Abstract
Floral nectar is considered the most important reward animal-pollinated plants offer to attract pollinators. Here we explore whether honeybees, which act as pollinators, affect the composition of bacterial communities in the nectar. Nectar and honeybees were sampled from two plant species: Amygdalus communis and Citrus paradisi. To prevent the contact of nectar with pollinators, C. paradisi flowers were covered with net bags before blooming (covered flowers). Comparative analysis of bacterial communities in the nectar and on the honeybees was performed by the 454-pyrosequencing technique. No significant differences were found among bacterial communities in honeybees captured on the two different plant species. This resemblance may be due to the presence of dominant bacterial OTUs, closely related to the Arsenophonus genus. The bacterial communities of the nectar from the covered and uncovered C. paradisi flowers differed significantly; the bacterial communities on the honeybees differed significantly from those in the covered flowers’ nectar, but not from those in the uncovered flowers’ nectar. We conclude that the honeybees may introduce bacteria into the nectar and/or may be contaminated by bacteria introduced into the nectar by other sources such as other pollinators and nectar thieves.
Introduction
High sugar concentration which generates high osmotic pressure [1], [2] and a nectar-related protein [3], [4] have been suggested as limiting factors for microbial growth in floral nectar. Despite these potentially restrictive factors, various microorganisms inhabit floral nectar: filamentous fungi, true yeasts, and yeast-like fungi [5]–[8]. Gilliam et al. [9] examined the floral nectar of three different plant species and found that out of 23 samples of Citrus nectar only three contained some gram-negative unidentified bacteria. They were unable to isolate bacteria from cotton (Gossypium spp.) and prickly pear cactus (Opuntia sp.) nectar flowers.
Recently we demonstrated for the first time that bacterial communities in nectar are abundant and diverse, and display significant variation among three plant species: Amygdalus communis, Citrus paradisi and Nicotiana glauca [10]. Bacterial counts (by DAPI staining) in the floral nectar of these plants were about 104–105 bacteria per one flower [10]. Following our study, Alvarez-Perez et al. [11] showed that bacteria are common inhabitants of the floral nectar of South African wild plants. Alvarez-Perez and Herrera [12] demonstrated that culturable communities of nectar microorganisms associated with wild Mediterranean plants from Southern Spain, are nonrandom assemblages of bacterial and yeast species.
Although many biologists have embraced microbial model systems as tools to address genetic and physiological questions, the explicit use of microbial communities as model systems in ecology has traditionally been more restricted [13]. Microorganisms represent the “unseen majority” of species, individuals and biomass in many ecosystems, hence may also play key roles in community and ecosystem function [14], [15]. As micro- and macro-organisms seem to follow the same general ecological laws and patterns, microbial model system studies could greatly impact our understanding of the ecology of plants and their pollinators. For example, microbial communities in nectar may affect the nectar’s chemical profile, thus directly controlling nectar consumption by flower visitors such as pollinators and nectar thieves, and thereby indirectly governing plant fitness [2], [8], [16], [17]. Brysch-Herzberg [1] examined yeast communities associated with nectar and flower-visiting insects. He concluded that yeasts may use flowers for mass reproduction and as a platform from which they will meet their next host insect for dispersal.
More than 200 plant species of the European flora are known to be visited by honeybees (Apis mellifera L.) [18]. The individual bee, however, tends to stick to one kind of flower over a certain period of time, and ingests nectar of this flower species only [19], [20]. Free [21], for example, found that 94% of all pollen foragers collected one pollen type during a foraging trip. Nectar foragers quickly learn flower attributes such as color, shape and odor, and use this information to land selectively on particular flowers [20].
Microbial communities in honeybee intestines have been studied intensively by cultivation-dependent techniques [22], [23] and molecular methods [24]–[27], yet as far as we know bacterial community composition (BCC) obtained from the honeybee surface and mouthparts has not been described. The bee uses its long proboscis to collect the nectar from floral nectaries. While feeding, the external surface of the bee’s other front organs may come into contact with the nectar. Thus, its surface bacteria, including those on its mouthparts, might play a role in the mutual relation of flower and bee.
The goal of our study was to understand the role of bees in shaping the BCC in floral nectar. Honeybee pollinators may transfer bacteria into and out of the nectar, thus manipulating the floral BCC. To better understand the relation of the BCCs on honeybees and in nectar, we studied two plant species in northern Israel (Amygdalus communis and Citrus paradisi), which are pollinated by honeybees (Apis mellifera), by both cultural and non-cultural methods (454-pyrosequencing). First we compared the BCC in the nectar of uncovered and covered C. paradisi flowers; next we compared the BCC on the bees captured on the two different plant species with the BCC of the floral nectar of the plant from which they were captured.
Methods
Study Site and Sample Collection
Floral nectar and honeybees were collected from two plant species: Citrus paradisi (grapefruit) and Amygdalus communis (almond), sampled respectively in January-February and March-April 2010. Sampling ranged across an area 20 km in diameter in open areas in northern Israel (around Oranim College, Tivon). We chose these two plant species because both are pollinated by the same honeybee species (A. mellifera). To prevent the contact of nectar with pollinators some flower buds of C. paradisi were entirely covered with net bags (45×30 cm nylon fly net) for a few days before blooming (covered flowers). Each plant was sampled on a different day. The nectar was collected from about 100 flowers of each sampled plant (five different A. communis plants and three different C. paradisi plants). Nectar collection from the covered and uncovered flowers was carried out simultaneously. About 700 µl nectar were collected from flowers of each individual plant with sterile tips under sterile conditions to avoid contamination. Three to four honeybees were captured from each sampled plant while they were visiting the flowers, and were transferred immediately to a sterile 50 ml falcon tube. Sterile saline water (15 ml; 0.85% NaCl), supplemented with 1% Glycerol and 1% Tween 80, was added and the tubes were sonicated for 4 min at 25°C in an ultrasonic cleaning bath (40 kHz; Bransonic 32, MRC, Israel) to dislodge bacteria from the bee surfaces. The resulting suspension was used to culture bacteria and extract DNA (as described below). In some cases, when the bees were transferred to the tube their mouthparts were accidentally pulled off, including the mandibles and the proboscis (tongue), and thus were exposed to the dislodging processes as well (as the bee’s mouthparts were not pulled off deliberately it is unlikely that the gut content contaminated the sample). In sum, five different A. communis plants were sampled (nectar was sampled from uncovered flowers and honeybees were captured on each plant) and three different C. paradisi plants (nectar was sampled from covered and uncovered flowers and honeybees were captured on each plant). The nectar samples from the uncovered flowers and from their honeybees were cultured and the 454-pyrosequencing technique was executed (details are given below). Nectar samples from covered flowers of C. paradisi plants were analyzed only by the 454-pyrosequencing technique.
DNA Extraction
The bacterial suspension resulting from bee sonication was centrifuged at 8,000 g for 10 min, and re-suspended in 200 µl saline. DNA was extracted from this saline solution (200 µl) and from nectar (100 µl). A DNA isolation kit (DNeasy Blood and Tissue, Qiagene, Germany) was used for DNA extraction, according to the manufacturer’s instructions.
Isolation and Identification of Culturable Bacteria from Nectar and Bees
Bee samples (after sonication) and nectar samples from uncovered flowers were serially diluted, and 0.1 ml aliquots were spread onto R2A agar (Himedia) and R2A agar supplemented with 20% sucrose. The plates were incubated under aerobic conditions at 30°C for 48 h, and then colony-forming units (cfu) were counted in the nectar samples from the uncovered flowers.
Individual colonies from the agar plates were randomly selected according to different morphologies, and streaked again on the appropriate agar plate from which the colony had been sampled to yield single colonies. The isolated colonies were sub-cultured at least five times before identification. Universal bacterial primers 11F and 1512R were used to amplify internal fragments of 16S rRNA genes, according to Felske et al. [28]. The procedure of the 16S rRNA gene amplification was performed in accordance with Izhaki et al. [29]. The amplified PCR products (approximately 1,500 bp) were sequenced in MCLAB laboratories (California, USA). The sequences length were at least 850 bp or more. For the identification of closest relatives, newly determined sequences were compared with those available in the EZtaxon software, version 2.1 (http://www.eztaxon.org; [30]).
Nucleotide Sequence Accession Numbers
The sequences from this study (cultured isolates) were deposited in the GenBank database under these accession numbers: JQ638273-JQ638275, JQ638280-JQ638283, JQ638299-JQ638320, JQ638324-JQ638329, JQ638331-JQ638336, JQ638339-JQ638343, JQ638347-JQ638352, JQ638355 and JQ638357-JQ638360.
454-pyrosequencing of 16S rRNA Genes
Bacterial diversity in nectar from covered and uncovered flowers (uncovered flowers were sampled only from C. paradisi) and on bees was analyzed by means of bacterial tag-encoded FLX 454-pyrosequencing (bTEFAP) [31], [32] of 16S rRNA genes (ten samples for A. communis and nine samples for C. paradisi). This technique was performed by the Research and Testing Laboratory (Lubbock, TX), based on RTL protocols (www.researchandtesting.com). Amplicon lengths were 200–450 bp from the 27F region numbered in relation to the E. coli rRNA gene (GGCGVACGGGTGAGTAA) (see Table S1 for full primers and barcode data). A total of ca. 10,000 sequences per sample was obtained.
Sequence Analysis
The data derived from the sequencing was processed using MOTHUR program (version 1.17.3) [33]. We used the trim.seqs script to remove the forward primer and the barcodes. We removed short sequences (<200 bp), sequences with ambiguous base calls, and sequences with homopolymer runs exceeding 6 bp. The raw data is available at the following website: https://sites.google.com/site/articalrawdata.
Sequences were aligned in MOTHUR against the Ribosomal Database Project (RDP) classifier (http://pyro.cme.msu.edu). We removed the taxon “Cyanobacteria” from the FASTA file using the remove.lineage script. After omitting chimeras and poor quality sequences, we compared the membership and structure of the 19 communities using an OTU-based approach. We generated a distance matrix (using the dist.seqs script which calculates uncorrected pairwise distances between aligned DNA sequences). Next we assigned our sequences to OTUs using the cluster script with the average neighbor algorithm. The cutoff level was 0.03.
This distance matrix was clustered into OTUs, compiling the OTUs to the most relevant taxonomic level based on percent identity (>97% species, 90%–97% genus, 85%–90% family, 80%–85% order, 77–80% phylum,<77% unclassified). Principal Coordinate Analysis (PCoA) and AMOVA analyses were performed with this distance matrix.
Rarefaction curves (as a measure of diversity) describing the number of OTUs observed as a function of sampling effort were calculated at 3% sequence divergence (using rarefaction.single script). We used the summary.single script to calculate the sample coverage and richness estimators. Richness is expressed as the number of observed OTUs. Richness was also estimated by the abundance-based coverage estimator (ACE), which is a nonparametric richness estimator based on distribution of abundant (>10) and rare (≤10) OTUs, and the richness estimator Chao1, which is a nonparametric richness estimator based on distribution of singletons and doubletons.
Results
Culturable Bacterial Community Composition in Nectar and on Bees
Bacterial colony-forming units (cfu) per ml flower nectar that were collected from two plant species A. communis and C. paradisi were 1.5 ×107 (±1.3×107) and 9.2 ×106 (±6.9×106) respectively. Representative bacterial isolates were identified from both nectar and body surface, including the mouthparts of the honeybees captured from the two studied plant species (Table 1). The bacterial colonies were selected for the purpose of locating the variety of different culturable bacterial species; therefore colonies of different appearance were chosen and identified. Bacterial species belonging to the Gammaproteobacteria, Actinobacteria and Bacilli classes were identified in the floral nectar. Species from these bacterial classes, and also from Alphaproteobacteria, were identified on bee surfaces and mouthparts (Table 1). One of the most abundant bacterial species in almost all the samples (except bees from C. paradisi) was a novel, unidentified Enterobateriaceae species, closely related to Phaseolibacter flectens [34] (Table 1).
Table 1. List of bacterial isolates from nectar and bees of Amygdalus communis and Citrus paradisi.
| Citrus paradisi | Amygdalus communis | ||||
| Class | Closest relative in GenBank database | Nectar | Bees | Bees | Nectar |
| Alpha-proteobacteria | Methylobacterium rhodesianum | 1 (99.8) | |||
| Methylobacterium populi | 1 (100) | ||||
| Gamma-proteobacteria | Acinetobacter lwoffii | 1 (95.9) | |||
| Erwinia amylovora | 1 (96.8) | ||||
| Klebsiella oxytoca | 1 (98.1) | ||||
| Pantoea eucrina | 1 (98.6) | ||||
| Pantoea septica | 2(98.7–99.1) | ||||
| Phaseolibacter flectens | 2 (96.6–96.7) | 5 (95.6–96.6) | 7 (95.9–96.8) | ||
| Actinobacteria | Microbacterium foliorum | 3 (98.9–99.1) | |||
| Micrococcus yunnanensis | 2 (99.8–99.9) | ||||
| Bacilli | Bacillus aryabhattai | 1 (100) | |||
| Bacillus cereus | 3 (99.5–100) | ||||
| Bacillus megaterium | 1 (99.7) | ||||
| Bacillus niabensis | 1 (96.8) | 1 (98.6) | |||
| Bacillus oceanisediminis | 1 (99.3) | ||||
| Bacillus safensis | 1 (100) | 1 (100) | |||
| Bacillus tequilensis | 1 (99.8) | ||||
| Lactobacillus kunkeei | 3 (100) | 6 (100) | |||
| Rummeliibacillus stabekisii | 1 (99.7) | ||||
| Staphylococcus epidermidis | 2 (100) | 1 (100) | |||
| Staphylococcus haemolyticus | 1 (100) | 1 (100) | |||
| Staphylococcus pasteuri | 3 (99.8–99.9) | ||||
| Terribacillus goriensis | 1 (100) | ||||
| Terribacillus saccharophilus | 1 (99.3) | ||||
The number before the parentheses indicates the number of isolates, the number within the parentheses indicates the percentage of the 16S rRNA gene similarities to the closest known species. Isolates with less than 97.5% 16S rRNA gene similarities to known species are most likely novel species and the name of their closest relative species is marked in bold. The isolates were identified by comparing their 16S rRNA gene sequences to that of the GenBank database (EZtaxon software version 2.0.http://147.47.212.35∶8080). The sequences were deposited in the GenBank database under the following accession numbers: JQ638273-JQ638275, JQ638280-JQ638283, JQ638299-JQ638320, JQ638324-JQ638329, JQ638331-JQ638336, JQ638339-JQ638343, JQ638347-JQ638352, JQ638355 and JQ638357-JQ638360.
454-pyrosequencing Analysis of Bacterial Community Composition in Nectar and on Bees
The 454-pyrosequencing analysis was used to characterize bacterial communities in nectar of uncovered flowers, nectar of covered flowers, and honeybee surfaces and mouthparts collected from three C. paradisi plants (nine samples in all, for C. paradisi). Bacterial communities were also characterized in the nectar of uncovered flowers and honeybee surfaces and mouthparts collected from five A. communis plants (ten samples in all for A. communis).
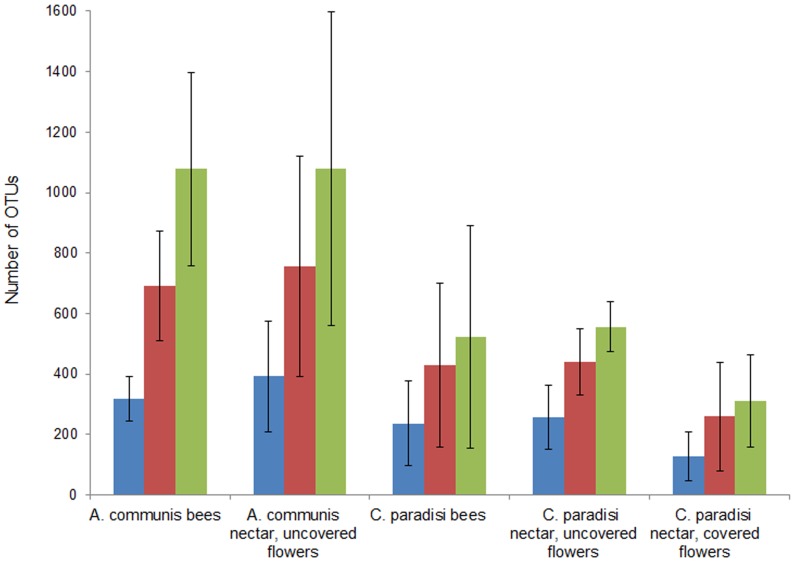
Sequences were assigned to species-level operational taxonomic units (OTUs) by means of a 97% pairwise-identity cutoff. Across all 19 samples we obtained 78,826 quality sequences. These were classified for a total of 3,330 unique bacterial operational taxonomic units (OTUs) at the 97% sequence-similarity level across all samples, with an average of 277 OTUs per sample. Rarefaction curves at 3% sequence divergence were calculated (Figs. S1 and S2). Some rarefaction curves did not saturate, indicating that the surveying effort did not cover the full extent of taxonomic diversity at this genetic distance. Coverage, Chao1 and ACE richness estimator are shown in Table S2. Bacterial species richness in the nectar and on bees from C. paradisi was much lower compared with those from A. communis (Table S2, Fig. 1). Full OTU data with their taxonomic classifications and their abundance within each sample are shown in Table S3.
Figure 1. Bacterial richness estimates of Amygdalus communis and Citrus paradisi representing different management types at a genetic distance of 3%.
Richness is expressed as the number of observed OTUs. In addition, richness was estimated by the abundance-based coverage estimator (ACE), which is a nonparametric richness estimator based on distribution of abundant (>10) and rare (≤10) OTUs, and the richness estimator Chao1, which is a nonparametric richness estimator based on distribution of singletons and doubletons. Richness prediction from ACE is colored green, richness prediction from Chao1 red, and richness observed blue.
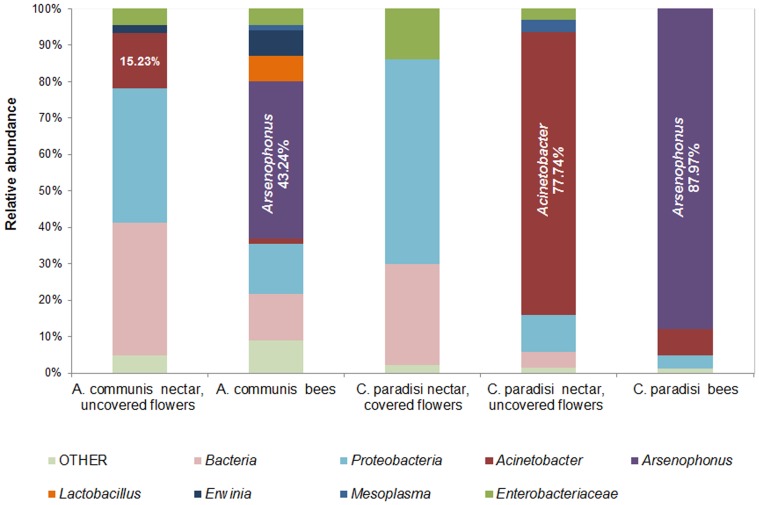
Most sequences from the nectar and bee samples were classified as Gammaproteobacteria (76% in average). The most dominant genus in the bee samples was Arsenophonus, with a frequency of 43% in bees collected from A. communis and of 88% in bees collected from C. paradisi (Fig. 2). Acinetobacter was the prevalent genus in the nectar of uncovered flowers extracted from both tree species, and it dominated the nectar of uncovered flowers of C. paradisi, with a frequency of 78% (its frequency in A. communis was 15%). The relative abundance of the taxonomic groups is shown in Fig. 2.
Figure 2. Relative abundances of bacterial taxonomic groups of all sequences derived from the Amygdalus communis and Citrus paradisi nectar samples and from honeybees captured on the two different plants.
Bacterial diversities of all nectar and bee samples were surveyed by 454-pyrosequencing of 16S rRNA genes. Full OTU data with their taxonomic classifications and their abundance within each sample is available in Table S3.
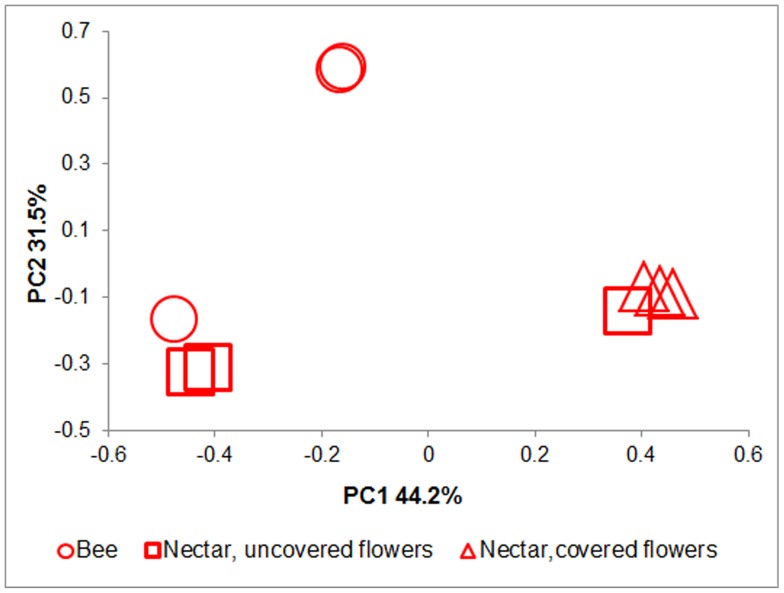
Samples of nectar from covered and uncovered flowers and bees captured from C. paradisi plants were analyzed separately (nine samples in all) and revealed that the BCC found in the nectar of uncovered flowers was similar to that found on the honeybees; the BCC from the covered flowers was significantly different from both BCCs described above (Fig. 3) (AMOVA between the nectar of uncovered and covered flowers F1,4 = 2.69; P<0.01; between covered flowers and bees F1,4 = 4.36; P<0.01; between bees and the nectar of uncovered flowers F1,4 = 1.92; P = 0.08).
Figure 3. Citrus paradisi bacterial diversity clustering by sample source.
No significant differences were found in the BCC between honeybees and nectar from uncovered flowers (F1,4 = 1.92 P = 0.08). The BCC of covered flowers was significantly different from that of uncovered flowers (F1,4 = 2.69; P<0.01) and honeybees (F1,4 = 4.36; P<0.01). Bacterial diversities of all samples were surveyed by 454-pyrosequencing of 16S rRNA genes. The first two principal coordinates (PC1 and PC2) from the principal coordinate analysis of weighted UniFrac are plotted for each sample.
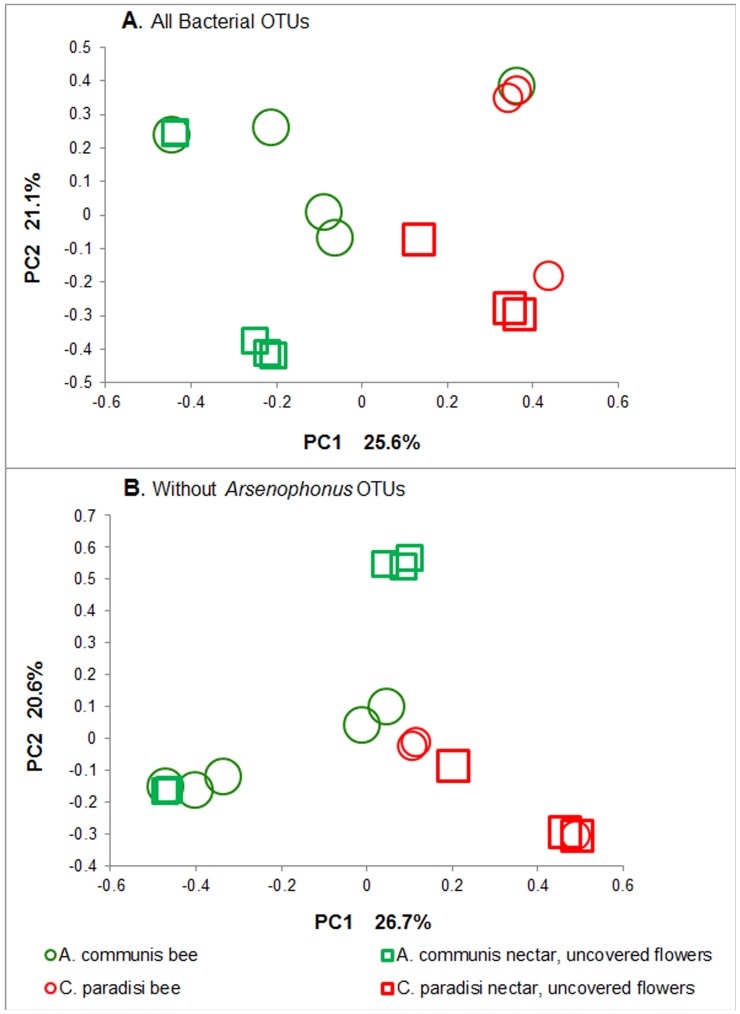
To compare the BCC of the two different plant species and of the bees captured on them, we excluded the covered flower data from the following analyses because they were derived from only one plant species. Principal component analysis (PCA) based on the relative abundance of the different bacterial phyla and classes attested to differences between the BCCs of the uncovered nectars of A. communis and C. paradisi flowers. The differences were found significant by AMOVA (F1,6 = 3.87; P = 0.012) (Fig. 4a). These two BCCs proved similar to those found on the honeybees captured from the respective plant species (supported by AMOVA: F1,8 = 1.61,P = 0.175 for A. communis; F1,4 = 1.93; P = 0.096 for C. paradisi).
Figure 4. Nectar bacterial diversity clustering by plant species and sample source.
(a) Analyses of all bacterial OTUs. (b) Analyses without Arsenophonus OTUs. (a) No significant differences were found in microbial communities among honeybees from the two different plants (F1,6 = 1.38 P = 0.191). However, each bee showed resembance only to the nectar of the plant it was captured on. (b) When data excluding the Arsenophonus OTUs were analyzed, significant differences were found in microbial communities among honeybees from the two different plants (F1,6 = 1.71 P = 0.013). Bacterial diversities of all samples were surveyed by 454-pyrosequencing of 16S rRNA genes. The first two principal coordinates (PC1 and PC2) from the principal coordinate analysis of weighted UniFrac are plotted for each sample. Each symbol represents a sample (circle, bee; square, nectar), colored by plant species (A. communis, green; C. paradisi, red). The variance explained by the PCs is indicated on the axes.
No significant differences were found between the BCCs of honeybees from the two plant species (supported by AMOVA: F1,6 = 1.38 P = 0.191) (Fig. 4a). This similarity may be explained by the dominant presence of bacterial species from the genus Arsenophonus on bees captured on the flowers of the two plant species (Fig. 2). All the Arsenophonus sequences were removed from the main FASTA file, and were aligned in MOTHUR against the SILVA reference alignment [51]. To determine whether the removal of the Arsenophonus sequences affected the sampling depth of the community, we recalculated diversity (coverage) and the richness estimator (ACE) (Table S2). Sample diversity remained unchanged and sample richness was reduced less than 2%. The Arsenophonus sequences were divided into 1,669 different OTUs, of which 1,512 (90.2%) showed more than 98% resemblance to known species (for example: accession numbers DQ508172, DQ517447, DQ517448 and AY264669).
To support the hypothesis that the presence of Arsenophonus was the reason for the similarities of the BCCs in honeybees from the two plant species, we reanalyzed our data after excluding all OTUs representing the genus Arsenophonus. The results shown in Fig. 4b demonstrate that without Arsenophonus, there were significant differences between the BCCs in honeybees from the two plant species (supported by AMOVA: F1,6 = 1.71, P = 0.013) (Fig. 4b). The reanalysis yielded no significant differences between the nectar of uncovered flowers and bees captured from the respective plant species (as was found before exclusion of the Arsenophonus OTUs) (F1,8 = 1.67; P = 0.146 for A. communis; F1,4 = 0.91; P = 0.421 for C. paradisi).
Discussion
Bacterial Community in Nectar
In the current study we demonstrate that the bacterial community composition (BCC) in the nectar of two plant species was distinct (Fig. 4b). Similar results were obtained by Fridman et al. [10]. This interspecific variability in nectar’s BCC is reasonable as its chemical profiles, including minerals, sugars, secondary metabolites and pH, differ markedly among plant species (for example: 35,36), and therefore are likely to stimulate the growth and development of unique bacterial communities. Previous studies showed pronounced interspecific variability of phyllosphere BCCs [37]–[42], so different plant species may promote the colonization of different bacterial communities. Future studies should focus on the mechanisms that shape the unique BCCs in the nectar of diverse plant species.
Acinetobacter dominated the BCC in the floral nectar of A. communis and C. paradise, and were also found on honeybees which foraged on their flowers (Fig. 2). Acinetobacter was also the dominant genus in the BCC of floral nectar in our previous study [10] and in the studies of Alvarez-Perez et al. [11], [12], [43], suggesting that Acinetobacter species are abundant in bacterial communities of floral nectar.
Bacterial Community on Bee Surfaces and Mouthparts
454-pyrosequencing of bacterial communities on bee surfaces revealed the presence of dominant OTUs belonging to the genus Arsenophonus; this caused the similarities between the BCCs in honeybees captured on the two different plant species (Fig. 4a). But when the sequences were analyzed without the Arsenophonus OTUs, the honeybees from the two plant species proved significantly different in their BCCs (Fig. 4b). The genus Arsenophonus is a widespread cluster of insect symbiotic bacteria [44], [45]. It has been described in honeybee intestines [25] but not on the body surfaces of bees or other insects. Nevertheless, it is possible that the source of these OTUs in the samples was the mandibles and the proboscis (tongue) that were pulled off and resulted in lysate traces of haemolymph and muscle cells which may have been released, together with the haemocoel. The presence of Arsenophonus in bee BCCs should be further studied.
Pyroseqencing analyses revealed that Lactobacillus spp. dominated the sample of bees captured from the A. communis plant (Fig 2). Lactobacillus kunkeei was also isolated and identified from the bees captured from both studied plant species (Table 1). This bacterial species was previously found in the stomach of honeybees [46], [47] and in flowers and honey [48]. However, Lactobacillus kunkeei was not isolated from the floral nectar of either of the studied plant. Lactobacillus kunkeei is an inhabitant of fructose-rich niches, so C. paradisi and A. communis nectar might not contain enough fructose for L. kunkeei growth.
Do Honeybees Play the Role of Bacterial Vector between Floral Nectars?
The pioneering results of our study demonstrate the possible role of honeybees as bacterial vectors between flowers within a plant species.
Comparison of the BCCs by the 454-pyrosequencing method in the C. paradisi plant (bees vs. covered vs. uncovered flowers, Fig. 3) revealed that the BCC in the nectar of “unvisited” flowers was significantly different from that found in exposed nectar and on the bees captured on these flowers. AMOVA of pyrosequencing data without Arsenophonus found a better separation in the BCC on bees trapped on the flowers of the two plant species than did the analysis with Arsenophonus present. The BCCs from uncovered flower nectar and from bee surfaces and mouthparts on each plant species did not differ (Fig. 4). These results demonstrate that bees found foraging for nectar on a specific plant species carry a unique bacterial flora that resembles the bacterial flora in that specific plant nectar; hence, bees may act as bacterial vectors. The distinct BCCs we found on bees from the two plant species may also be explained by the fact that the plant species have different blossoming periods. Detzel and Wink [19] and Gruter et al. [20] noted that the individual bee tends to stick to one kind of flower over a certain period of time; however, it is possible that bees first visit A. communis (flowering around January), and begin visiting C. paradisi as it begins to blossom (around March), towards the end of the A. communis blossoming period.
Based on these results we suggest that honeybees have their own endogenous bacterial communities, as already demonstrated by others [24]–[27], [49]. Recently, McFrederick et al. [50] showed that honeybees and bumblebees have host-specific Lactobacillus associates. Nevertheless, apart from the fact that honeybees have their specific endogenous bacterial communities, the results of the current study suggest that they acquire bacteria from the environment they visit, such as the nectar of the flower of a specific plant species that they pollinate in a certain period. This behavior may cause the transfer of bacteria in and out of the nectar, thereby changing the BCC in the floral nectar. Furthermore, pollinator exposure gave rise to significant changes in the chemical profile of the nectar, such as sucrose–fructose balance and nectar pH [17], [52]. These results support our suggestion that the honeybee pollinator may possibly introduce bacteria into the nectar and thus may positively or negatively affect nectar quality and plant fitness.
In conclusion, we demonstrate that the BCCs in nectar are abundant and diverse (Table 1; Figs. 1 and 2). We also demonstrate that uncovered flowers show different bacterial populations than do covered flowers, indicating that the bacterial community in floral nectar is affected by insects which visit the flowers. The honeybee pollinator may introduce bacteria into, or acquire bacteria from, the nectar. Further research is needed to buttress this hypothesis and to explore the role of bacteria in the nectar in attracting or repelling honeybees or other nectar visitors.
Supporting Information
Rarefaction curves indicating the observed number of operational taxonomic units (OTUs) at a genetic distance of 3% in different Amygdalus communis nectar samples (a) and bee samples (b). Capital letters B and N represent the sample origin: B- bee; N- floral nectar.
(TIF)
Rarefaction curves indicating the observed number of operational taxonomic units (OTUs) at a genetic distance of 3% in different Citrus paradisi samples. Capital letters B, C and U represent the sample origin: B- bee; C- nectar from covered flowers; U- nectar from uncovered flowers.
(TIF)
Sequences of primers and barcodes that were used for 454-pyrosequencing of the 16S rRNA gene.
(DOC)
Coverage, chao1 and ACE richness estimator.
(DOC)
OUT’s data. OTU abundances and taxonomic classifications within each nectar or bee sample. Data was obtained using the 454 pyrosequencing.
(XLSX)
Acknowledgments
We thank Adi Halpern for her assistance in generating the figures. We very much appreciate the critical comments of the anonymous reviewers which proved to be very helpful.
Funding Statement
This study was supported by grants from the Israel Science Foundation (ISF, grants 189/08 and 1094/12). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Brysch-Herzberg M (2004) Ecology of yeasts in plant-bumblebee mutualism in Central Europe. FEMS Microbiol Ecol 50: 87–100. [DOI] [PubMed] [Google Scholar]
- 2. Herrera CM, Pozo MI (2010) Nectar yeasts warm the flowers of a winter-blooming plant. Proc R Soc B 277: 1827–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carter C, Shafir S, Yehonatan L, Palmer RG, Thornburg R (2006) A novel role for proline in plant floral nectars. Naturwiss 93: 72–79. [DOI] [PubMed] [Google Scholar]
- 4. Gonzalez-Teuber M, Heil M (2009) Nectar chemistry is tailored for both attraction of mutualists and protection from exploiters. Plant Signal Behav 4: 809–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Phaff HJ, Starmer WT, Miranda M, Miller MW (1978) Pichia-heedii, a new species of yeast indigenous to necrotic cacti in North American Sonoran-Desert. Int J Syst Bacteriol 28: 326–331. [Google Scholar]
- 6. Sandhu DK, Waraich MK (1985) Yeasts associated with pollinating bees and flower nectar. Microb Ecol 11: 51–58. [DOI] [PubMed] [Google Scholar]
- 7. Lachance MA, Starmer WT, Rosa CA, Bowles JM, Barker JSF, et al. (2001) Biogeography of the yeasts of ephemeral flowers and their insects. FEMS Yeast Res 1: 1–8. [DOI] [PubMed] [Google Scholar]
- 8. Herrera CM, de Vega C, Canto A, Pozo MI (2009) Yeasts in floral nectar: a quantitative survey. Ann Bot 103: 1415–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gilliam M, Moffett JO, Kauffeld NK (1983) Examination of floral nectar of citrus, cotton, and Arizona desert plants for microbes. Apidologie 14 299–302. [Google Scholar]
- 10. Fridman S, Izhaki I, Gerchman Y, Halpern M (2012) Bacterial communities in floral nectar. Environ Microbiol Report 4: 97–104. [DOI] [PubMed] [Google Scholar]
- 11. Alvarez-Perez S, Herrera CM, de Vega C (2012) Zooming-in on floral nectar: a first exploration of nectar-associated bacteria in wild plant communities. FEMS Microbiol Ecol 80: 591–602. [DOI] [PubMed] [Google Scholar]
- 12. Alvarez-Pérez S, Herrera CM (2013) Composition, richness and nonrandom assembly of culturable bacterial-microfungal communities in floral nectar of Mediterranean plants. FEMS Microbiol Ecol 83: 685–699. [DOI] [PubMed] [Google Scholar]
- 13. Jessup CM, Kassen R, Forde SE, Kerr B, Buckling A, et al. (2004) Big questions, small worlds: microbial model systems in ecology. Trends Ecol Evol 19: 189–197. [DOI] [PubMed] [Google Scholar]
- 14. Whitman WB, Coleman DC, Wiebe WJ (1998) Prokaryotes: the unseen majority. Proc Natl Acad Sci U S A 95: 6578–6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fuhrman JA (2009) Microbial community structure and its functional implications. Nature 459: 193–199. [DOI] [PubMed] [Google Scholar]
- 16. Herrera CM, Garcia IM, Perez R (2008) Invisible floral larcenies: microbial communities degrade floral nectar of bumble bee-pollinated plants. Ecology 89: 2369–2376. [DOI] [PubMed] [Google Scholar]
- 17. Vannette RL, Gauthier MPL, Fukami T (2012) Nectar bacteria, but not yeast, weaken a plant–pollinator mutualism. Proc R Soc B 280: 20122601 doi:10.1098/rspb.2012.2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maurizio A, Grafi I (1969) Das Tracht- pflanzenbuch, Ehrenwirth Verlag, Munich, West Germany.
- 19. Detzel A, Wink M (1993) Attraction, deterrence or intoxication of bees (Apis mellifera) by plant allelochemicals. Chemo ecol 4: 8–18. [Google Scholar]
- 20. Gruter C, Moore H, Firmin N, Helantera H, Ratnieks FL (2011) Flower constancy in honey bee workers (Apis mellifera) depends on ecologically realistic rewards. J Exp Biol 214: 1397–1402. [DOI] [PubMed] [Google Scholar]
- 21. Free JB (1963) The flower constancy of honeybees. J Anim Ecol 32: 119–131. [Google Scholar]
- 22. Gilliam M, Buchmann SL, Lorenz BJ, Schmalzel RJ (1990) Bacteria belonging to the genus Bacillus associated with 3 species of solitary bees. Apidologie 21: 99–105. [Google Scholar]
- 23. Gilliam M (1997) Identification and roles of non-pathogenic microflora associated with honey bees. FEMS Microbiol Lett 155: 1–10. [Google Scholar]
- 24. Jeyaprakash A, Hoy MA, Allsopp MH (2003) Bacterial diversity in worker adults of Apis mellifera capensis and Apis mellifera scutellata (Insecta: Hymenoptera) assessed using 16S rRNA sequences. J Invertebr Pathol 84: 96–103. [DOI] [PubMed] [Google Scholar]
- 25. Babendreier D, Joller D, Romeis J, Bigler F, Widmer F (2007) Bacterial community structures in honeybee intestines and their response to two insecticidal proteins. FEMS Microbiol Ecol 59: 600–610. [DOI] [PubMed] [Google Scholar]
- 26. Martinson VG, Moy J, Moran NA (2012) Establishment of characteristic gut bacteria during development of the honeybee worker. Appl Environ Microbiol 78: 2830–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koch H, Abrol DP, Li J, Schmid-Hempel P (2013) Diversity and evolutionary patterns of bacterial gut associates of corbiculate bees. Mol Ecol doi: 10.1111/mec.12209. [DOI] [PubMed]
- 28. Felske A, Rheims H, Wolterink A, Stackebrandt E, Akkermans AD (1997) Ribosome analysis reveals prominent activity of an uncultured member of the class Actinobacteria in grassland soils. Microbiol 143: 2983–2989. [DOI] [PubMed] [Google Scholar]
- 29. Izhaki I, Fridman S, Gerchman Y, Halpern M (2013) Variability of bacterial community composition on leaves between and within plant species. Curr Microbiol. 66: 227–235. [DOI] [PubMed] [Google Scholar]
- 30. Chun J, Lee JH, Jung Y, Kim M, Kim S, et al. (2007) EzTaxon: a web-based tool for the identification of prokaryotes based on 16S ribosomal RNA gene sequences. Int Sys Evol Microbiol 57: 2259–2261. [DOI] [PubMed] [Google Scholar]
- 31. Bailey MT, Walton JC, Dowd SE, Weil ZM, Nelson RJ (2010) Photoperiod modulates gut bacteria composition in male Siberian hamsters (Phodopussungorus). Brain Behav Immun 24: 577–84. [DOI] [PubMed] [Google Scholar]
- 32. Pitta DW, Pinchak E, Dowd SE, Osterstock J, Gontcharova V, et al. (2010) Rumen bacterial diversity dynamics associated with changing from bermudagrass hay to grazed winter wheat diets. Microb Ecol 59: 511–522. [DOI] [PubMed] [Google Scholar]
- 33. Schloss PD, Westcott SL, Ryabin T, Hall JH, Hartmann M, et al. (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75: 7537–7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Halpern M, Fridman S, Aizenberg-Gershtein Y, Izhaki I (2013) Transfer of Pseudomonas flectens Johnson (1956) to Phaseolibacter gen. nov., in the family Enterobacteriaceae, as Phaseolibacter flectens comb. nov. Int J Syst Evol Microbiol 63: 268–273. [DOI] [PubMed] [Google Scholar]
- 35.Baker HG, Baker I (1983) A brief historical review of the chemistry of floral nectar. In: Bentley B, Elias TS, editors. The biology of nectaries. Columbia University Press, NY. Pp. 126–152.
- 36. Tadmor-Melamed H, Markman S, Arieli A, Distl M, Wink M, et al. (2004) Limited ability of Palestine Sunbirds Nectariniaosea to cope with pyridine alkaloids in nectar of Tree Tobacco Nicotianaglauca . Funct Ecol 18: 844–850. [Google Scholar]
- 37. Yang CH, Crowley DE, Borneman J, Keen NT (2001) Microbial phyllosphere populations are more complex than previously realized. Proc Natl Acad Sci USA 98: 3889–3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lambais MR, Crowley DE, Cury JC, Bull RC, Rodrigues RR (2006) Bacterial diversity in tree canopies of the Atlantic forest. Science 312: 1917. [DOI] [PubMed] [Google Scholar]
- 39. Ruppel S, Krumbein A, Schreiner M (2008) Composition of the phyllospheric microbial populations on vegetable plants with different glucosinolate and carotenoid compositions. Microb Ecol 56: 364–372. [DOI] [PubMed] [Google Scholar]
- 40. Redford AJ, Bowers RM, Knight R, Linhart Y, Fierer N (2010) The ecology of the phyllosphere: geographic and phylogenetic variability in the distribution of bacteria on tree leaves. Environ Microbiol 12: 2885–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Izhaki I, Fridman S, Gerchman Y, Halpern M (2012) Variability of bacterial community composition on leaves between and within plant species. Current Microbiol 66: 227–235. [DOI] [PubMed] [Google Scholar]
- 42. Kim M, Singh D, Lai-Hoe A, Go R, Abdul Rahim R, et al. (2012) Distinctive phyllosphere bacterial communities in tropical trees. Microb Ecol 63: 674–681. [DOI] [PubMed] [Google Scholar]
- 43. Alvarez-Perez S, Lievens B, Jacquemyn H, Herrera CM (2013) Acinetobacter nectaris sp. nov. and Acinetobacter boissieri sp. nov., two novel bacterial species isolated from floral nectar of wild Mediterranean insect-pollinated plants. Int J Syst Evol Microbiol 63: 1532–1539. [DOI] [PubMed] [Google Scholar]
- 44. Novakova E, Hypsa V, Moran NM (2009) Arsenophonus, an emerging clade of intracellular symbionts with a broad host distribution. BMC Microbiol 9: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilkes TE, Duron O, Darby AC, Hypsa V, Novakov E, et al. (2011) The Genus Arsenophonus. In: Zchori-Fein E, Bourtzis K, editors. Manipulative Tenants: Bacteria Associated with Arthropods. Taylor & Francis Inc. 225–244.
- 46. Olofsson TC, Vasquez A (2008) Detection and identification of a novel lactic acid bacterial flora within the honey stomach of the honeybee Apis mellifera . Curr Microbiol 57: 356–363. [DOI] [PubMed] [Google Scholar]
- 47. Vasquez A, Forsgren E, Fries I, Paxton RJ, Flaberg E, et al. (2012) Symbionts as major modulators of insect health: lactic acid bacteria and honeybees. PLoS ONE 7: e33188 doi:10.1371/journal.pone.0033188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Endo A, Irisawa T, Futagawa-Endo Y (2011) Characterization and emended description of Lactobacillus kunkeei as a fructophilic lactic acid bacterium. Int J Syst Evol Microbiol doi: 10.1099/ijs.0.031054–0. [DOI] [PubMed]
- 49. Moran NA, Hansen AK, Powell JE, Sabree ZL (2012) Distinctive gut microbiota of honey bees assessed using deep sampling from individual worker bees. PLoS ONE 7: e36393 doi:10.1371/journal.pone.0036393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McFrederick QS, Cannone JJ, Robin RG, Kellner K, Plowes RM, et al. (2013) Host specificity between Hymenoptera and lactobacilli is the exception rather than the rule. Appl Environ Microbiol 79: 1803–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig W, et al. (2007) SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res 35: 7188–7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Canto A, Herrera CM, Garcia IM, Perez R, Vaz M (2011) Intra plant variation in nectar traits in Helleborusfoetidus(Ranunculaceae) as related to floral phase, environmental conditions and pollinator exposure. Flora 206: 668–675. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Rarefaction curves indicating the observed number of operational taxonomic units (OTUs) at a genetic distance of 3% in different Amygdalus communis nectar samples (a) and bee samples (b). Capital letters B and N represent the sample origin: B- bee; N- floral nectar.
(TIF)
Rarefaction curves indicating the observed number of operational taxonomic units (OTUs) at a genetic distance of 3% in different Citrus paradisi samples. Capital letters B, C and U represent the sample origin: B- bee; C- nectar from covered flowers; U- nectar from uncovered flowers.
(TIF)
Sequences of primers and barcodes that were used for 454-pyrosequencing of the 16S rRNA gene.
(DOC)
Coverage, chao1 and ACE richness estimator.
(DOC)
OUT’s data. OTU abundances and taxonomic classifications within each nectar or bee sample. Data was obtained using the 454 pyrosequencing.
(XLSX)