Abstract
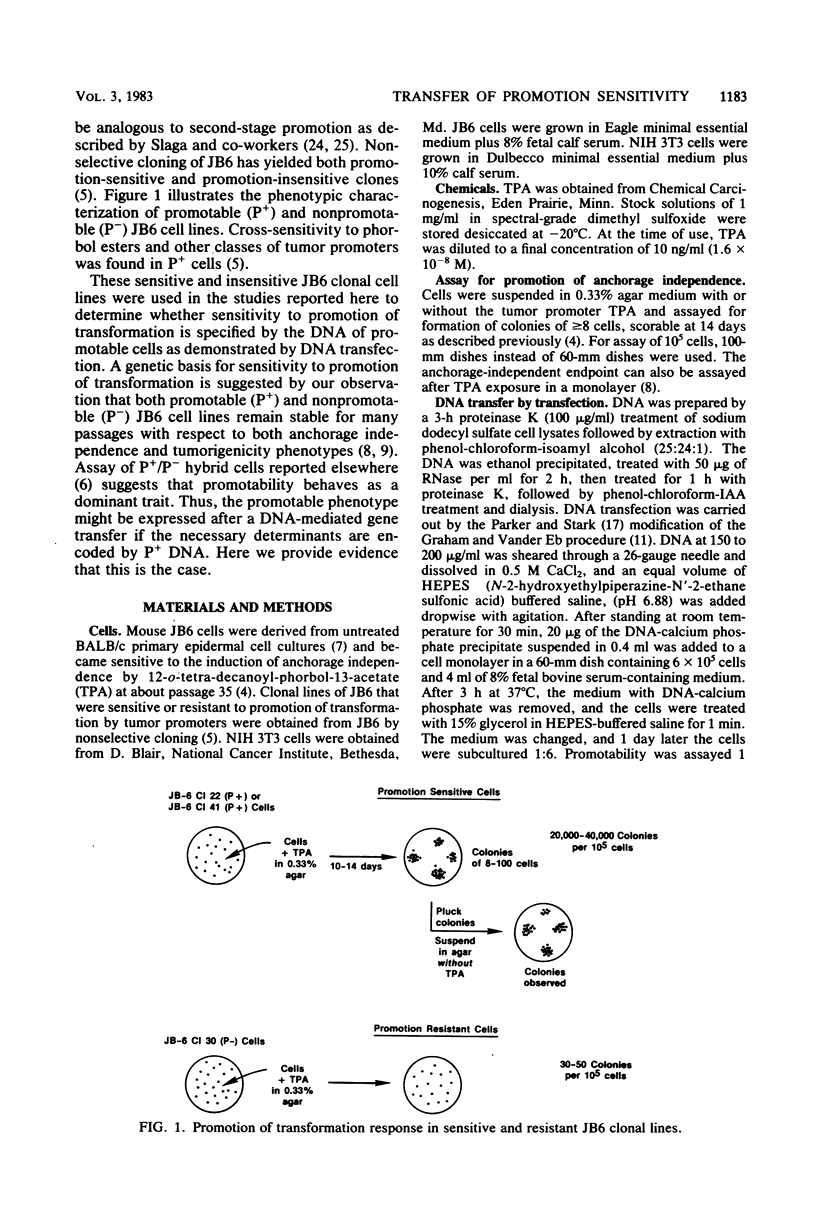
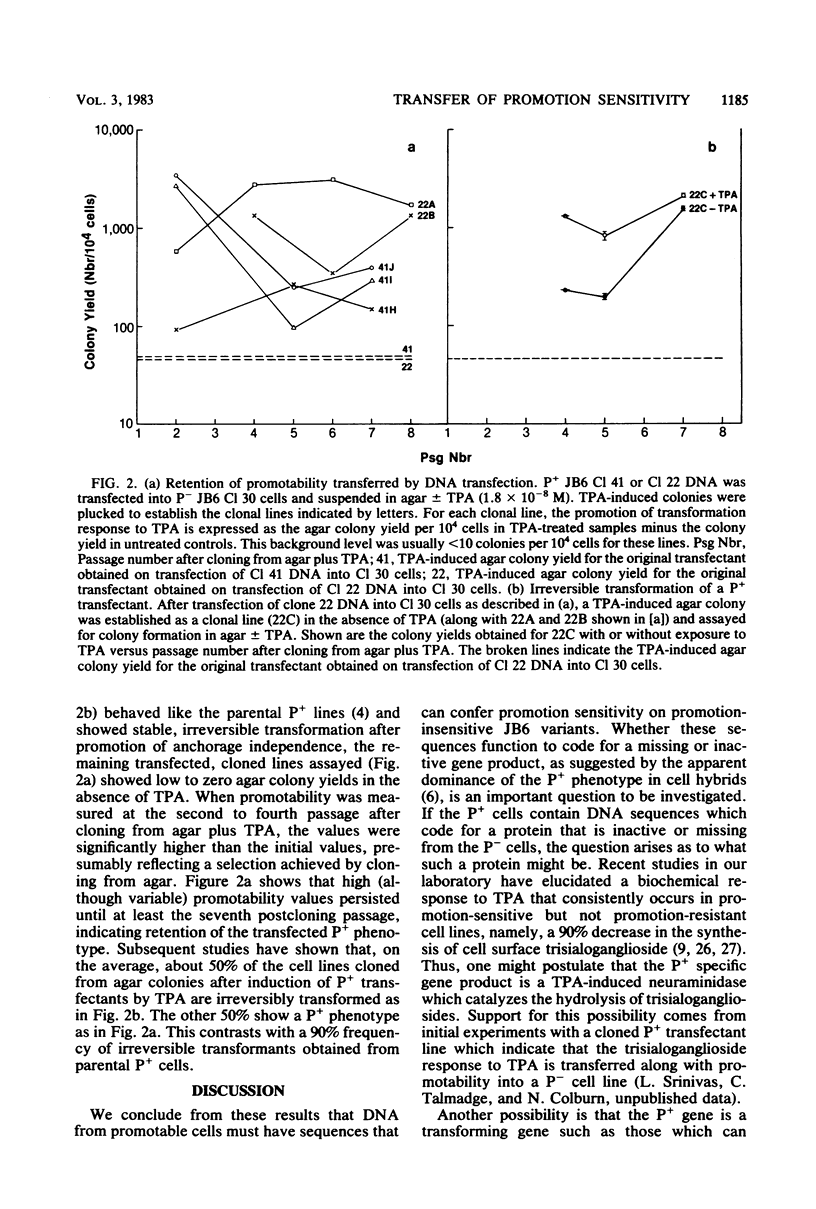
Sensitivity to promotion of transformation by tumor promoters in mouse epidermal JB6 cells appears to have a genetic basis since the phenotypes of both promotable and nonpromotable JB6 cells derived from a common parent line are stable. Hybridization of promotable (P+) and nonpromotable (P−) cells previously indicated that promotability appears to behave as a dominant trait. These results suggest that it should be possible to find DNA sequences which specify sensitivity to promotion of anchorage independence by 12-o-tetradecanoyl-phorbol-13-acetate (TPA). Cellular DNA isolated from one of two P+ lines, JB6 Cl 41 or JB6 Cl 22, was CaPO4 precipitated and used to transfect the P− cell line JB6 Cl 30. At 7 days posttransfection, the cells were suspended in agar with or without TPA at 1.6 × 10−8 M and assayed 10 days later for TPA-dependent colony formation. Untreated or Cl 30 DNA-treated P− JB6 Cl 30 cells yielded 40 to 50 colonies per 105 cells. In contrast, transfection of Cl 30 cells with “P+ DNA” derived from either Cl 41 or Cl 22 yielded 200 to 500 TPA-induced colonies per 105 cells, or a five- to eightfold enhancement of promotability. The enhanced promotability obtained after transfection with P+ DNA was stable, as judged by the retention of promotability for at least eight passages in cell lines derived from TPA-induced agar colonies. Other transfectants showed irreversible transformation by TPA, as observed in the parental P+ lines. When NIH 3T3 cells instead of the putative preneoplastic JB6 Cl 30 cells were used as recipients for transfection of P+ DNA, no evidence for acquisition of promotability was obtained. P− JB6 Cl 25, like Cl 30, also permitted expression of transfected P+ DNA. These results suggest that sensitivity to phorbol ester promotion of transformation in JB6 cells is determined by DNA sequence(s) present in the P+ DNA and requires recipient cells of the appropriate phenotype for expression.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boutwell R. K. The function and mechanism of promoters of carcinogenesis. CRC Crit Rev Toxicol. 1974 Jan;2(4):419–443. doi: 10.3109/10408447309025704. [DOI] [PubMed] [Google Scholar]
- Colburn N. H., Bruegge W. F., Bates J. R., Gray R. H., Rossen J. D., Kelsey W. H., Shimada T. Correlation of anchorage-independent growth with tumorigenicity of chemically transformed mouse epidermal cells. Cancer Res. 1978 Mar;38(3):624–634. [PubMed] [Google Scholar]
- Colburn N. H., Former B. F., Nelson K. A., Yuspa S. H. Tumour promoter induces anchorage independence irreversibly. Nature. 1979 Oct 18;281(5732):589–591. doi: 10.1038/281589a0. [DOI] [PubMed] [Google Scholar]
- Colburn N. H., Koehler B. A., Nelson K. J. A cell culture assay for tumor-promoter-dependent progression toward neoplastic phenotype: detection of tumor promoters and promotion inhibitors. Teratog Carcinog Mutagen. 1980;1(1):87–96. doi: 10.1002/tcm.1770010109. [DOI] [PubMed] [Google Scholar]
- Colburn N. H., Talmadge C. B., Gindhart T. D. Transfer of phorbol ester promotability by transfection of DNA from promotable into nonpromotable cells. Prog Nucleic Acid Res Mol Biol. 1983;29:107–110. doi: 10.1016/s0079-6603(08)60436-5. [DOI] [PubMed] [Google Scholar]
- Colburn N. H. Tumor promotion and preneoplastic progression. Carcinog Compr Surv. 1980;5:33–56. [PubMed] [Google Scholar]
- Colburn N. H., Wendel E., Srinivas L. Responses of preneoplastic epidermal cells to tumor promoters and growth factors: use of promoter-resistant variants for mechanism studies. J Cell Biochem. 1982;18(3):261–270. doi: 10.1002/jcb.1982.240180302. [DOI] [PubMed] [Google Scholar]
- Fürstenberger G., Berry D. L., Sorg B., Marks F. Skin tumor promotion by phorbol esters is a two-stage process. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7722–7726. doi: 10.1073/pnas.78.12.7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hopkins N., Besmer P., DeLeo A. B., Law L. W. High-frequency cotransfer of the transformed phenotype and a tumor-specific transplantation antigen by DNA from the 3-methylcholanthrene-induced Meth A sarcoma of BALB/c mice. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7555–7559. doi: 10.1073/pnas.78.12.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klobutcher L. A., Miller C. L., Ruddle F. H. Chromosome-mediated gene transfer results in two classes of unstable transformants. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3610–3614. doi: 10.1073/pnas.77.6.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane M. A., Sainten A., Cooper G. M. Activation of related transforming genes in mouse and human mammary carcinomas. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5185–5189. doi: 10.1073/pnas.78.8.5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Expression of a bacterial gene in mammalian cells. Science. 1980 Sep 19;209(4463):1422–1427. doi: 10.1126/science.6251549. [DOI] [PubMed] [Google Scholar]
- Murray M. J., Shilo B. Z., Shih C., Cowing D., Hsu H. W., Weinberg R. A. Three different human tumor cell lines contain different oncogenes. Cell. 1981 Aug;25(2):355–361. doi: 10.1016/0092-8674(81)90054-4. [DOI] [PubMed] [Google Scholar]
- Parker B. A., Stark G. R. Regulation of simian virus 40 transcription: sensitive analysis of the RNA species present early in infections by virus or viral DNA. J Virol. 1979 Aug;31(2):360–369. doi: 10.1128/jvi.31.2.360-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perucho M., Goldfarb M., Shimizu K., Lama C., Fogh J., Wigler M. Human-tumor-derived cell lines contain common and different transforming genes. Cell. 1981 Dec;27(3 Pt 2):467–476. doi: 10.1016/0092-8674(81)90388-3. [DOI] [PubMed] [Google Scholar]
- Pulciani S., Santos E., Lauver A. V., Long L. K., Robbins K. C., Barbacid M. Oncogenes in human tumor cell lines: molecular cloning of a transforming gene from human bladder carcinoma cells. Proc Natl Acad Sci U S A. 1982 May;79(9):2845–2849. doi: 10.1073/pnas.79.9.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scangos G. A., Huttner K. M., Silverstein S., Ruddle F. H. Molecular analysis of chromosome-mediated gene transfer. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3987–3990. doi: 10.1073/pnas.76.8.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih C., Padhy L. C., Murray M., Weinberg R. A. Transforming genes of carcinomas and neuroblastomas introduced into mouse fibroblasts. Nature. 1981 Mar 19;290(5803):261–264. doi: 10.1038/290261a0. [DOI] [PubMed] [Google Scholar]
- Shih C., Shilo B. Z., Goldfarb M. P., Dannenberg A., Weinberg R. A. Passage of phenotypes of chemically transformed cells via transfection of DNA and chromatin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5714–5718. doi: 10.1073/pnas.76.11.5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilo B. Z., Weinberg R. A. Unique transforming gene in carcinogen-transformed mouse cells. Nature. 1981 Feb 12;289(5798):607–609. doi: 10.1038/289607a0. [DOI] [PubMed] [Google Scholar]
- Slaga T. J., Fischer S. M., Nelson K., Gleason G. L. Studies on the mechanism of skin tumor promotion: evidence for several stages in promotion. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3659–3663. doi: 10.1073/pnas.77.6.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaga T. J., Klein-Szanto A. J., Fischer S. M., Weeks C. E., Nelson K., Major S. Studies on mechanism of action of anti-tumor-promoting agents: their specificity in two-stage promotion. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2251–2254. doi: 10.1073/pnas.77.4.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas L., Colburn N. H. Ganglioside changes induced by tumor promoters in promotable JB6 mouse epidermal cells: antagonism by an antipromoter. J Natl Cancer Inst. 1982 Mar;68(3):469–473. [PubMed] [Google Scholar]
- Srinivas L., Gindhart T. D., Colburn N. H. Tumor-promoter-resistant cells lack trisialoganglioside response. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4988–4991. doi: 10.1073/pnas.79.16.4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]



