Abstract
Aging is considered to be driven by the so called senescence pathways, especially the mTOR route, although there is almost no information on its activity in aged tissues. Aging also induces Ca2+ signal alterations, but information regarding the mechanisms for these changes is almost inexistent. We investigated the possible involvement of the mTOR pathway in the age-dependent changes on Ca2+ stores mobilization in colonic smooth muscle cells of young (4 month old) and aged (24 month old) guinea pigs. mTORC1 activity was enhanced in aged smooth muscle, as revealed by phosphorylation of mTOR and its direct substrates S6K1 and 4E-BP1. Mobilization of intracellular Ca2+ stores through IP3R or RyR channels was impaired in aged cells, and it was facilitated by mTOR and by FKBP12, as indicated by the inhibitory effects of KU0063794 (a direct mTOR inhibitor), rapamycin (a FKBP12-mediated mTOR inhibitor) and FK506 (an FKBP12 binding immunosuppressant). Aging suppressed the facilitation of the Ca2+ mobilization by FKBP12 but not by mTOR, without changing the total expression of FKBP12 protein. In conclusion, or study shows that in smooth muscle aging enhances the constitutive activity of mTORC1 pathway and impairs Ca2+ stores mobilization by suppression of the FKBP12-induced facilitation of Ca2+ release.
Keywords: mTOR, Ca2+ signal, FKBP12, colon, smooth muscle
INTRODUCTION
Aging can be viewed as a quasi-programmed process driven by changes in signaling pathways, among which the mTOR pathway stands out as a key factor [1; 2]. This kinase forms part of a energy-sensing signaling pathway and can assemble two types of heteromeres, mTORC1 and mTORC2, the former involved in aging based on the lifespan-extending effects of the mTOR inhibitor rapamycin (reviewed in [3]). mTORC1 is activated by nutrients, insulin and the PI3K/Akt pathway, and can be inhibited by nutrients deprivation, AMPK activation [4] and FKBP12 [5; 6], the target for the immunosuppressant rapamycin. mTOR has been proposed to convert arrest to senescence (geroconversion) [7; 8]. In spite of this, there is very little information regarding the mTOR pathway status in aged tissues [9; 10].
One of the functional consequences of aging is the alteration of different aspects of calcium signals, which play a key role in multiple cellular functions, from contraction or secretion to gene regulation and cell fate and proliferation. Therefore, these changes are the basis for important alterations linked to aging [11-15]. Calcium signals consist of cytosolic Ca2+ concentration ([Ca2+]i) increases due either to activation of Ca2+ entry from extracellular space (through Ca2+ channels activated by voltage, Ca2+ stores depletion or receptors), or to Ca2+ release from intracellular stores. Mobilization of intracellular stores is mediated by IP3R and RyR channels, activated respectively by the intracellular messengers IP3 and cADPribose. The subsequent [Ca2+]i decrease to resting levels is operated by active extrusion to extracellular medium or reuptake into intracellular organelles.
Most of available evidence on the effects of aging on [Ca2+]i has focused on Ca2+ clearing mechanisms [15-17] and influx of extracellular Ca2+ [15; 18; 19], but it is rather limited and controversial for Ca2+ release. In neurons, the most studied model for age-related changes in Ca2+ signals, there is evidence that aging enhances Ca2+ release from intracellular stores through RyR and IP3R calcium channels [13], similar to reports in cardiomyocytes [20]. In aged smooth muscle cells both inhibition [21] and enhancement [22; 23] have been reported.
Regarding the mechanisms underlying the effects of age on [Ca2+]i signals, knowledge is rather superficial, most of the studies addressing only a description of the signal alterations. Some authors have proposed that mitochondrial modifications or redox imbalance are the origin for Ca2+ signal disfunction [14; 24]. Interactions between some mTOR pathway elements and Ca2+ release imply that the mTOR pathway could be involved in the age-related [Ca2+]i modifications: Ca2+ release from intracellular stores can be modulated by mTOR kinase [25-28], and its inhibitor FKBP12 is a known modulator of Ca2+ release channels [29]. More explicit, a recent study hypothesizes that in aging neurons down-regulation of FKBP12 activates mTOR and enhances RyR mediated Ca2+ release [5], explaining the Ca2+ signal phenotype typical of aged neurons [12].
The aim of this study was to investigate the involvement of mTOR and FKBP12 in the age-induced changes in Ca2+ release in smooth muscle cells. Our data indicate that aging reduces the intracellular Ca2+ release, an effect accompanied by loss of a facilitatory effect of FKBP12 on Ca2+ mobilization which was independent on the expression of this protein. mTOR facilitated Ca2+ release both in young and aged cells and showed enhanced activity in aged cells.
RESULTS
Effect of aging on mTOR pathway activation
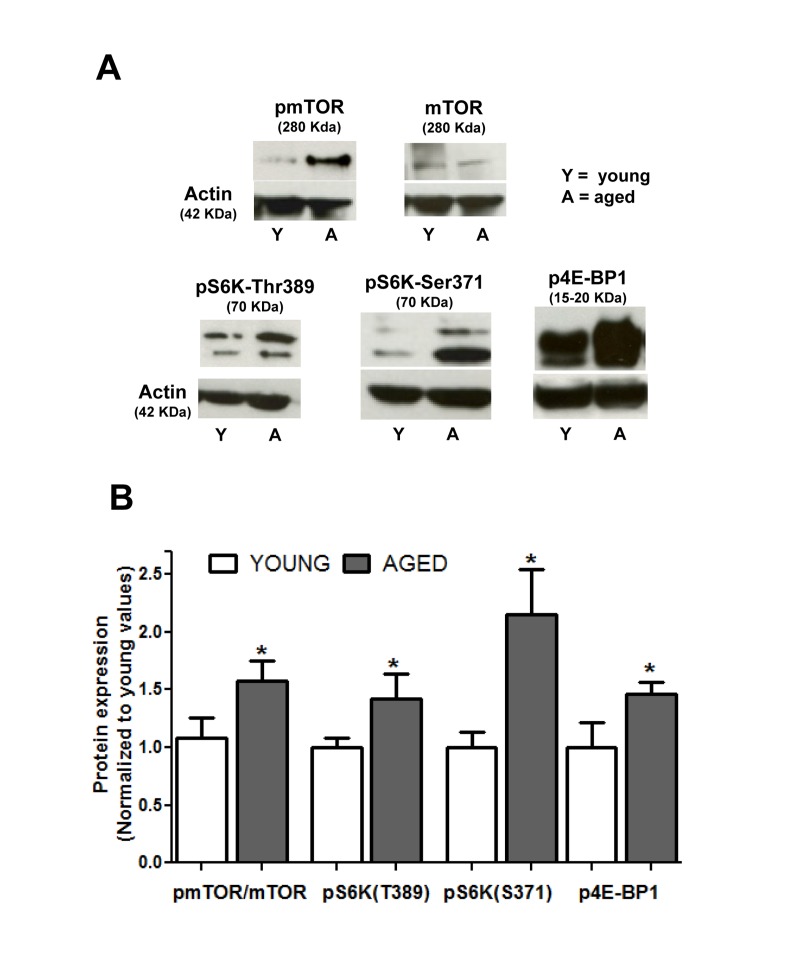
Since changes in the mTOR route have been proposed to be associated to the aging process [30], we investigated the constitutive activation of this pathway in the non stimulated smooth muscle layer of guinea pig colon. Activation of mTORC1 can be reliably determined by phosphorylation of Ser 2448 [31]. Figure 1 shows that Ser2448 phosphorylation was significantly enhanced in the aged colon muscle layer compared to the young colon (p < 0.05). To confirm that mTOR pathway was enhanced in aged muscle, we determined the level of phosphorylation of two direct substrates of mTOR, p70S6K1 and 4E-BP1, which are respectively activated and inhibited upon phosphorylation by mTORC1 kinase activity. We found that aged muscle showed higher levels of the phosphorylated forms of both 4E-BP1 and p70S6K1, the latter including phosphorylation of two different residues specific for mTOR kinase activity [32] (Fig. 1).
Figure 1. Aging enhances the constitutive activity of the mTOR pathway in colon smooth muscle.
(A) Representative immunoblotts showing expression of mTOR and phosphorylated (active) forms of mTOR, p70S6K1 (at Thr389 and Ser371) and 4E-BP1 in non stimulated muscular layer of colon. Figures in parenthesis are the molecular weight of each band as judged from the molecular weight marker run in each assay. (B) Histogram depicting phosphorylation of mTOR and its substrates in young and aged colon. Values are average ± sem of arbitrary units normalized to mean average values of young colon. * p< 0.05, n = 6.
Modification of Ca2+ signals in aged smooth muscle cells
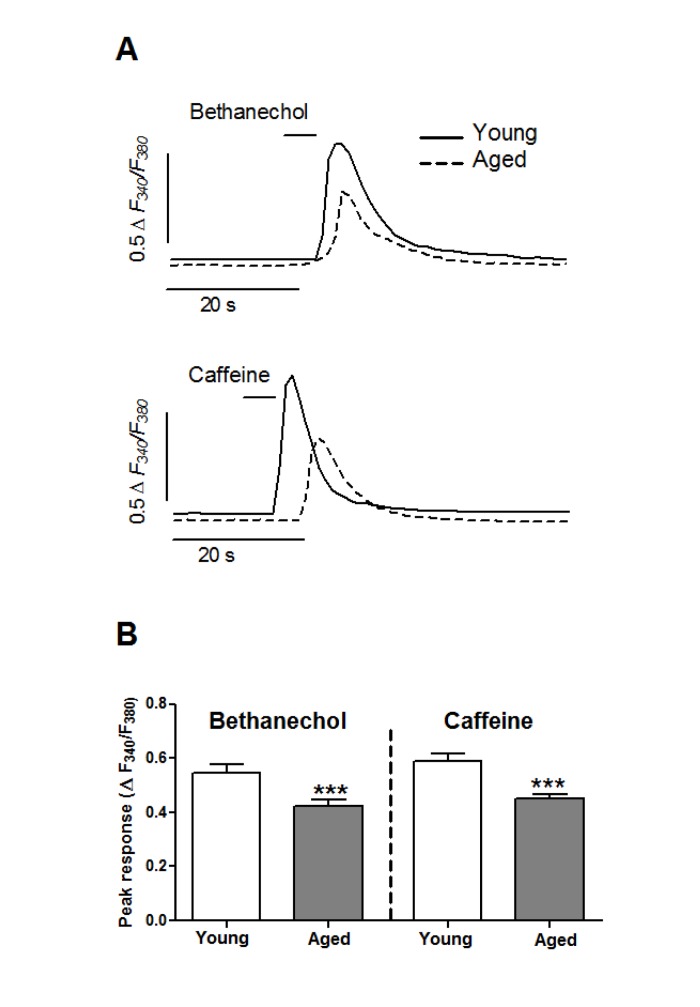
To study the effects of aging in the mobilization of Ca2+ from intracellular stores we challenged colon smooth muscle cells with bethanechol, known to release Ca2+ through IP3R in this cell type [33; 34], or with caffeine, which releases Ca2+ through RyR [35]. [Ca2+]i signals induced by mobilization of intracellular Ca2+ pools in smooth muscle cells are characterized by an initial [Ca2+]i peak followed by gradual decline towards pre-stimulus levels, as shown in Figure 2. Bethanechol caused a transient [Ca2+]i increase which was smaller in aged (0.418 ± 0.024 ΔF340/F380, n =1 88) than in young cells (0.546 ±0 .028 ΔF340/F380, n = 117, p <0.005). Compared to bethanechol, the effect of caffeine in young cells was also transient but larger in amplitude (0.588 ± 0.027 ΔF340/F380, n = 165, p < 0.05), and showed a similar decrease in aged cells (0.450 ± 0.018 ΔF340/F380, n = 286, p <0.005).
Figure 2. Aging inhibits the mobilization of Ca2+ stores in colonic smooth muscle cells.
(A) Isolated cells were challenged with a short pulse of bethanechol (0.1 mM) or caffeine (10 mM) to release Ca2+ from intracellular pools through IP3R and RyR channels, respectively. Traces are representative of average responses in young and aged cells. (B) Average ± sem response (ΔF340/F380) from young (8 animals, 117 and 165 cells) and aged (6 animals, 188 and 286 cells) guinea pigs.
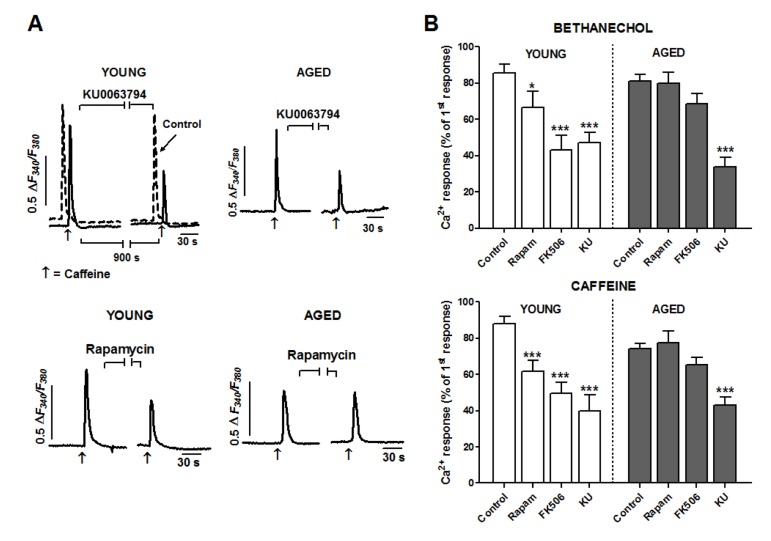
To assess a possible role of mTOR in Ca2+ pools mobilization, we used a double pulse protocol: either bethanechol or caffeine was applied twice separated by a 15 minutes interval to allow for recovery of the response and acute application of inhibitors (see Fig. 3). In control experiments the amplitude of the second stimulus was close to 90 % respect to the first one in young cells (bethanechol: 85.60 ± 4.82 % respect to the first pulse, n = 54; caffeine: 88.10 ± 4.16 %, n = 74), and close to 80% in aged cells (bethanechol: 81.13 ± 3.59 %, n = 89; caffeine: 74.24 ± 2.87 %, n= 130) (Fig. 3).
Figure 3. Aging reduces facilitation of Ca2+ release by FKBP12 but not by mTOR kinase in smooth muscle cells.
(A) Cells were stimulated with two pulses of caffeine (10 μM separated by a 15 min interval to apply inhibitors (indicated by horizontal lines) or normal medium (control trace). For the sake of comparison, the control trace in top left panel is shifted to the left. Traces are representative of the effects of KU0063794 (5 μM) or rapamycin (5 μM) on young (left column) or aged cells (right column). (B) Histogram summarizing the effects of the three inhibitors (KU: KU0063794, 5 μM; rapamycin 5 μM; FK506 10 μM) on the bethanechol (0.1 mM) and caffeine (10 mM) evoked Ca2+ responses in young and aged cells. Two-ways ANOVA showed significant effect for treatment (bethanechol: F = 22.4, p < 0.005; caffeine: F = 21.9, p < 0.005), which was modified by age (bethanechol: F = 4.0, p < 0.01; caffeine: F = 5.0, p < 0.01). Asterisks denote significance for planned comparisons between groups of interest. * p<0.05, ** p< 0.01, *** p< 0.005. n= 17-130 cells from 6 (aged) or 8 (young) animals.
To inhibit mTOR we used two different types of compounds: rapamycin, a frequently used mTOR inhibitor which binds to FKBP12 protein to inhibit mTOR kinase, and KU0063794, a second generation inhibitor acting directly at the kinase domain of mTOR. When applied to cells from young guinea pigs, KU0063794 (5 μM) significantly decreased (p < 0.005) the responses to bethanechol and to caffeine (by around 50% each, see Fig. 3). This inhibition was also present in aged cells, indicating that mTOR kinase activity has a facilitating role on IP3R- and RyR-mediated Ca2+ release and that this facilitation is not affected by aging. In the case of rapamycin (5 μM), its inhibitory effect in young cells was qualitatively similar to that of KU0063794 though smaller in amplitude (around 23 and 30% for bethanechol and caffeine, respectively; Fig. 3). On the contrary, in aged cells the inhibition was strongly attenuated or even suppressed, suggesting that the mechanisms of action of rapamycin on the Ca2+ mobilization are altered in aged cells.
To explore the differential influence of age on KU0063794 and rapamycin effects on Ca2+ mobilization, we treated colon muscle cells with the compound FK506, which binds FKBP12 without inhibiting mTOR activity. In young cells FK506 (10 μM) reduced clearly (p < 0.005) the responses to bethanechol and caffeine by approximately 50 and 60% respectively (Fig. 3). Similar to rapamycin, FK506 failed to inhibit significantly the responses in aged cells. Therefore, our data strongly suggest that aging alters the FKBP12-mediated facilitation of calcium signal, but not the facilitation induced by mTOR.
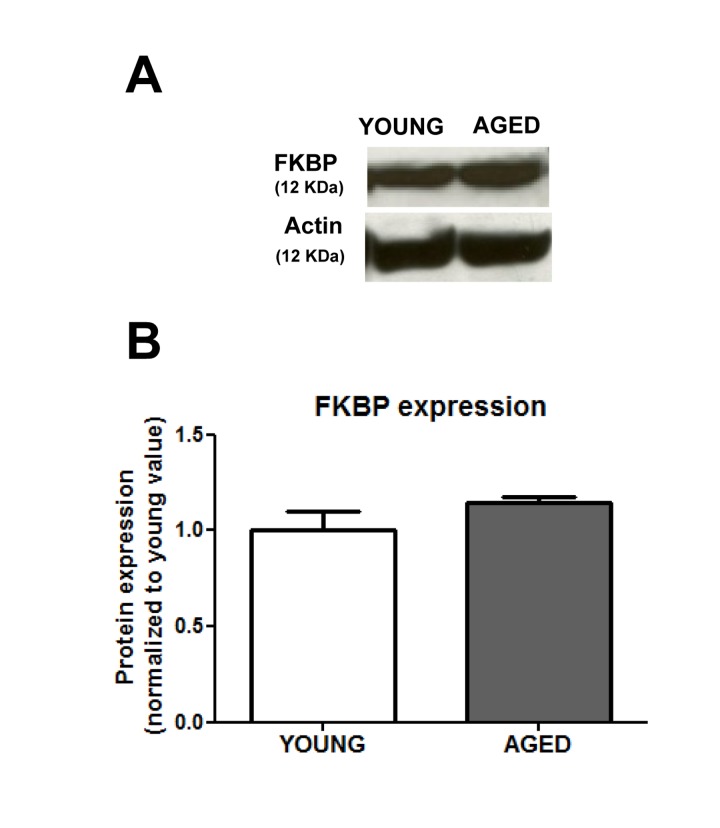
In view of our data, and given that a recent report shows an age-related decrease in FKBP12 expression [5], we explored a possible down-regulation of FKBP12 expression in the colonic smooth muscle layer of aged guinea pigs. Figure 4 shows, however, that in the aged colon there is a slight and non-significant (p < 0.09) increase in FKBP12 rather than a decrease in the expression of this protein.
Figure 4. Aging does not change significantly the expression of FKBP12 protein.
(A) Representative immunoblotts and (B) average ± sem values (arbitrary units) (n=6) of the FKBP12 expression in muscular layer of colon from young and aged guinea pigs.
DISCUSSION
The present study shows that in colonic smooth muscle aging enhances the activity of the mTOR pathway and inhibits the mobilization of Ca2+ stores, an effect likely associated to the alteration of the facilitating effect of FKBP12 protein on Ca2+ mobilization.
Aging is considered a process regulated by longevity pathways among other factors. The mTOR route is an energy-sensing system whose inhibition with rapamycin prolongs lifespan in a number of models, resembling the effect of caloric restriction [3], hitherto the most successful intervention to extend lifespan. We describe here that in resting conditions aged smooth muscle cells show a significant increase in the expression and phosphorylation of mTOR, and in the phosphorylation of its targets p70S6K1 and 4B-EP1. To our knowledge, the only precedent of age-induced mTOR constitutive activity are hippocampal neurons in mice [9] and an enhancement of pS6 phosphorylation in male mice heart compared to females [10]. In human heart no effect of age has been reported [36]. Our findings are in keeping with a previous report showing increased mTOR pathway responses in senescent cultured cells [30]. Data from gene expression studies are conflicting. Rat hippocampus shows enhanced mTOR gene expression ([37], their supplementary data), but recent array studies on the expression of genes linked to the mTOR pathway in blood samples from human cohorts suggest that aging down-regulates this route [38], although this report found no decrease in mTOR gene expression for and Protor1, a protein forming mTOR complexes, was actually up-regulated. Another study in a cohort of long-lived nonagenarians [39] reported that increased longevity was associated to slightly reduced expression of mTOR gene, but the pattern of the gene expression for the mTOR complex proteins was unclear: for mTORC1 complex, protein Raptor was impaired but PRAS40 was enhanced, and for mTORC2 complexes only Protor2 was enhanced. Whether these discrepancies are due to methodological or to tissue-specific differences (see [9]) is unclear and need further investigation. In any case, our findings support the theory that aging is a quasi-programmed process driven by signaling routes [1]: enhanced mTOR levels would drive and accompany aging, so that the more long-lived individuals display lower levels [10, 38]. As inhibition of mTOR had the same influence in aged and young cells, it is unlikely, however, that our finding of an age-mediated decrease of Ca2+ release is due to the activation of mTOR pathway.
Given that Ca2+ signals are a key regulator in multiple cellular functions, age-related changes in this parameter are relevant for the aging process [15]. For example, age-related changes in Ca2+ influx [18; 19; 40] could reduce Ca2+ content of the stores, a condition known to alter several cellular functions [41], and can change gene expression [42; 43]. Although in striated muscle the effects of aging on Ca2+ signals seem to be relatively settled (enhanced Ca2+ sparks and impaired Ca2+ release upon stimulation; reviewed in [44]), reports in smooth muscle are very limited and somewhat conflicting. Initial contractility studies proposed enhanced release from intracellular stores in colon and arterial strips [22; 23] but not in gallbladder [45]. Results from [Ca2+]i determination in isolated cells showed that aging decreases intracellular Ca2+ release in arterial and detrusor muscle cells [21; 46] but not in gallbladder cells [18]. We report here that in colonic smooth muscle cells aging impairs IP3R-and RyR-mediated Ca2+ release, suggesting that age influences this signaling system in a tissue-specific way.
At the moment there is very little information regarding the mechanisms causing age-related changes in Ca2+ signals. Because its facilitator role in Ca2+ release through IP3R and RyR channels [25; 27-29], modifications of mTOR and FKBP12 function are plausible candidates to mediate the age-induced Ca2+ signal changes. While there is no information regarding the mechanism of action of mTOR on Ca2+ release, the complex effects of FKBP12 are due to direct interaction with the channels and/or to inhibition of calcineurin, a known regulator of Ca2+ release. In fact, we describe here that both IP3 and RyR signals are impaired by inhibitors targeted to mTOR kinase (KU0063794), to FKBP12 (FK506) or to both (rapamycin, that binds FKBP12 to a regulatory domain of mTOR kinase). Our results agree with a previous work in colon myocytes for IP3-mediated signals [28], but are opposite for RyR-mediated signal [35]. The discrepancy could be due to differences in the age of the animals (we use 5 months old, more mature than other reports [35]) or to the experimental design (we use single pulses of stimulation instead of choosing cells responding to repetitive stimulation).
The finding that aging does reduce only the effect of the FKBP12-targeted inhibitors rapamycyin and FK506, indicates for the first time that aging could impair Ca2+ signals by alteration of the facilitation of Ca2+ release by FKBP12. On the contrary, the enhanced activity of mTOR and its facilitating effect on the Ca2+ signal in aged cells rules out an involvement of mTOR in the impairment of the Ca2+ mobilization. Although a recent report in hippocampus [37] describes that age-related Ca2+ deregulation is due to down-regulation of FKBP12, which operates there as an inhibitor of RyR-mediated Ca2+ release [5], we found that FKBP12 expression was not decreased in aged cells. Assuming that FKBP12 interaction to mTOR was normal in aged cells one would expect an inhibition of the signal comparable to that of KU0063794, which was not the case. It his therefore likely that aging simply alters the functional association of FKBP12 with Ca2+ release channels and even with mTOR activity. The differences in the effects of experimental manipulations of the mTOR pathway could be due to cell-specific changes in the regulation of aging. For example, in aged hypothalamus only some neuronal populations show enhanced mTOR activity in aged rodents [9]. The exact link between changes in FKBP12 expression and functional facilitation of Ca2+ release, out of the scope of the present study, deserves further investigation and could be related to the constitutive activation of mTOR pathway in aged cells.
In conclusion, this study shows that in smooth muscle the mTOR kinase activity is increased by aging, but although mTOR facilitates Ca2+ mobilization, aged cells present lower responses to agonists as the result of the loss of FKBP12-induced facilitation of Ca2+ release.
METHODS
Animals and cell isolation
Female guinea pigs (Dunkin-Hartley), housed in light (12 h light-dark cycle) and temperature (20°C) controlled conditions and with ad libitum access to water and food, were divided into two groups according to age: young adults (5 months old, average weight 1048.3 ± 82.7 g) and aged (28 month old, average weight 912.9 ± 22.6 g). The experiments were performed according to European guidelines for animal research and approved by the Animal Ethics Committees of the University of Extremadura.
Approximately 20 mg of the circular and longitudinal smooth muscle layer of the colon was cut into small pieces and incubated for 35 min at 37°C in enzyme solution (ES, for composition see Solutions and drugs) supplemented with 1 mg/mL BSA, 1 mg/mL papain and 1 mg/mL dithioerythritol. The tissue was then transferred to fresh ES containing 1 mg/mL BSA, 1 mg/mL collagenase and 100 μm CaCl2 and incubated for 10 min at 37°C. After washing with cold ES, single smooth muscle cells were mechanically isolated using a fire-polished pipette. Cell suspensions were kept in ES at 4°C until use, generally within 6 h. Cell viability (90%), as assessed by trypan blue staining was the same in all groups of animals.
Cell loading and [Ca2+]i determination
[Ca2+]i was determined by epifluorescence microscopy (Eclipse TE2000-S; Nikon, Melville, NY, USA) at room temperature using the ratiometric Ca2+ indicator fura-2. Isolated cells were loaded with 4 μM fura 2-AM at room temperature for 15 min. After loading, cells were perfused with Na+-HEPES solution in the absence or presence of the experimental agents. Cells were illuminated with a monochromator (Optoscan; Cairn Research, Faversham, UK) at 340-380 nm 1 Hz cycles, and the emitted fluorescence was captured with a digital camera (ORCAII-ERG; Hamamatsu Spain, Barcelona, Spain) and recorded using dedicated software (Metafluor; Universal Imaging, Molecular Devices, Downingtown, PA, USA). After background subtraction, fluorescence ratio (F340/F380) was calculated pixel by pixel and used to estimate the changes in [Ca2+]i. A calibration of the ratio for [Ca2+]i was not performed in view of the many uncertainties related to the binding properties of fura 2 with Ca2+ inside of smooth muscle cells.
Analysis of protein expression and phosphorylation by Western blot
Small pieces (~2 mg of dry weight) were quickly frozen, pulverized in liquid nitrogen, extracted in lysis buffer (for composition see Solutions and drugs) and then sonicated for 5 s. Lysates were centrifuged at 10000 g for 15 min at 4°C to remove nuclei and intact cells and the protein concentration was measured. Protein extracts (30 μg) were heat-denatured at 95°C for 5 min with DTT, electrophoresed on 7.5% and 15% polyacrylamide-SDS gels and then transferred to nitrocellulose membranes. Membranes were blocked for 1 h at room temperature using 10% bovine serum albumin (BSA) and incubated overnight at 4°C with affinity-purified polyclonal antibodies against mTOR, phospho-mTOR (Ser2448), phospho-4E-BP1, phospho-p70 S6 kinase (Ser371 and Thr389) (Cat#9862, Cell Signaling, Boston, MA, USA) and FKBP12 (#PA1-026A, Thermo Scientific, MA, USA).
A mouse anti-actin antibody (A2066, Sigma-Aldrich) was used to control for protein loading and to normalize expression of proteins of interest. After washing, the membranes were incubated for 1 h at room temperature with anti-mouse (1:10000; Amersham Biosciences, Bucks, UK) or anti-rabbit (1:7000; Santa Cruz Biotechnology) IgG-horseradish peroxidase conjugated secondary antibodies.
Bands were visualized using the supersignal west pico chemiluminescent substrate (Pierce, Rockford, IL, USA), quantified using the software gel-pro analyzer (4.0, Media Cybernetics, Bethesda, MD, USA) and normalized to α-tubulin content. For comparison purposes between young and aged samples, all the α-tubulin-corrected values were normalized to the average of young samples of the same assay.
In mTOR phosphorylation assay, two similar gels were run and one membrane was incubated with the antibody against the total protein and the other with an antiphospho-protein of interest.
Solutions and drugs
Na+-HEPES solution (in mM): 10 HEPES, 140 NaCl, 4.7 KCl, 2 CaCl2, 2 MgCl2 and 10 D-glucose (pH 7.3). The Ca2+ -free Na+-HEPES solution included EGTA (1 mm) instead of CaCl2. ES (in mM): 10 HEPES, 55 NaCl, 5.6 KCl, 80 sodium glutamate, 2 MgCl2 and 10 D-glucose (pH 7.3). Lysis buffer (in mM): Tris-HCl 40, NaCl 400, 0.2% SDS and 10% glycerol supplemented with protease and phosphatase inhibitors (4-(2-aminoethyl) benzenesulfonyl fluoride, E-64, bestatin, leupeptin, aprotinin and sodium vanadate).
Drugs and chemicals were obtained from the following sources: bethanechol, caffeine and phosphatase inhbitors from Sigma-Aldrich (Madrid, Spain); fura 2-AM from Molecular Probes (Life Technologies, Madrid, Spain), rapamycin and KU-63794 from Calbiochem (VWR, Madrid, Spain), FK-506 from Cayman (VWR, Madrid, Spain), collagenase from Fluka (Madrid, Spain) and papain from Worthington Biochemical (Lakewood, NJ,USA).
Quantification and statistics
Results are expressed as means ± standard error of the mean (sem) of n cells or blots. [Ca2+]i responses are expressed as increases in the ratio of fura-2 fluorescence (ΔF340/F380). To compare two groups, paired t test was used to assess the effect of treatment. The effects of age and experimental treatments were tested using a two-way analysis of variance, followed by planned comparisons between selected groups. Differences were considered significant at p < 0.05.
Acknowledgments
Supported by BFU2011-24365, RETICEF RD12/0043/ 0016, FEDER and Junta de Extremadura (GR10009).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- Blagosklonny MV. Answering the ultimate question “What is the Proximal Cause of Aging?”. Aging (Albany. NY) 2012;4:899–916. doi: 10.18632/aging.100525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. Aging: ROS or TOR. Cell Cycle. 2008;7:3344–3354. doi: 10.4161/cc.7.21.6965. [DOI] [PubMed] [Google Scholar]
- Camello PJ, Camello-Almaraz C, Pozo MJ. Pharmacological approaches to improve ageing. Pharmacology. Gallelli L, editor. InTech. 2012:257–282. [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling at a glance. J. Cell Sci. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gant JC, Chen KC, Norris CM, Kadish I, Thibault O, Blalock EM, Porter NM, Landfield PW. Disrupting function of FK506-binding protein 1b/12.6 induces the Ca(2)+-dysregulation aging phenotype in hippocampal neurons. J. Neurosci. 2011;31:1693–1703. doi: 10.1523/JNEUROSCI.4805-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffer CA, Tang W, Wong H, Santillan A, Patterson RJ, Martinez LA, Tejada-Simon MV, Paylor R, Hamilton SL, Klann E. Removal of FKBP12 enhances mTOR-Raptor interactions, LTP, memory, and perseverative/repetitive behavior. Neuron. 2008;60:832–845. doi: 10.1016/j.neuron.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontieva OV, Blagosklonny MV. DNA damaging agents and p53 do not cause senescence in quiescent cells, while consecutive re-activation of mTOR is associated with conversion to senescence. Aging (Albany. NY) 2010;2:924–935. doi: 10.18632/aging.100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. Cell cycle arrest is not yet senescence, which is not just cell cycle arrest: terminology for TOR-driven aging. Aging (Albany. NY) 2012;4:159–165. doi: 10.18632/aging.100443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SB, Tien AC, Boddupalli G, Xu AW, Jan YN, Jan LY. Rapamycin ameliorates age-dependent obesity associated with increased mTOR signaling in hypothalamic POMC neurons. Neuron. 2012;75:425–436. doi: 10.1016/j.neuron.2012.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontieva OV, Paszkiewicz GM, Blagosklonny MV. Mechanistic or mammalian target of rapamycin (mTOR) may determine robustness in young male mice at the cost of accelerated aging. Aging (Albany. NY) 2012;4:899–916. doi: 10.18632/aging.100528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toescu EC, Verkhratsky A. Parameters of calcium homeostasis in normal neuronal ageing. J. Anat. 2000;197(Pt 4):563–569. doi: 10.1046/j.1469-7580.2000.19740563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gant JC, Sama MM, Landfield PW, Thibault O. Early and simultaneous emergence of multiple hippocampal biomarkers of aging is mediated by Ca2+-induced Ca2+ release. J. Neurosci. 2006;26:3482–3490. doi: 10.1523/JNEUROSCI.4171-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault O, Gant JC, Landfield PW. Expansion of the calcium hypothesis of brain aging and Alzheimer's disease: minding the store. Aging Cell. 2007;6:307–317. doi: 10.1111/j.1474-9726.2007.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toescu EC, Verkhratsky A. Ca2+ and mitochondria as substrates for deficits in synaptic plasticity in normal brain ageing. J. Cell Mol. Med. 2004;8:181–190. doi: 10.1111/j.1582-4934.2004.tb00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toescu EC, Vreugdenhil M. Calcium and normal brain ageing. Cell Calcium. 2010;47:158–164. doi: 10.1016/j.ceca.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Martinez-Serrano A, Blanco P, Satrustegui J. Calcium binding to the cytosol and calcium extrusion mechanisms in intact synaptosomes and their alterations with aging. J. Biol. Chem. 1992;267:4672–4679. [PubMed] [Google Scholar]
- Gomez-Pinilla PJ, Pozo MJ, Baba A, Matsuda T, Camello PJ. Ca2+ extrusion in aged smooth muscle cells. Biochem. Pharmacol. 2007;74:860–869. doi: 10.1016/j.bcp.2007.06.037. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla PJ, Camello-Almaraz C, Moreno R, Camello PJ, Pozo MJ. Melatonin treatment reverts age-related changes in Guinea pig gallbladder neuromuscular transmission and contractility. J. Pharmacol. Exp. Ther. 2006;319:847–856. doi: 10.1124/jpet.106.109256. [DOI] [PubMed] [Google Scholar]
- Xiong Z, Sperelakis N, Noffsinger A, Fenoglio-Preiser C. Changes in calcium channel current densities in rat colonic smooth muscle cells during development and aging. Am. J. Physiol. 1993;265:C617–C625. doi: 10.1152/ajpcell.1993.265.3.C617. [DOI] [PubMed] [Google Scholar]
- Howlett SE, Grandy SA, Ferrier GR. Calcium spark properties in ventricular myocytes are altered in aged mice. Am. J. Physiol Heart Circ. Physiol. 2006;290:H1566–H1574. doi: 10.1152/ajpheart.00686.2005. [DOI] [PubMed] [Google Scholar]
- Del CC, Ostrovskaya O, McAllister CE, Murray K, Hatton WJ, Gurney AM, Spencer NJ, Wilson SM. Effects of aging on Ca2+ signaling in murine mesenteric arterial smooth muscle cells. Mech. Ageing Dev. 2006;127:315–323. doi: 10.1016/j.mad.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Rubio C, Moreno A, Briones A, Ivorra MD, D'Ocon P, Vila E. Alterations by age of calcium handling in rat resistance arteries. J. Cardiovasc. Pharmacol. 2002;40:832–840. doi: 10.1097/00005344-200212000-00004. [DOI] [PubMed] [Google Scholar]
- Lopes GS, Ferreira AT, Oshiro ME, Vladimirova I, Jurkiewicz NH, Jurkiewicz A, Smaili SS. Aging-related changes of intracellular Ca2+ stores and contractile response of intestinal smooth muscle. Exp. Gerontol. 2006;41:55–62. doi: 10.1016/j.exger.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Xiong J, Camello PJ, Verkhratsky A, Toescu EC. Mitochondrial polarisation status and [Ca2+]i signalling in rat cerebellar granule neurones aged in vitro. Neurobiol. Aging. 2004;25:349–359. doi: 10.1016/S0197-4580(03)00123-4. [DOI] [PubMed] [Google Scholar]
- Fregeau MO, Regimbald-Dumas Y, Guillemette G. Positive regulation of inositol 1,4,5-trisphosphate-induced Ca2+ release by mammalian target of rapamycin (mTOR) in RINm5F cells. J. Cell Biochem. 2011;112:723–733. doi: 10.1002/jcb.23006. [DOI] [PubMed] [Google Scholar]
- Dargan SL, Lea EJ, Dawson AP. Modulation of type-1 Ins(1,4,5)P3 receptor channels by the FK506-binding protein, FKBP12. Biochem. J. 2002;361:401–407. doi: 10.1042/0264-6021:3610401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan D, McCarron JG. Regulation by FK506 and rapamycin of Ca2+ release from the sarcoplasmic reticulum in vascular smooth muscle: the role of FK506 binding proteins and mTOR. Br. J. Pharmacol. 2009;158:1112–1120. doi: 10.1111/j.1476-5381.2009.00369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan D, Currie S, Bradley KN, Muir TC, McCarron JG. In smooth muscle, FK506-binding protein modulates IP3 receptor-evoked Ca2+ release by mTOR and calcineurin. J. Cell Sci. 2005;118:5443–5451. doi: 10.1242/jcs.02657. [DOI] [PubMed] [Google Scholar]
- Macmillan D. FK506 binding proteins: Cellular regulators of intracellular Ca(2+) signalling. Eur J. Pharmacol. 2013;700:181–193. doi: 10.1016/j.ejphar.2012.12.029. [DOI] [PubMed] [Google Scholar]
- Chen Y, Wang J, Cai J, Sternberg P. Altered mTOR signaling in senescent retinal pigment epithelium. Invest Ophthalmol. Vis. Sci. 2010;51:5314–5319. doi: 10.1167/iovs.10-5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp J, Manning G, Hunter T. TORC-specific phosphorylation of mammalian target of rapamycin (mTOR): phospho-Ser2481 is a marker for intact mTOR signaling complex 2. Cancer Res. 2009;69:1821–1827. doi: 10.1158/0008-5472.CAN-08-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh M, Pullen N, Brennan P, Cantrell D, Dennis PB, Thomas G. Regulation of an activated S6 kinase 1 variant reveals a novel mammalian target of rapamycin phosphorylation site. J. Biol. Chem. 2002;277:20104–20112. doi: 10.1074/jbc.M201745200. [DOI] [PubMed] [Google Scholar]
- Macmillan D, Chalmers S, Muir TC, McCarron JG. IP3-mediated Ca2+ increases do not involve the ryanodine receptor, but ryanodine receptor antagonists reduce IP3-mediated Ca2+ increases in guinea-pig colonic smooth muscle cells. J. Physiol. 2005;569:533–544. doi: 10.1113/jphysiol.2005.096529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LB, Buxton IL. Muscarinic receptors in canine colonic circular smooth muscle. II. Signal transduction pathways. Mol. Pharmacol. 1991;40:952–959. [PubMed] [Google Scholar]
- Macmillan D, Currie S, McCarron JG. FK506-binding protein (FKBP12) regulates ryanodine receptor-evoked Ca2+ release in colonic but not aortic smooth muscle. Cell Calcium. 2008;43:539–549. doi: 10.1016/j.ceca.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Niemann B, Pan R, Teschner M, Boening A, Silber RE, Rohrbach S. Age and obesity-associated changes in the expression and activation of components of the AMPK signaling pathway in human right atrial tissue. Exp. Gerontol. 2013;48:55–63. doi: 10.1016/j.exger.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Kadish I, Thibault O, Blalock EM, Chen KC, Gant JC, Porter NM, Landfield PW. Hippocampal and cognitive aging across the lifespan: a bioenergetic shift precedes and increased cholesterol trafficking parallels memory impairment. J. Neurosci. 2009;29:1805–1816. doi: 10.1523/JNEUROSCI.4599-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harries LW, Fellows AD, Pilling LC, Hernandez D, Singleton A, Bandinelli S, Guralnik J, Powell J, Ferrucci L, Melzer D. Advancing age is associated with gene expression changes resembling mTOR inhibition: evidence from two human populations. Mech. Ageing Dev. 2012;133:556–562. doi: 10.1016/j.mad.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passtoors WM, Beekman M, Deelen J, van der BR, Maier AB, Guigas B, Derhovanessian E, van HD, de Craen AJ, Gunn DA, Pawelec G, Slagboom PE. Gene expression analysis of mTOR pathway: association with human longevity. Aging Cell. 2013;12:24–31. doi: 10.1111/acel.12015. [DOI] [PubMed] [Google Scholar]
- Xiong Z, Sperelakis N, Noffsinger A, Fenoglio-Preiser C. Ca2+ currents in human colonic smooth muscle cells. Am. J. Physiol. 1995;269:G378–G385. doi: 10.1152/ajpgi.1995.269.3.G378. [DOI] [PubMed] [Google Scholar]
- Mekahli D, Bultynck G, Parys JB, De SH, Missiaen L. Endoplasmic-reticulum calcium depletion and disease. Cold Spring Harb. Perspect. Biol. 2011;3 doi: 10.1101/cshperspect.a004317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuribara M, Eijsink VD, Roubos EW, Jenks BG, Scheenen WJ. BDNF stimulates Ca2+ oscillation frequency in melanotrope cells of Xenopus laevis: contribution of IP3-receptor-mediated release of intracellular Ca2+ to gene expression. Gen. Comp Endocrinol. 2010;169:123–129. doi: 10.1016/j.ygcen.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Morales S, Diez A, Puyet A, Camello P, Camello-Almaraz C, Bautista J, Pozo MJ. Calcium controls smooth muscle TRPC gene transcription via the CAMK/calcineurin-dependent pathways. Am. J. Physiol Cell Physiol. 2006 doi: 10.1152/ajpcell.00096.2006. [DOI] [PubMed] [Google Scholar]
- Weisleder N, Ma J. Altered Ca2+ sparks in aging skeletal and cardiac muscle. Ageing Res. Rev. 2008;7:177–188. doi: 10.1016/j.arr.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka J, Murakami M, Nichols GA, Cooper CW, Greeley GH, Jr., Thompson JC. Age-related changes in gallbladder contractility and cytoplasmic Ca2+ concentration in the guinea pig. Am. J. Physiol. 1993;264:G624–G629. doi: 10.1152/ajpgi.1993.264.4.G624. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla PJ, Pozo MJ, Camello PJ. Aging differentially modifies agonist-evoked mouse detrusor contraction and calcium signals. Age (Dordr.) 2011;33:81–88. doi: 10.1007/s11357-010-9163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]