Abstract
The goal of this work was to experimentally quantify the geometric accuracy of a novel real-time 3D target localisation method using sequential kV imaging combined with respiratory monitoring for clinically realistic arc and static field treatment delivery and target motion conditions. A general method for real-time target localisation using kV imaging and respiratory monitoring was developed. Each dimension of internal target motion T(x, y, z;t) was estimated from the external respiratory signal R(t) through the correlation between R(ti) and the projected marker positions p(xp, yp;ti) on kV images by a state-augmented linear model: T(x, y, z;t)=aR(t)+bR(t − τ)+c. The model parameters, a, b, c, were determined by minimizing the squared fitting error Σ||p(xp, yp;ti) − P(θi)·(aR(ti)+bR(ti − τ)+c)||2 with the projection operator P(θi). The model parameters were first initialized based on acquired kV arc images prior to MV beam delivery. This method was implemented on a Trilogy linear accelerator consisting of an OBI x-ray imager (operating at 1 Hz) and Real-time Position Monitoring (RPM) system (30 Hz). Arc and static field plans were delivered to a moving phantom programmed with measured lung tumour motion from 10 patients. During delivery, the localisation method determined the target position and the beam was adjusted in real time via dynamic multileaf collimator (DMLC) adaptation. The beam-target alignment error was quantified by segmenting the beam aperture and a phantom-embedded fiducial marker on MV images and analysing their relative position. With the localisation method, the root-mean-squared errors of the 10 lung tumour traces ranged from 0.7–1.3 mm and 0.8–1.4 mm during the single arc and 5-field static beam delivery, respectively. Without the localisation method, these errors ranged from 3.1–7.3 mm. In summary, a general method for real-time target localisation using kV imaging and respiratory monitoring has been experimentally investigated for arc and static field delivery. The average beam-target error was 1 mm.
Introduction
Three dimensional knowledge of tumour location during abdominal and thoracic radiotherapy is an important component of motion management whether the treatment delivery utilizes motion inclusive, gating or tumour tracking methods. A wide variety of real-time or continuous localisation methods are either available or under development. The diversity of systems includes radiofrequency, radioisotope, ultrasound and MRI, in addition to the optical, kV and MV imaging systems available on conventional accelerators. In this study, we experimentally investigated a method for real-time localisation and delivery adaptation, that uses a commercially-available, gantry-mounted kV imaging system combined with optical respiratory monitoring.
The rationale for utilizing a method combining kV imaging with optical respiratory monitoring was its widespread clinical availability: kV imaging is available from most major linear accelerator vendors including Accuracy, BrainLab, Elekta and Varian. kV imaging enables image-based target localisation and geometry monitoring independently of the megavoltage treatment beam, at a cost of imaging dose. Stereoscopic kV imaging has been explored for intrafraction radiotherapy guidance (Shirato et al., 2000b; Shirato et al., 2000a; Berbeco et al., 2004; Hoogeman et al., 2009; Kamino et al., 2006), yet most commercial systems do not offer stereoscopic kV imaging. A 3D localisation method using a single gantry-mounted kV imager has been experimentally studied by Poulsen et al. (Poulsen et al., 2010a; Poulsen et al., 2010c), yielding geometric uncertainties of approximately 1.5 mm for arc and static delivery to abdominal, thoracic and prostate targets. In order to reduce the imaging dose, it is appealing to combine kV imaging with an external respiratory signal and develop an internal/external correlation model (Murphy et al., 2007). Respiratory monitors are widely available in medicine and relatively cheap devices, such as a web-cam (Siwiak et al., 2009) and the iPod accelerometer (Lam et al., 2009) have been used to provide a useful respiratory signal.
Pioneering work combining internal and external signals using stereoscopic x-ray imaging and a respiratory signal for target localisation was performed by Schweikard et al. (Schweikard et al., 2000) and incorporated into the Cyberknife linear accelerator. Several further models have been proposed (Murphy, 2004; Isaksson et al., 2005; Kanoulas et al., 2007) and one has been integrated into a prototype dynamic multileaf collimator (DMLC) tracking system and experimentally investigated (Cho et al., 2011). However, not all IGRT systems have imagers capable of synchronous stereoscopic imaging. Furthermore, even for those systems capable of stereoscopic imaging, it may not be optimal to image synchronously to balance targeting accuracy and imaging dose. The challenge with using a single imager for target localisation is that the position along the imaging beam is difficult to determine. In an earlier work, Cho et al. (Cho et al., 2008) described a method of combining a single kV imager with a respiratory signal, where the position along the imaging beam was estimated by building a correlation model between the unresolved component and the two other components projected on the imager from prior information of the 3D target trajectory. This method was evaluated for fixed beam simulation studies. More recently, a new sequential model has been developed in which the internal/external correlated model is updated by a minimization of the difference between the measured projections and the estimated trajectory projected into the imaging geometry (Cho et al., 2010). The method is general enough to be applicable to a wide variety of IGRT systems and to be used for both arc and static field delivery, but only simulation results have been reported so far.
The purpose of this study was to experimentally quantify the geometric accuracy of a novel real-time 3D target localisation method (Cho et al., 2010) using sequential kV imaging combined with respiratory monitoring for clinically realistic arc and static field treatment delivery and target motion conditions. Compared to previous localization methods that rely partly on MV images, the current method allows arbitrary intensity modulation. Compared to localization methods that rely only on x-ray images, the current method results in reduced imaging dose due to the addition of external respiratory monitoring.
Method and materials
Target localisation algorithm
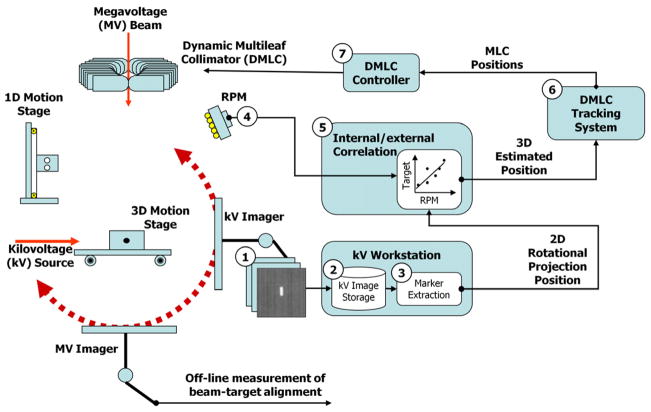
The theory and simulation results of the localisation method have previously been described (Cho et al., 2010). They are reviewed briefly here to support the experimental investigation in the current work. The 3D target position T(x, y, z;t) is estimated from the respiratory signal R(t) through the correlation between R(ti) and the projected marker positions p(xp, yp;ti) on kV images by a state-augmented linear model (Ruan et al., 2008): T(x, y, z;t)=aR(t)+bR(t − τ)+c. The model parameters, a, b, c, are determined by solving a least-squares estimation of the 2D error Σ||p(xp, yp;ti) − P(θi)·(aR(ti)+bR(ti − τ)+c)||2. Here, P(θi) is a projection operator onto the i-th kV image of the gantry angle θi and the lag time τ was set 0.5 s. The model is initiated by acquiring kV projections in an arc of 120° prior to MV beam delivery. The steps, equations and term definitions are shown in Figure 1.
Figure 1.
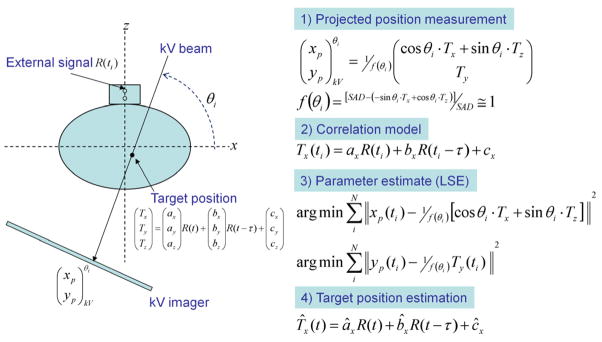
Schematic diagram showing the procedure of real-time target localisation. Each direction of internal target motion T(x, y, z;t), was estimated from the external respiratory signal R(t) through the correlation between R(ti) and the projected marker positions p(xp, yp;ti) on kV images by a state-augmented linear model: T(x, y, z;t)=aR(t − τ)+c. The model parameters were determined by solving a least-squares estimation (LSE) of the error Σ||p(xp, yp;ti) − P(θi)·(aR(ti) +bR(ti − τ)+c)||2 with projection operator P(θi) (Cho et al., 2010). Prior to treatment, rotational kV imaging of 120° was sequentially acquired to initialize the estimation model and start the estimation. The model parameters were updated every time a new kV image was acquired with the kV projection data covering 120-degree angular span. SAD = source-axis distance (100 cm in this study).
Experimental system and method
The localisation method is integrated into a prototype DMLC tracking system consisting of an OBI x-ray imager (operating at 1 Hz in this study), RPM respiratory signal (30 Hz) and Trilogy linac equipped with a Millennium 120-leaf MLC (Varian Medical Systems, Palo Alto, CA). The process is shown in Figure 2. In step 1 of Figure 2 the kV images are acquired, then saved (step 2). An in-house developed marker extraction program opens the images and segments the marker positions (step 3). The 2D marker positions and the projection angle, along with the respiratory signal (step 4) are sent to the 3D position estimation model (step 5) described above. It also includes a motion prediction module (Srivastava et al., 2007) applied to the respiratory signal to compensate for the 160 ms system latency (the time between the occurrence of motion and the MLC response). The prediction algorithm uses a modified linear adaptive filter and reduces the error induced by the latency by 30% on average. The calculated 3D target location is sent to the DMLC tracking program (step 6) where the DMLC positioning algorithm (Sawant et al., 2008) integrates the 3D target location with the planned leaf sequence. The updated leaf sequence is then sent to the DMLC controller (step 7) and the MLC to align the beam with the target. Note that the 160 ms system latency is the same as that measured for the RPM input (Keall et al., 2006), rather than the 1090 ms latency of the 1Hz OBI input alone (Poulsen et al., 2010a; Poulsen et al., 2010b). The time difference between the signals is accounted for in the model by synchronising them together. The effect is a 930 ms delay in the updating of the model, but not in the use of the model for real-time 3D target localisation.
Figure 2.
Flowchart for the real-time target localisation and beam adaptation measurements.
Each experiment started with training of the prediction algorithm to compensate the 160ms system delay. This training involved acquiring the external respiratory signal (RPM in our case) for 40 seconds. Subsequently, the initial correlation model between the marker position on kV images and synchronised RPM signal was built by rotating the gantry through 120 degrees with the kV imager on.
Ten tumour trajectories and associated external respiratory signals with a motion range larger than 10 mm were selected from 160 tumour trajectories (Suh et al., 2008). The dataset was acquired from 46 thoracic and abdominal tumour patients treated by a CyberKnife Synchrony system. The mean (range) of the peak-to-peak motion of the selected trajectories was 6.4 (1.2 – 20.8) mm, 13.0 (1.0 – 20.4) mm, and 5.7 (1.8 – 13.7) mm in the left-right (LR), superior-interior (SI) and anterior-posterior (AP) directions, respectively. The mean (range) of their average breathing cycles was 3.8 (2.7 – 4.9) sec. Note, this dataset was the same as that used for a previous experimental tracking study combining kV, MV and the respiratory signal (Cho et al., 2011).
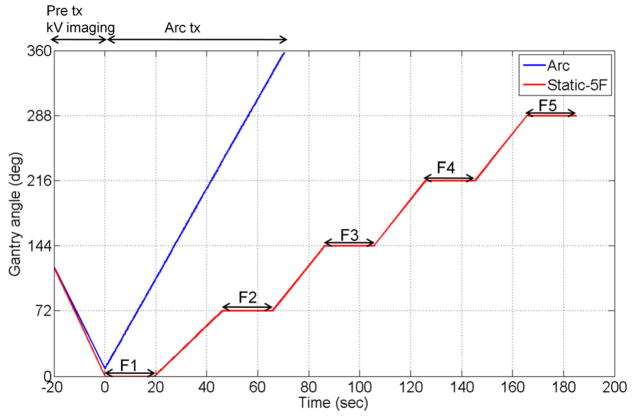
A 358°-arc plan and a static 5-field plan were delivered to a 4D phantom from Washington University (Malinowski et al., 2007). The phantom consists of a 3D moving phantom which was programmed to reproduce each of the 10 tumour trajectories, and an independent 1D moving phantom that was simultaneously programmed to reproduce the patient-measured anterior-posterior external abdominal breathing motion. The arc plan started in a counter-clock wise manner with the gantry starting at 1° and ending at 359°. The 5 beam angles of the static plan were 0, 72, 144, 216, and 288° (Varian scale). The timeline of the gantry angle position for the arc and static fields is shown in Figure 3. The beam delivery time was set to 60 seconds for the arc plan and 20 seconds for each of the 5 fields in the static plan. The MLC-shaped circular field was used for both plans. Fluoroscopic kV images were acquired at 1Hz perpendicular to the treatment beam axis during a 120° pre-treatment gantry rotation, during treatment delivery and, for the static 5-field plan, during inter-field gantry rotations. The 120° rotational kV imaging prior to treatment was used to initialize the correlation model for both the arc and static plans, as shown in the −20 to 0 second part of Figure 3. It was based on the finding of the previous simulation study (Cho et al., 2010) that at least 120° rotational kV imaging is necessary for minimal parallax error. For the same reason, the data window for the model computation was fixed so that the projection data covered an angular span of 120° instead of fixing the time interval. Therefore, the estimation error would likely be higher for the static plan than the arc plan because the temporal change of the correlation would not be adapted to as promptly.
Figure 3.
Gantry angle vs. time for the arc and static 5-field beam delivery experiments. Prior to treatment onset, from −20 to 0 seconds in the plot, rotational kV images over 120° are acquired at 1 Hz along with the respiratory signal and are used to initialize the localisation method. For arc delivery, the MV beam was delivered in ~72 seconds from gantry angle 1° to 359°. For the 5-field treatment the MV beam was delivered for 20s at each of 5 even-spaced gantry angles.
The accuracy of the localisation method was investigated by quantifying the beam-target positional error, during arc and static beam delivery, for each of the ten traces. The MV imager was used as an independent measurement tool where the beam aperture and the target position, represented by a fiducial marker, were visible in each image. The MV images were acquired in cine mode at 3.4 Hz, which was determined empirically to obtain appropriate kV image quality while the MV beam was on. This measurement method has been described previously (Cho et al., 2009). The beam-target alignment error with real-time localisation was quantified by measuring the geometric distance between the centre of the circular beam aperture and fiducial marker on each MV image as a function of time. The error without real-time localization was quantified as the distance between the fiducial marker and the position of the beam aperture centre as it would have been if no MLC refitting were applied.
Results
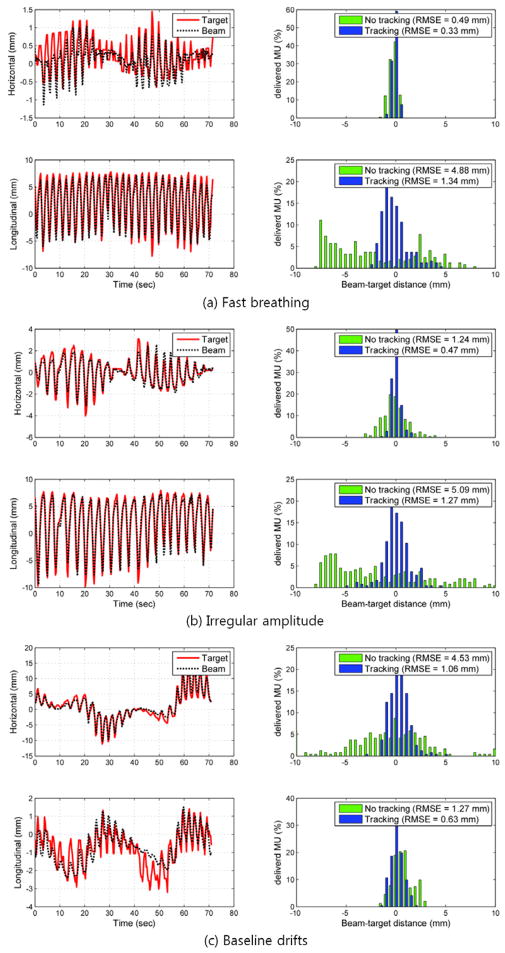
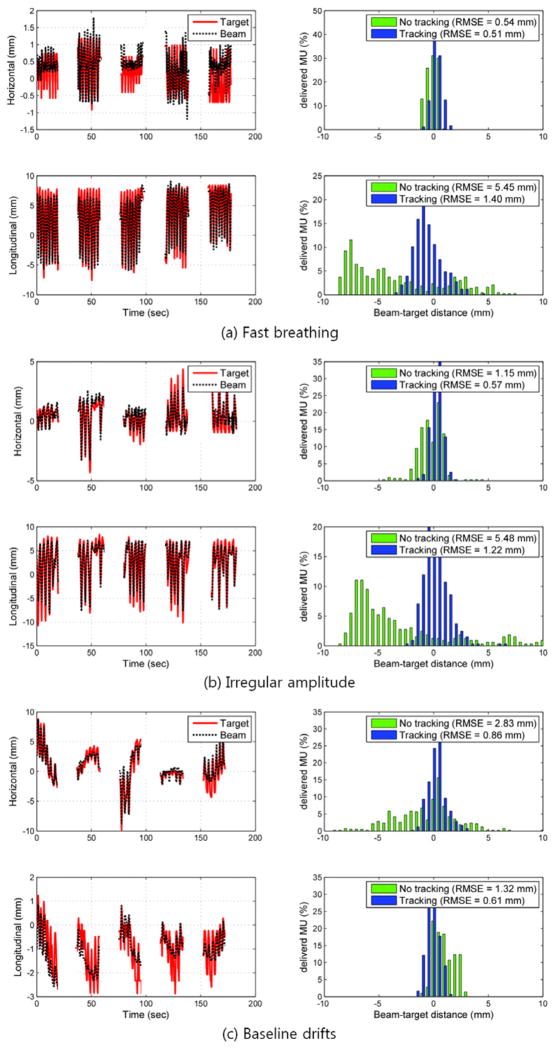
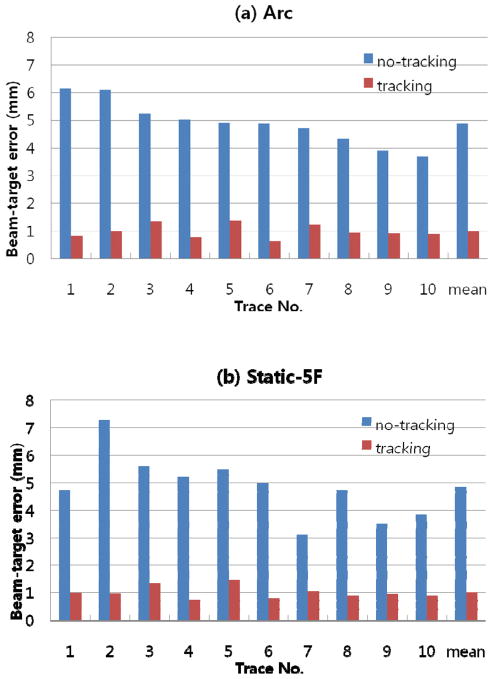
Typical examples of the target and aperture position as a function of time are shown in Figure 4 and Figure 5 for the arc and static beam delivery, respectively. They are the same trajectory examples chosen in the previous tracking study combining kV, MV and the respiratory signal (Cho et al., 2011), to represent characteristics of fast breathing, irregular amplitude, and baseline drift in respiratory motion. In Figure 4, the apparent variation in the horizontal motion with time is due to the changing motion projection during the treatment delivery with the rotating gantry. The 2D root-mean-squared (rms) beam-target errors for all 10 tumour motion traces during both the arc and static field delivery with and without real-time localisation are summarized in Figure. 6. The overall error was substantially reduced with real-time localisation: the beam-target 2D error of 4.9 mm ±1.0 mm (1σ) without real-time localisation was reduced to 1.0 mm ±0.2 mm (1σ) with localisation for both the arc and static beam delivery, approximately an 80% reduction in both cases. In addition to an overall reduction in beam-target error, the patient to patient variation without real-time localisation of 4.2 mm (range 3.1–7.3mm) was reduced to 1.0 mm (range 0.7–1.4 mm). This reduction in the patient-to-patient variability indicates that a more stable estimate can be made of the margin needed to account for targeting errors.
Figure 4.
The MV imager measured target position, aperture position and tracking error of three typical tumour motion traces during arc delivery featuring (a) fast breathing, (b) irregular amplitude and (c) baseline drifts during the beam delivery. The left column shows the trajectory of the marker and the following MLC aperture in each direction on MV images: the horizontal position corresponds to motion perpendicular to the MV imager in the axial patient plane; the longitudinal position corresponds to motion in the SI direction. The right column shows histograms of tracking error without (no tracking) and with (tracking) real-time localisation.
Figure 5.
The MV imager measured target position, aperture position and tracking error of three typical tumour motion traces during 5-field delivery featuring (a) fast breathing, (b) irregular amplitude, and (c) baseline drifts during the beam delivery. The left column shows the trajectory of the marker and the following MLC aperture in each direction on MV images, where the longitudinal position corresponds to motion in the SI direction. The right column shows histograms of tracking error without (no tracking) and with (tracking) real-time localisation.
Figure 6.
Summary of the 2D root-mean-square tracking errors for the ten lung tumour motion traces measured using cine MV images during the (a) arc and (b) static 5-field beam delivery without (no tracking) and with (tracking) real-time localisation. DMLC tracking integrated with the proposed localisation method showed ~1mm of mean root-mean square error irrelevant of the beam delivery modes or the range of motion.
The residual localisation error is mainly attributed to the accuracy of the correlation model, the system latency (partially corrected using the prediction algorithm), gantry sag and the alignment of the kV imager with respect to the beam isocentre. To learn more about the contributions from each of the components, their performances were separately investigated. To examine the accuracy of the correlation model, each time a kV or MV image was acquired the measured position projected on the image was compared with the position estimated by the correlation model using the synchronised RPM signal. The estimation accuracy of the correlation model for the all experiments was ~0.5 mm in each direction. It demonstrates that even with a single kV imager the overall accuracy was similar to a previous study using the respiratory signal and both kV and MV imagers for stereoscopic imaging (Cho et al., 2011). This value is already close to the limit of the machine accuracy, i.e., gantry sag and the alignment of the kV imager with respect to the beam isocentre. It should be noted that another important factor determining the accuracy of the correlation model would be the degree of strength in the individual correlation between the internal target movement and the external respiratory signal.
To assess the performance of the prediction algorithm, the predicted external respiratory signals were recorded with the measured values. The prediction error was computed from the difference between the predicted value and the measured value shifted 160 ms. Overall, the error due to the system latency was reduced by 30% by applying the prediction algorithm, even though the error due to the system latency was small, <1mm, because of the relatively short latency.
Discussion
Many devices and methods are available for real-time target localisation. However, the clinical integration of these methods to control the treatment beam and align the radiation beam and treatment target is to date limited to the Cyberknife and Vero systems. In this study, a new real-time 3D localisation method (Cho et al., 2010) was integrated into a prototype DMLC-based tumour tracking system to obtain the first experimental results of the real-time localisation algorithm. The localisation method utilizes data from a kV imager and an optical respiratory monitoring signal, both widely available on many IGRT platforms. The localization method is fully compatible with intensity modulation (IMRT and VMAT) since the use of MV portal images is avoided. Tt allows a large reduction in the imaging frequency and, thus the imaging dose as compared to methods that solely relying on x-ray imaging. Experimental validation of the localisation method was performed using clinically realistic arc and static field treatment delivery conditions to a phantom containing an implanted marker. The phantom was programmed to reproduce measured tumour motion data. An independent axis was programmed to reproduce synchronously measured respiratory motion. Average beam-target geometric errors of 1 mm were observed.
This method, in its current form, requires marker implantation which is similar to other marker-based methods including those employed by BrainLab, Calypso, Cyberknife, and Navotek. Potentially, marker-free kV imaging could be used to image the tumour location directly (Lin et al., 2009; Berbeco et al., 2005). However, any uncertainties in the tumour segmentation will be translated to beam-target alignment errors. In applying these results to a clinical scenario, the biggest difference is the accuracy of marker segmentation. For the current application, a marker was embedded in a uniform phantom, making the marker segmentation simpler and more reliable than clinical segmentation in which there is a heterogeneous background.
An important clinical issue with using kV for guidance is the additional imaging dose. From AAPM Task Group 75 (Murphy et al., 2007), the dose per image based on the Synchrony system is 0.05–0.25 mGy. In addition to ensuring the exposure settings are optimal, large dose reductions can be made by limiting the kV field to the target and reducing the image frequency. In this experimental study, the kV imager was operating at 1 Hz. From Figure 3, this would result in 100 images for the arc treatment and 200 images for the static field treatment. In the simulation paper of this localisation method, when the imaging frequency was reduced from once per second to once every 10 seconds, a 25% increase in geometric error was observed, at the reduction of an order of magnitude of imaging dose (Cho et al., 2010). The optimal kV imaging frequency for clinical implementation which trades off accuracy with dose remains to be determined.
Should clinical marker segmentation prove similar in accuracy to these experiments, we can anticipate that the total system accuracy could be improved to have sub-mm error on a system specifically designed for real-time tracking of respiratory motion. For example, reducing the system latency (160 ms for this system), better kV/MV alignment, lower gantry sag (or gantry sag compensation by MLC adjustment) and improved prediction algorithms would all help to reduce the beam-target error. Given that the inter-observer variations in target delineation (4–10 mm) (Steenbakkers et al., 2006) are of the order of the error of the treatment accuracy without real-time localisation, further reductions in error at the sub-mm level may have limited clinical benefit at this time.
Conclusion
The first experimental study of a novel real-time 3D target localisation method using sequential kV imaging combined with optical respiratory monitoring on a Varian linear accelerator has been performed. Average geometric errors of 1mm were observed for clinically realistic arc and static field treatment delivery conditions for 10 patient-derived tumour and respiratory motion traces. These errors could be further reduced with improved kV/MV alignment and lower system latency. The localisation method can also be applied to other IGRT systems, including those from Accuray, BrainLab and Elekta.
Acknowledgments
The authors gratefully acknowledge the support of US NIH/NCI R01-93626 and an NHMRC Australia Fellowship. Byungchul Cho was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-26369). Thank you to Julie Baz, University of Sydney, for reviewing the manuscript. The authors thank the following contributors from Varian Medical Systems: Herbert Cattell, for substantial development of the DMLC adaptation program, Hassan Mostafavi and Alexander Sloutsky, for the marker extraction software used for offline image analysis and Sergey Povzner for the RPM communication interface development.
References
- Berbeco RI, Jiang SB, Sharp GC, Chen GT, Mostafavi H, Shirato H. Integrated radiotherapy imaging system (IRIS): design considerations of tumour tracking with linac gantry-mounted diagnostic x-ray systems with flat-panel detectors. Phys Med Biol. 2004;49:243–55. doi: 10.1088/0031-9155/49/2/005. [DOI] [PubMed] [Google Scholar]
- Berbeco RI, Mostafavi H, Sharp GC, Jiang SB. Towards fluoroscopic respiratory gating for lung tumours without radiopaque markers. Physics in medicine and biology. 2005;50:4481. doi: 10.1088/0031-9155/50/19/004. [DOI] [PubMed] [Google Scholar]
- Cho B, Poulsen PR, Keall PJ. Real-time tumor tracking using sequential kV imaging combined with respiratory monitoring: a general framework applicable to commonly used IGRT systems. Physics in medicine and biology. 2010;55:3299–316. doi: 10.1088/0031-9155/55/12/003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho B, Poulsen PR, Sawant A, Ruan D, Keall PJ. Real-time target position estimation using stereoscopic kilovoltage/megavoltage imaging and external respiratory monitoring for dynamic multileaf collimator tracking. International journal of radiation oncology, biology, physics. 2011;79:269–78. doi: 10.1016/j.ijrobp.2010.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho B, Poulsen PR, Sloutsky A, Sawant A, Keall PJ. First demonstration of combined kV/MV image-guided real-time dynamic multileaf-collimator target tracking. International journal of radiation oncology, biology, physics. 2009;74:859–67. doi: 10.1016/j.ijrobp.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho B, Suh Y, Dieterich S, Keall PJ. A monoscopic method for real-time tumour tracking using combined occasional x-ray imaging and continuous respiratory monitoring. Physics in medicine and biology. 2008;53:2837. doi: 10.1088/0031-9155/53/11/006. [DOI] [PubMed] [Google Scholar]
- Hoogeman M, Prévost J-B, Nuyttens J, Pöll J, Levendag P, Heijmen B. Clinical Accuracy of the Respiratory Tumor Tracking System of the CyberKnife: Assessment by Analysis of Log Files. International Journal of Radiation Oncology*Biology*Physics. 2009;74:297–303. doi: 10.1016/j.ijrobp.2008.12.041. [DOI] [PubMed] [Google Scholar]
- Isaksson M, Jalden J, Murphy MJ. On using an adaptive neural network to predict lung tumor motion during respiration for radiotherapy applications. Med Phys. 2005;32:3801–9. doi: 10.1118/1.2134958. [DOI] [PubMed] [Google Scholar]
- Kamino Y, Takayama K, Kokubo M, Narita Y, Hirai E, Kawawda N, Mizowaki T, Nagata Y, Nishidai T, Hiraoka M. Development of a four-dimensional image-guided radiotherapy system with a gimbaled X-ray head. International journal of radiation oncology, biology, physics. 2006;66:271–8. doi: 10.1016/j.ijrobp.2006.04.044. [DOI] [PubMed] [Google Scholar]
- Kanoulas E, Aslam JA, Sharp GC, Berbeco RI, Nishioka S, Shirato H, Jiang SB. Derivation of the tumor position from external respiratory surrogates with periodical updating of the internal/external correlation. Phys Med Biol. 2007;52:5443–56. doi: 10.1088/0031-9155/52/17/023. [DOI] [PubMed] [Google Scholar]
- Keall PJ, Cattell H, Pokhrel D, Dieterich S, Wong KH, Murphy MJ, Vedam SS, Wijesooriya K, Mohan R. Geometric accuracy of a real-time target tracking system with dynamic multileaf collimator tracking system. International journal of radiation oncology, biology, physics. 2006;65:1579–84. doi: 10.1016/j.ijrobp.2006.04.038. [DOI] [PubMed] [Google Scholar]
- Lam K, Hadley S, Balter J. IBreathe: Monitoring respiratorion with the accelerometer in an iPod Touch. Med Phys. 2009;36:2489. (abstract) [Google Scholar]
- Lin T, Li R, Tang X, Dy JG, Jiang SB. Markerless gating for lung cancer radiotherapy based on machine learning techniques. Physics in medicine and biology. 2009;54:1555. doi: 10.1088/0031-9155/54/6/010. [DOI] [PubMed] [Google Scholar]
- Malinowski K, Noel C, Lu W, Lechleiter K, Hubenschmidt J, Low D, Parikh P. Development of the 4D Phantom for patient-specific, end-to-end radiation therapy QA. Phys Med Imag. 2007;6510:65100E–9. [Google Scholar]
- Murphy MJ. Tracking moving organs in real time. Semin Radiat Oncol. 2004;14:91–100. doi: 10.1053/j.semradonc.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Murphy MJ, Balter J, Balter S, BenComo JJA, Das IJ, Jiang SB, Ma CM, Olivera GH, Rodebaugh RF, Ruchala KJ, Shirato H, Yin F-F. The management of imaging dose during image-guided radiotherapy: Report of the AAPM Task Group 75. Medical physics. 2007;34:4041–63. doi: 10.1118/1.2775667. [DOI] [PubMed] [Google Scholar]
- Poulsen PR, Cho B, Ruan D, Sawant A, Keall PJ. Dynamic multileaf collimator tracking of respiratory target motion based on a single kilovoltage imager during arc radiotherapy. International journal of radiation oncology, biology, physics. 2010a;77:600–7. doi: 10.1016/j.ijrobp.2009.08.030. [DOI] [PubMed] [Google Scholar]
- Poulsen PR, Cho B, Sawant A, Keall PJ. Implementation of a new method for dynamic multileaf collimator tracking of prostate motion in arc radiotherapy using a single kV imager. International journal of radiation oncology, biology, physics. 2010b;76:914–23. doi: 10.1016/j.ijrobp.2009.06.073. [DOI] [PubMed] [Google Scholar]
- Poulsen PR, Cho B, Sawant A, Ruan D, Keall PJ. Dynamic MLC tracking of moving targets with a single kV imager for 3D conformal and IMRT treatments. Acta Oncol. 2010c;49:1092–100. doi: 10.3109/0284186X.2010.498438. [DOI] [PubMed] [Google Scholar]
- Ruan D, Fessler JA, Balter JM, Berbeco RI, Nishioka S, Shirato H. Inference of hysteretic respiratory tumor motion from external surrogates: a state augmentation approach. Physics in medicine and biology. 2008;53:2923. doi: 10.1088/0031-9155/53/11/011. [DOI] [PubMed] [Google Scholar]
- Sawant A, Venkat R, Srivastava V, Carlson D, Povzner S, Cattell H, Keall P. Management of three-dimensional intrafraction motion through real-time DMLC tracking. Medical physics. 2008;35:2050–61. doi: 10.1118/1.2905355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweikard A, Glosser G, Bodduluri M, Murphy MJ, Adler JR. Robotic motion compensation for respiratory movement during radiosurgery. Comput Aided Surg. 2000;5:263–77. doi: 10.1002/1097-0150(2000)5:4<263::AID-IGS5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Shirato H, Shimizu S, Kitamura K, Nishioka T, Kagei K, Hashimoto S, Aoyama H, Kunieda T, Shinohara N, Dosaka-Akita H, Miyasaka K. Four-dimensional treatment planning and fluoroscopic real-time tumor tracking radiotherapy for moving tumor. Int J Radiat Oncol Biol Phys. 2000a;48:435–42. doi: 10.1016/s0360-3016(00)00625-8. [DOI] [PubMed] [Google Scholar]
- Shirato H, Shimizu S, Kunieda T, Kitamura K, van Herk M, Kagei K, Nishioka T, Hashimoto S, Fujita K, Aoyama H, Tsuchiya K, Kudo K, Miyasaka K. Physical aspects of a real-time tumor-tracking system for gated radiotherapy. Int J Radiat Oncol Biol Phys. 2000b;48:1187–95. doi: 10.1016/s0360-3016(00)00748-3. [DOI] [PubMed] [Google Scholar]
- Siwiak D, Berger J, Yang Y. Catch Your Breath - musical biofeedback for breathing regulation. New Interfaces for Musical Expression: 9th Internation Conference; Pittsburgh, PA. 2009. [Google Scholar]
- Srivastava V, Keall P, Sawant A, Suh Y. Accurate Prediction of Intra-Fraction Motion Using a Modified Linear Adaptive Filter. Med Phys. 2007;34:2546. [Google Scholar]
- Steenbakkers RJ, Duppen JC, Fitton I, Deurloo KE, Zijp LJ, Comans EF, Uitterhoeve AL, Rodrigus PT, Kramer GW, Bussink J, De Jaeger K, Belderbos JS, Nowak PJ, van Herk M, Rasch CR. Reduction of observer variation using matched CT-PET for lung cancer delineation: a three-dimensional analysis. Int J Radiat Oncol Biol Phys. 2006;64:435–48. doi: 10.1016/j.ijrobp.2005.06.034. [DOI] [PubMed] [Google Scholar]
- Suh Y, Dieterich S, Cho B, Keall PJ. An analysis of thoracic and abdominal tumour motion for stereotactic body radiotherapy patients. Physics in medicine and biology. 2008;53:3623–40. doi: 10.1088/0031-9155/53/13/016. [DOI] [PubMed] [Google Scholar]