Abstract
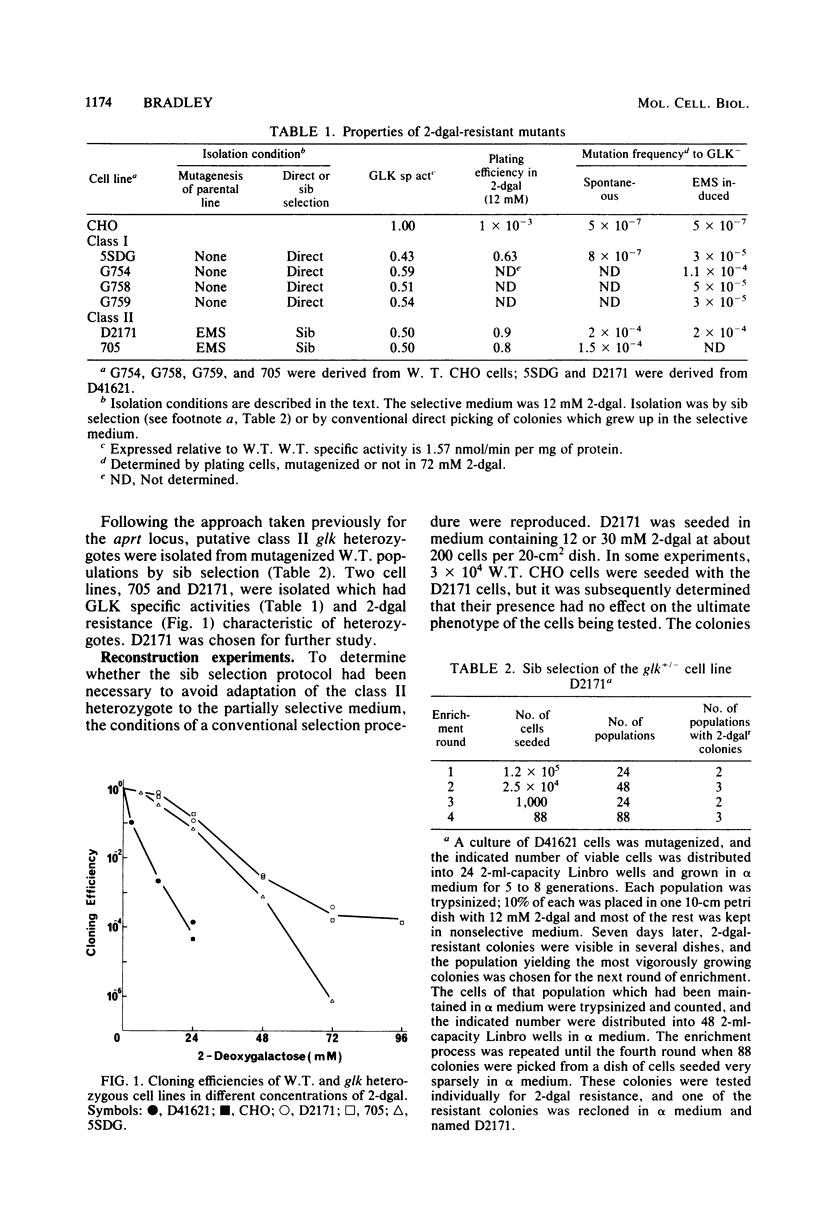
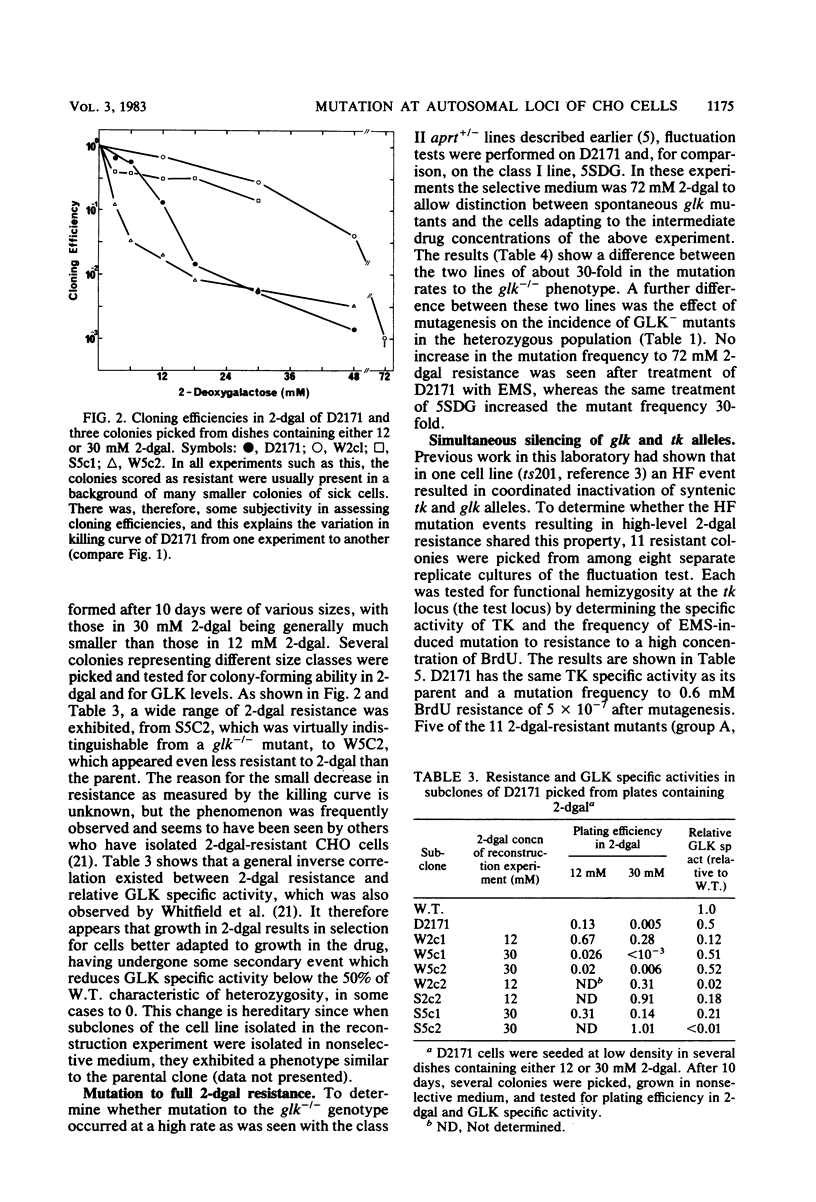
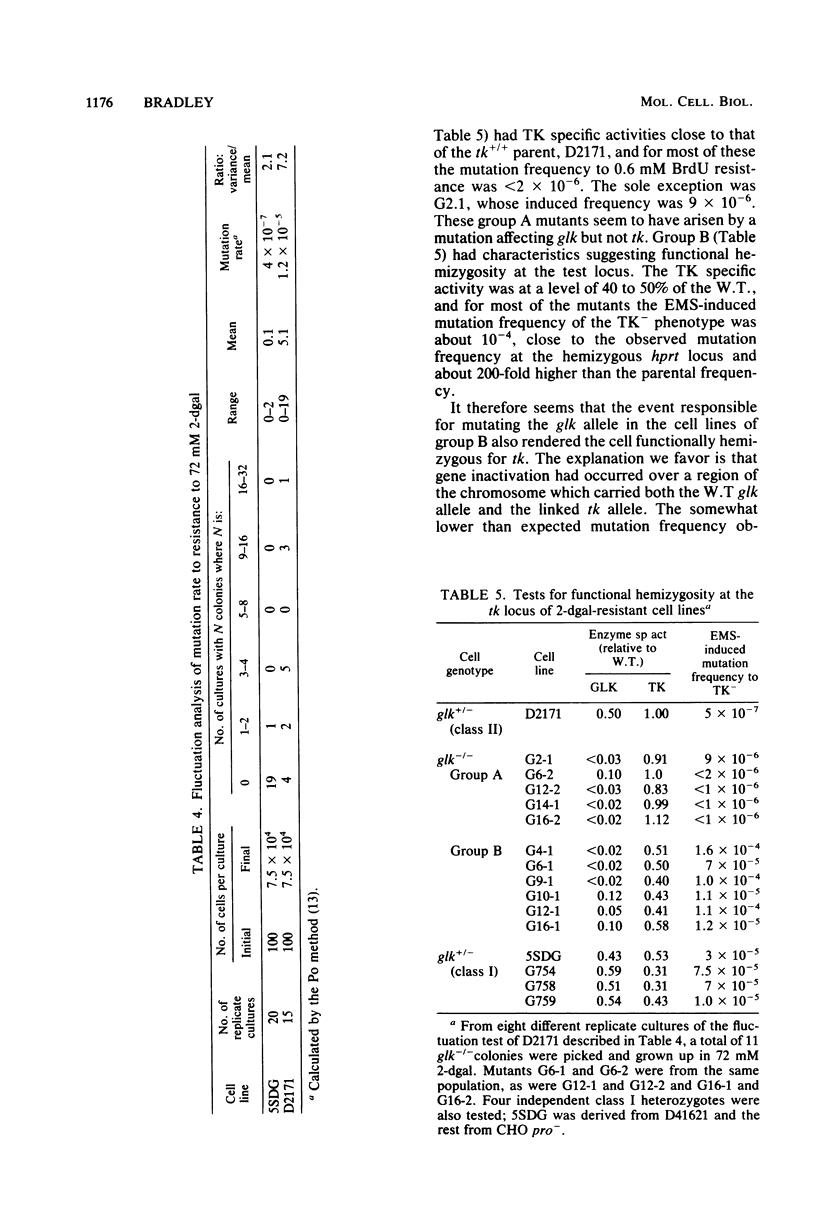
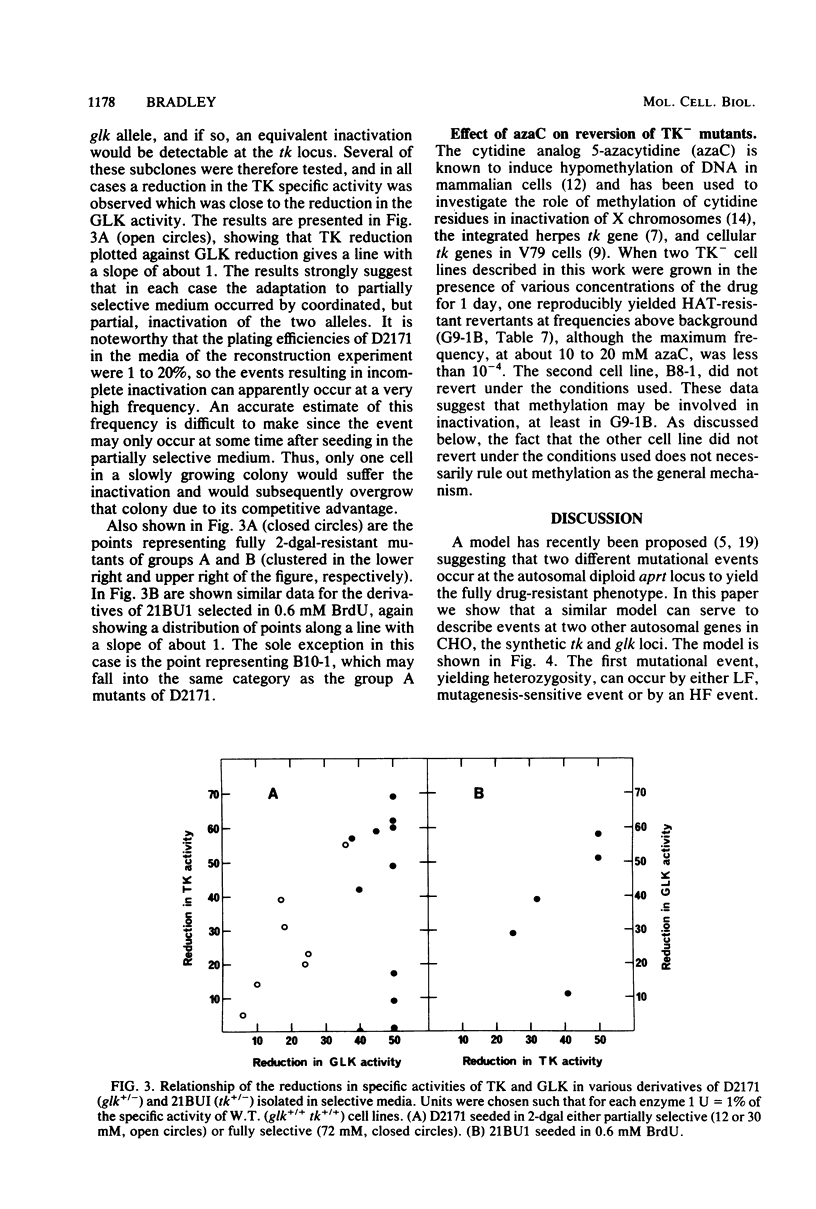
Two classes of cell lines heterozygous at the galactokinase (glk) locus have been isolated from Chinese hamster ovary cells. Class I, selected by plating nonmutagenized wild-type cells at low density in medium containing 2-deoxygalactose at a partially selective concentration, underwent subsequent mutation to the glk-/- genotype at a low frequency (approximately 10(-6) per cell), which was increased by mutagenesis. Class II heterozygotes, isolated by sib selection from mutagenized wild-type cells, had a higher spontaneous frequency of mutation to the homozygous state (approximately 10(-4) per cell), which was not affected by mutagenesis. About half of the glk-/- mutants derived from a class II heterozygote, but not the heterozygote itself, were functionally hemizygous at the syntenic thymidine kinase (tk) locus. Similarly, a tk+/- heterozygote with characteristics analogous to the class II glk+/- cell lines underwent high-frequency mutation to tk-/-, and most of these mutants, but not the tk+/- heterozygote, were functionally hemizygous at the glk locus. A model is proposed, similar to that for the mutational events at the adenine phosphoribosyl transferase locus (W. E. C. Bradley and D. Letovanec, Somatic Cell Genet. 8:51-66, 1982), of two different events, high and low frequency, being responsible for mutation at either of the linked loci tk and glk. The low-frequency event may be a point mutation, but the high-frequency event, in many instances, involves coordinated inactivation of a portion of a chromosome carrying the two linked alleles. Class II heterozygotes would be generated as a result of a low-frequency event at one allele, and class I heterozygotes would be generated by a high-frequency event. Supporting this model was the demonstration that all class I glk+/- lines examined were functionally hemizygous at tk.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adair G. M., Carver J. H., Wandres D. L. Mutagenicity testing in mammalian cells. I. Derivation of a Chinese hamster ovary cell line heterozygous for the adenine phosphoribosyltransferase and thymidine kinase loci. Mutat Res. 1980 Sep;72(2):187–205. doi: 10.1016/0027-5107(80)90035-4. [DOI] [PubMed] [Google Scholar]
- Bradley W. E., Dinelle C., Charron J., Langelier Y. Bromodeoxyuridine resistance in CHO cells occurs in three discrete steps. Somatic Cell Genet. 1982 Mar;8(2):207–222. doi: 10.1007/BF01538678. [DOI] [PubMed] [Google Scholar]
- Bradley W. E., Letovanec D. High-frequency nonrandom mutational event at the adenine phosphoribosyltransferase (aprt) locus of sib-selected CHO variants heterozygous for aprt. Somatic Cell Genet. 1982 Jan;8(1):51–66. doi: 10.1007/BF01538650. [DOI] [PubMed] [Google Scholar]
- Bradley W. E. Reversible inactivation of autosomal alleles in Chinese hamster cells. J Cell Physiol. 1979 Nov;101(2):325–340. doi: 10.1002/jcp.1041010212. [DOI] [PubMed] [Google Scholar]
- Campbell C. E., Worton R. G. Segregation of recessive phenotypes in somatic cell hybrids: role of mitotic recombination, gene inactivation, and chromosome nondisjunction. Mol Cell Biol. 1981 Apr;1(4):336–346. doi: 10.1128/mcb.1.4.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough D. W., Kunkel L. M., Davidson R. L. 5-Azacytidine-induced reactivation of a herpes simplex thymidine kinase gene. Science. 1982 Apr 2;216(4541):70–73. doi: 10.1126/science.6175023. [DOI] [PubMed] [Google Scholar]
- Eves E. M., Farber R. A. Chromosome segregation is frequently associated with the expression of recessive mutations in mouse cells. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1768–1772. doi: 10.1073/pnas.78.3.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M., Collier K. Phenotypic evolution of cells resistant to bromodeoxyuridine. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4206–4210. doi: 10.1073/pnas.77.7.4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M. Induction of thymidine kinase in enzyme-deficient Chinese hamster cells. Cell. 1982 Jun;29(2):483–492. doi: 10.1016/0092-8674(82)90165-9. [DOI] [PubMed] [Google Scholar]
- Jones G. E., Sargent P. A. Mutants of cultured chinese hamster cells deficient in adenine phosphoribosyl transferase. Cell. 1974 May;2(1):43–54. doi: 10.1016/0092-8674(74)90007-5. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980 May;20(1):85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- Mohandas T., Sparkes R. S., Shapiro L. J. Reactivation of an inactive human X chromosome: evidence for X inactivation by DNA methylation. Science. 1981 Jan 23;211(4480):393–396. doi: 10.1126/science.6164095. [DOI] [PubMed] [Google Scholar]
- Roberts J. M., Axel R. Gene amplification and gene correction in somatic cells. Cell. 1982 May;29(1):109–119. doi: 10.1016/0092-8674(82)90095-2. [DOI] [PubMed] [Google Scholar]
- Rosenstraus M., Chasin L. A. Isolation of mammalian cell mutants deficient in glucose-6-phosphate dehydrogenase activity: linkage to hypoxanthine phosphoribosyl transferase. Proc Natl Acad Sci U S A. 1975 Feb;72(2):493–497. doi: 10.1073/pnas.72.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddle F. H. A new era in mammalian gene mapping: somatic cell genetics and recombinant DNA methodologies. Nature. 1981 Nov 12;294(5837):115–120. doi: 10.1038/294115a0. [DOI] [PubMed] [Google Scholar]
- Siminovitch L. On the nature of hereditable variation in cultured somatic cells. Cell. 1976 Jan;7(1):1–11. doi: 10.1016/0092-8674(76)90249-x. [DOI] [PubMed] [Google Scholar]
- Simon A. E., Taylor M. W., Bradley W. E., Thompson L. H. Model involving gene inactivation in the generation of autosomal recessive mutants in mammalian cells in culture. Mol Cell Biol. 1982 Sep;2(9):1126–1133. doi: 10.1128/mcb.2.9.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon A. E., Taylor M. W. High-frequency mutation at the adenine phosphoribosyltransferase locus in Chinese hamster ovary cells due to deletion of the gene. Proc Natl Acad Sci U S A. 1983 Feb;80(3):810–814. doi: 10.1073/pnas.80.3.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. H., Fong S., Brookman K. Validation of conditions for efficient detection of HPRT and APRT mutations in suspension-cultured Chinese hamster ovary cells. Mutat Res. 1980 Feb;74(1):21–36. doi: 10.1016/0165-1161(80)90188-0. [DOI] [PubMed] [Google Scholar]
- Whitfield C. D., Buchsbaum B., Bostedor R., Chu E. H. Inverse relationship between galactokinase activity and 2-deoxygalactose resistance in Chinese hamster ovary cells. Somatic Cell Genet. 1978 Nov;4(6):699–713. doi: 10.1007/BF01543159. [DOI] [PubMed] [Google Scholar]
- Wright J. A., Lewis W. H., Parfett C. L. Somatic cell genetics: a review of drug resistance, lectin resistance and gene transfer in mammalian cells in culture. Can J Genet Cytol. 1980;22(4):443–496. doi: 10.1139/g80-056. [DOI] [PubMed] [Google Scholar]


