinez reports no disclosures.
Dr. LaFaver reports no disclosures.
Dr. Edwards reports no disclosures.
Dr. Mehta reports no disclosures.
Ms. Collins reports no disclosures.
Dr. Harrison reports no disclosures.
Dr. Haggard reports no disclosures.
Dr. Hallett serves as Chair of the Medical Advisory Board for and receives honoraria and funding for travel from the Neurotoxin Institute. He may accrue revenue on US Patent #6,780,413 B2 (Issued: August 24, 2004): Immunotoxin (MAB-Ricin) for the treatment of focal movement disorders, and US Patent #7,407,478 (Issued: August 5, 2008): Coil for Magnetic Stimulation and methods for using the same (H-coil); in relation to the latter, he has received license fee payments from the NIH (from Brainsway) for licensing of this patent. He receives royalties from publishing from Blackwell Publisher, Cambridge University Press, Springer Verlag, Taylor & Francis Group, Oxford University Press, John Wiley & Sons, Massachusetts Medical Society, Wolters Kluwer, and Elsevier. He has received honoraria for lecturing from Columbia University and the Parkinson and Aging Research Foundation. Dr. Hallett's research at the NIH is largely supported by the NIH Intramural Program. Supplemental research funds came from the US Army via the Henry Jackson Foundation, Ariston Pharmaceutical Company via a Cooperative Research and Development Agreement (CRADA) with NIH, and the Kinetics Foundation and BCN Peptides, S.A., via Clinical Trials Agreements (CTA) with NIH.
Dr. Voon serves on the editorial board of Movement Disorders.
References
- 1.American Psychiatric Association Task Force on DSM-IV. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 2.Hallett M. Physiology of psychogenic movement disorders. J Clin Neurosci. 2010;17:959–965. doi: 10.1016/j.jocn.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haggard P, Clark S, Kalogeras J. Voluntary action and conscious awareness. Nat Neurosci. 2002;5:382–385. doi: 10.1038/nn827. [DOI] [PubMed] [Google Scholar]
- 4.Libet B, Gleason CA, Wright EW, Pearl DK. Time of conscious intention to act in relation to onset of cerebral activity (readiness-potential). The unconscious initiation of a freely voluntary act. Brain. 1983;106:623–642. doi: 10.1093/brain/106.3.623. [DOI] [PubMed] [Google Scholar]
- 5.Edwards MJ, Moretto G, Schwingenschuh P, Katschnig P, Bhatia KP, Haggard P. Abnormal sense of intention preceding voluntary movement in patients with psychogenic tremor. Neuropsychologia. 2011;49:2791–2793. doi: 10.1016/j.neuropsychologia.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 6.Wilke C, Synofzik M, Lindner A. The valence of action outcomes modulates the perception of one's actions. Conscious Cogn. 2012;21:18–29. doi: 10.1016/j.concog.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Voon V, Brezing C, Gallea C, et al. Emotional stimuli and motor conversion disorder. Brain. 2010;133:1526–1536. doi: 10.1093/brain/awq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore JW, Ruge D, Wenke D, Rothwell J, Haggard P. Disrupting the experience of control in the human brain: pre-supplementary motor area contributes to the sense of agency. Proc Biol Sci. 2010;277:2503–2509. doi: 10.1098/rspb.2010.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fahn S, Williams DT. Psychogenic dystonia. Adv Neurol. 1988;50:431–455. [PubMed] [Google Scholar]
- 10.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 11.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 12.Haggard P, Clark S, Kalogeras J. Voluntary action and conscious awareness. Nature Neuroscience. 2002;5:382–385. doi: 10.1038/nn827. [DOI] [PubMed] [Google Scholar]
- 13.Moore JW, Wegner DM, Haggard P. Modulating the sense of agency with external cues. Consciousness and Cognition. 2009;18:1056–1064. doi: 10.1016/j.concog.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Voss M, Moore J, Hauser M, Gallinat J, Heinz A, Haggard P. Altered awareness of action in schizophrenia: a specific deficit in predicting action consequences. Brain. 2010;133:3104–3112. doi: 10.1093/brain/awq152. [DOI] [PubMed] [Google Scholar]
- 15.Edwards MJ, Adams RA, Brown H, Parees I, Friston KJ. A Bayesian account of 'hysteria'. Brain. 2012 doi: 10.1093/brain/aws129. published online May 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voon V, Brezing C, Gallea C, Hallett M. Aberrant supplementary motor complex and limbic activity during motor preparation in motor conversion disorder. Mov Disord. 2011;26:2396–2403. doi: 10.1002/mds.23890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voon V, Gallea C, Hattori N, Bruno M, Ekanayake V, Hallett M. The involuntary nature of conversion disorder. Neurology. 2010;74:223–228. doi: 10.1212/WNL.0b013e3181ca00e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moretto G, Walsh E, Haggard P. Experience of agency and sense of responsibility. Conscious Cogn. 2011;20:1847–1854. doi: 10.1016/j.concog.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 19.Haggard P, Clark S. Intentional action: conscious experience and neural prediction. Conscious Cogn. 2003;12:695–707. doi: 10.1016/s1053-8100(03)00052-7. [DOI] [PubMed] [Google Scholar]
- 20.Tsakiris M, Haggard P. Awareness of somatic events associated with a voluntary action. Exp Brain Res. 2003;149:439–446. doi: 10.1007/s00221-003-1386-8. [DOI] [PubMed] [Google Scholar]