Myeloid-derived suppressor cells are increased with age and elevated in donors with a history of cancer; an age-related effect has never been shown in humans.
Keywords: aging, elderly, inflammaging, mortality
Abstract
As we age, the composition of our peripheral leukocytes changes dramatically. Many of these alterations contribute to the general immune dysfunction that burdens the elderly, which in turn, contributes to increased susceptibility to disease. MDSCs represent a heterogeneous population of immunosuppressive leukocytes that are elevated in the peripheral blood of cancer patients. Given the relation between cancer incidence and age, this study examined the frequency of peripheral blood CD33(+)HLA-DR(−) MDSCs across three cohorts: healthy adults (19–59 years old), community-dwelling seniors (61–76 years old), and frail elderly (67–99 years old). This analysis is the first to demonstrate that MDSCs and specifically the CD11b(+)CD15(+) MDSC subset are increased with age. Proinflammatory cytokines that are required for the differentiation of MDSCs (e.g., TNF-α, IL-6, and IL-1β) were similarly found to be increased in the serum of the frail elderly. Furthermore, the proportion of MDSCs and the CD11b(+)CD15(+) subset were found to be elevated significantly in elderly donors with a history of cancer. This age-related elevation in the frequency of MDSCs may contribute to the increased cancer incidence that occurs with age. Further investigation into the functional consequences of elevated MDSCs will provide valuable insight into the progression of age-related pathologies.
Introduction
Profound changes occur to the proportions and phenotype of peripheral white blood cells with age and correlate with age-related pathologies, such as Alzheimer's and Parkinson's diseases [1] and rheumatoid arthritis [2]. Whereas reports of alterations with age for specific subsets of monocytes, DCs, T/B lymphocytes, and NK cells are fairly common (reviewed in ref. [3]), there is a paucity of studies examining rare yet functionally relevant populations, such as the recently discovered myeloid-derived suppressor cell (MDSC).
MDSCs are a heterogeneous population of immunosuppressive, myeloid lineage cells in the peripheral blood [4]. In mice, these cells are defined as monocytic (CD11b+Ly6G−Ly6Chigh) or granulocytic (CD11b+LyG6+Ly6Clow), both of which paradoxically express arginase and iNOS, and are suppressive of T cell function [4]. The analogous human population is also suppressive to T cell proliferation, expresses arginase-1 and iNOS, and is commonly defined as CD33+HLA-DR− and lineage (CD3, CD19, CD56)-negative [5]. Some studies also describe these cells as expressing CD11b and similarly, subdivide them into monocytic (CD14+) and granulocytic (CD15+) categories [6, 7]. Readily identified in the blood of healthy adults [7], MDSCs can also be induced in vitro by proinflammatory cytokines or tumor cell conditioned media [8].
The majority of observational studies comparing MDSC frequency does so in relation to cancers, such as renal and lung carcinoma, of which CD33+HLA-DR−CD11b+ [9–12] and CD33+HLA-DR− [13, 14] populations are found to be significantly increased. Furthermore, MDSC frequency has also been found to be associated with clinical scores, such as tumor stage, burden, and metastasis [10, 15]. Enioutina and colleagues [16] recently reported increased levels of tissue and circulating blood MDSCs in aged mice; however, this has yet to be shown in aged humans. Similar findings in humans would be apt, given that average cancer mortality rate [17] and cancer incidence [18] are known to increase with age.
In addition to cancer as a common underlying theme, a chronic inflammatory environment bridges MDSCs and aging. Proinflammatory cytokines, such as TNF-α and IL-6, are known to be increased in the serum of the elderly and also correlate with the incidence of myriad age-related pathologies, such as cardiovascular disease, cognitive decline, cancer, and general physical disability (reviewed in ref. [19]). Furthermore, serum IL-6 has been shown to be correlated with MDSC frequency in patients with gastrointestinal cancer [20], and as mentioned above, proinflammatory cytokines are able to induce the differentiation of MDSCs in vitro [5, 8, 21].
To determine whether advanced age is associated with the frequency of circulating MDSCs, the following study examined a cohort of adults (19–59 years old; n=41), community-dwelling seniors (61–76 years old; n=45), and frail elderly (67–99 years old; n=131). This cross-sectional design allows for the analysis of each cellular subset within three immunologically relevant life stages, from young adulthood into advanced age, when frailty and the need for assisted living can be expected [22, 23]. Our analysis indicates that MDSCs and the CD11b+CD15+ MDSC subset are increased with age. Interestingly, proinflammatory cytokines, such as TNF-α, IL-6, and IL-1β, related to differentiation of MDSCs, were increased in the serum of the frail elderly. Furthermore, the proportion of MDSCs and the CD11b+CD15+ subset was found to be elevated significantly in elderly donors with a history of cancer.
MATERIALS AND METHODS
Healthy adults (n=41, median age=32 years, range 19–59, male:female 19:22) and community-dwelling seniors (n=45, median age=69 years, range 61–76, male:female 15:30) were recruited from Hamilton, Ontario, Canada, between November and May 2012. Frail elderly participants (n=131, median age=87 years, range 67–99, male:female 34:97) were recruited from five local nursing homes between November 2011 and January 2012, and all were vulnerable based on the Frail Scale [24]. Individuals on immunosuppressive medication or chemotherapy or undergoing radiation therapy were excluded. PBMCs were isolated from heparinized blood by ficoll-density centrifugation and cryopreserved at −140°C in human AB serum (Lonza, Basel, Switzerland) and 10% DMSO. These studies were approved by the McMaster Research Ethics Board, and written, informed consent was obtained for all participants.
Antibody staining was performed as described previously [25] using a Biomek NXP Laboratory Automation Workstation (Beckman Coulter, Brea, CA, USA) and analyzed using a BD LSR II (BD Biosciences, San Jose, CA, USA). CountBright absolute counting beads (Life Technologies, Ontario, Canada) were used to calculate the frequency of CD45+ cells. Antibodies included: CD33-FITC, CD15-PE-Cy7, CD11bAPC, CD3-PE, CD19-PE, and CD56-PE (BD Biosciences, Ontario, Canada); CD14-Pacific Blue (BioLegend, San Diego, CA, USA); and HLA-DR-PerCp-Cy5.5 and CD45-eFluor605NC (eBioscience, San Diego, CA, USA). With the exception of CD45+ leukocytes, all cells were lineage (CD3, CD19, CD56)-negative. Fluorescent-minus-one and isotype controls were used for gating, as represented in Supplemental Fig. 1. No significant differences in CD45+ leukocytes were identified between the age groups; thus, all other subpopulations are presented as the percentage of CD45-expressing leukocytes. All analyses were performed in FlowJo 7.6.4 (Tree Star, Ashland, OR, USA).
Serum cytokine levels in cryopreserved serum from sex-matched adults (22–45 years old; n=10) and frail elderly (68–91 years old; n=30) donors were measured using the proinflammatory 7-Plex ELISA (Meso Scale Discovery, Rockville, MD, USA), accordingly, to the manufacturer's instructions.
CD33+ cells were isolated from fresh PBMCs by positive selection using EasySep magnetic-bead purification, whereas monocytes were isolated by negative selection (Stem Cell Technologies, Brtish Columbia, Canada). Histological assessment was performed by Shandon CytoSpin centrifugation, followed by modified Wright-Giemsa staining (Accustain; Sigma, St. Louis, MO, USA). For qPCR, RNA was extracted by the illustra RNA Mini Spin kit (GE Healthcare Life Sciences, Pittsburgh, PA, USA), followed by cDNA synthesis (Superscript II, Life Technologies) and analysis using GoTaq qPCR Master Mix (Promega, Madison, WI, USA). Primers can be found in the Supplemental information.
All statistical analyses were performed in R 2.11.1 (R Development Core Team, 2011). For immunophenotyping, age groups were compared by linear regression on log-transformed values, adjusting for sex. Resultant P values were adjusted for multiple testing using the Benjamini-Hochberg procedure. Log transformation was deemed necessary to approximate normality, according to Pearson's χ2 normality test. Age-stratified, community-dwelling seniors and frail elderly were compared by linear regression on log-transformed values, adjusting for sex. Associations to cancer history were derived using linear regression on log-transformed values, adjusting for age, sex, and cohort. For serum cytokine analysis, age groups were compared by the Kruskal-Wallis test with adjustments for multiple testing by FDR.
RESULTS AND DISCUSSION
Although it has been demonstrated that the elderly have altered profiles of circulating myeloid cells, the frequency of circulating MDSCs has not been investigated. To determine whether MDSC frequency changes with age, peripheral blood was collected from a cohort of healthy adults (aged 19–59 years), community-dwelling seniors (aged 61–76 years old), and advanced age, frail elderly (aged 67–99 years). The latter group is crucial to provide a realistic model of our aging population, as for individuals 85 and older, nearly 20% reside in nursing homes, and approximately one-half can be considered physically frail [22, 23]. Frailty is considered a reliable marker of immune health in the elderly [26] and has been linked to chronic, low-grade inflammation [27] and alterations in leukocyte function [28] and frequency [29]. As an association between frailty and MDSC levels has yet to be investigated, a cross-sectional study was performed in age-stratified senior and frail elderly donors.
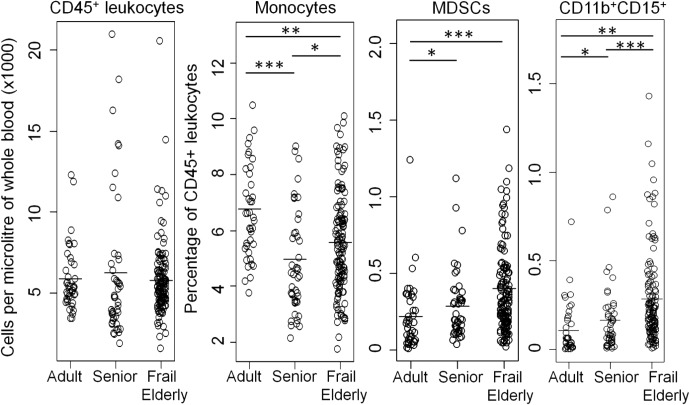
The absolute number of CD45+ leukocytes was not altered across adults, seniors, and frail elderly donors, whereas the proportion of total monocytes was reduced with age, as has been demonstrated previously [30, 31] (Fig. 1). Two populations of MDSCs were measured: the parent CD33+HLA-DR− population, as well as the CD33+HLA-DR−CD11b+CD15+ subset. MDSCs (CD33+HLA-DR−) and the CD11b+CD15+ MDSC subset were found to be increased significantly in seniors (0.29±0.03 and 0.17±0.03, respectively) and the frail elderly (0.40±0.03 and 0.29±0.02, respectively) as compared with healthy adults (0.22±0.03 and 0.11±0.03, respectively; Fig. 1). As many studies on MDSC frequency have been performed in PBMCs, we also compared PBMCs from a subset of adults and frail elderly. As expected, similar, significant trends were observed for MDSCs and the CD11b+CD15+ subset (Supplemental Fig. 2). We characterized the CD33+ fraction of PBMCs to further substantiate the phenotype of leukocytes being defined as MDSCs. Indeed, CD33+ PBMCs were observed to be enriched for cells with a nuclear staining pattern (Supplemental Fig. 3A) and expression profile (Supplemental Fig. 3B), described previously to be characteristic of human MDSCs [5, 7, 8, 32–34]. No significant differences were identified for the frequency of CD33+HLA-DR− MDSCs between seniors and frail elderly of similar age, but the CD11b+CD15+ subset was increased significantly in the frail elderly when compared with aged-matched seniors (Table 1). This suggests a potential effect of nursing home residence or frailty in MDSC frequency. However, as a result of the experimental design and patient consent for the present study, the exact nature of this association is unknown.
Figure 1. MDSCs and CD11b+CD15+ MDSCs are increased with age.
The frequency of CD45+ leukocytes and proportions of monocytes, MDSCs, and the CD11b+CD15+ subsets were measured in adults, community-dwelling seniors, and frail elderly. Comparisons were performed by linear regression on log-transformed values, adjusting for sex, with P values adjusted to control for FDR. ***P < 0.001; **P < 0.01; *P < 0.05.
Table 1. Comparison of Age-Stratified Senior and Frail Elderly Donors and Associations with Previous Cancer Status.
| Characteristic | Seniors | Frail elderly | Pval | Previous cancer status |
Pval | |
|---|---|---|---|---|---|---|
| Negative | Positive | |||||
| Sample size | 24 | 26 | 148 | 23 | ||
| Age range (median) | 68–76 (71) | 67–80 (74) | 61–99 (84) | 68–99 (86) | ||
| CD45+ leukocytes | 6811 ± 961 | 5440 ± 298 | NS | 5844 ± 257 | 6077 ± 471 | NS |
| Monocytes | 5.29 ± 0.41 | 4.55 ± 0.30 | NS | 5.42 ± 0.37 | 5.45 ± 0.16 | NS |
| MDSCs | ||||||
| CD33+HLA-DR− | 0.29 ± 0.04 | 0.33 ± 0.05 | NS | 0.35 ± 0.02 | 0.56 ± 0.10 | 0.031 |
| CD11b+CD15+ subset | 0.16 ± 0.04 | 0.24 ± 0.05 | 0.046 | 0.23 ± 0.02 | 0.44 ± 0.09 | 0.030 |
Means and se are presented. CD45+ leukocytes are presented as per microliter of whole blood, whereas values for all other subsets are relative to values established for CD45+ leukocytes. Comparisons were performed by linear regression on log-transformed values, adjusting for age, sex, and cohort (where applicable, see Materials and Methods). Comparison-wise P values (Pval) are presented.
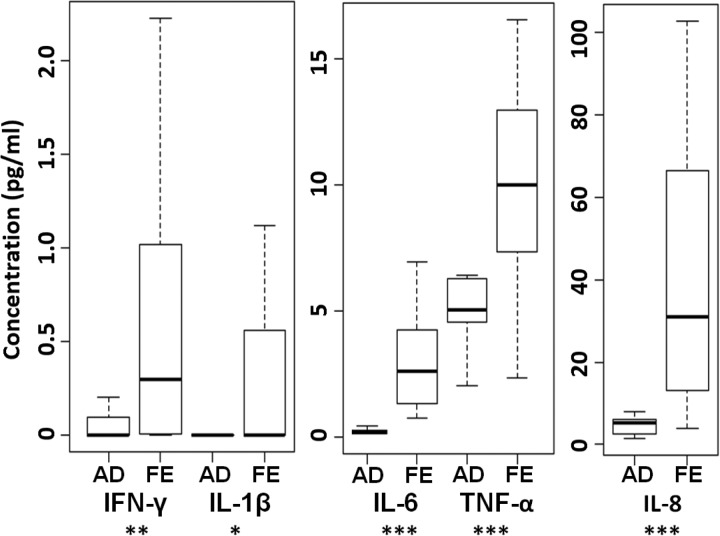
Lechner and colleagues [5] showed that proinflammatory cytokines, namely IL-6, IL-1β, and TNF-α, are able to induce differentiation of MDSCs in combination with GM-CSF. With age, circulating levels of IL-6, IL-1β, and TNF-α increase, and this is commonly referred to as “inflammaging” (reviewed in ref. [35]). Although it is yet to be reported that levels of serum GM-CSF change with age, it is feasible that chronically elevated levels of TNF-α and IL-6 contribute to the frequency of circulating MDSCs. As there are no previous reports regarding the serum cytokine profile of the frail elderly, we compared a sub-sample of our cohort to sex-matched, healthy adult donors using a highly sensitive, multiplexed ELISA. Of the proinflammatory cytokines assayed, IFN-γ, IL-1β, IL-6, TNF-α, and IL-8 were elevated significantly in the frail elderly (Fig. 2), whereas IL-12p70 was not significantly different (data not shown). It is feasible that MDSC frequency is influenced by the proinflammatory serum microenvironment observed in aged and frail individuals; however, larger studies are needed to test this theory.
Figure 2. Serum concentrations of proinflammatory cytokines are increased in the frail elderly (FE; n=30, average age=80) relative to adult donors (AD; n=10, average age=31).
For IFN-γ, 60% of adult donors did not meet the limit of detection; for IL-1β, all of the adults and 60% of frail elderly donors did not meet the limit of detection. Differences determined by the Kruskal-Wallis test with adjustments to control for FDR. ***P < 0.001; **P < 0.01; *P < 0.05.
MDSCs are studied primarily in the context of cancer, and as cancer incidence [18] and mortality rates [17] increase with age, we compared MDSC levels of elderly donors with and without a history of cancer. Of the 171 community-dwelling and frail elderly donors, 23 had a history of cancer, and the most common sites included breast (26%), lung (17%), prostate (17%), skin (9%), and colon (9%). At the time of this study, all were in partial or complete remission. After adjusting for age, sex, and cohort, the frequency of CD33+HLA-DR− MDSCs and the CD11b+CD15+ subsets was found to be increased significantly in donors with a history of cancer (Table 1). To our knowledge, this is the first study to show that circulating levels of MDSCs remain increased for individuals in cancer remission. Whether the increase in cancer incidence and mortality with age is related to an elevation in MDSCs or whether MDSC levels remain elevated after an individual is in remission warrants further investigation. It is unclear, for example, whether elevated levels of MDSCs are resolved during remission of all cancers, as a recent study on patients with large B cell lymphoma demonstrated that the elevated levels of MDSCs observed during cancer returned to normal following successful treatment [36]. The age range of patients in this study was broad (20–89 years old), and hence, our observations of MDSC levels remaining elevated after remission may be a phenomenon confined to the elderly.
In summary, the present study has shown novel associations for peripheral blood MDSCs in the elderly. Not only are these cells increased with age, but also, they are elevated in aged individuals with a history of cancer. As CD11b+ MDSC subsets have been shown to be potently suppressive of T cell proliferation and readily produce ROS upon stimulation, it is possible that CD11b+CD15+ MDSCs are prominent contributors to the depression of adaptive T cell responses [37, 38] and increases in oxidative damage by ROS (reviewed in ref. [39]) that are known to occur with age. CD15+ MDSC subsets are major producers of ROS [7], and hence, the significant increase of this subset in the frail elderly as compared with similarly aged community-dwelling seniors may contribute to the elevated levels of superoxide anion (a major species of ROS) observed in the frail elderly [27]. Furthermore, given the immunosuppressive nature of these cells, complementary studies investigating their role in age-associated immune decline are essential.
Supplementary Material
ACKNOWLEDGMENTS
Funding for this study was supported by the CIHR, the IIDR, and the McMaster Immunology Research Centre. C.P.V. is funded by the 2011 M. G. DeGroote and 2012 Canadian Lung Association (Canadian Thoracic Society) postdoctoral fellowships. J.J. receives salary support from CIHR.
The authors acknowledge Amy Bartholomew for nursing assistance and Dr. Frédéric Geissmann for advice on the immunophenotyping protocol.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- CIHR
- Canadian Institutes of Health Research
- FDR
- false discovery rate
- IIDR
- Michael G. DeGroote Institute for Infectious Disease Research
- MDSC
- myeloid-derived suppressor cell
- qPCR
- quantitative PCR
AUTHORSHIP
C.P.V. designed experiments, performed all research and analyses, and wrote the manuscript. J.J. organized and managed the nursing home cohorts and edited the final manuscript. J.M. performed flow cytometry staining. M.G.D., M.H., and A.L. performed research. M.L. organized and managed the nursing home cohorts, edited the final manuscript, and provided research funding. J.L.B. provided facilities, edited the final manuscript, and provided research funding. D.M.E.B. supervised all research, designed experiments, edited the manuscript, and provided research funding.
REFERENCES
- 1. Rosenkranz D., Weyer S., Tolosa E., Gaenslen A., Berg D., Leyhe T., Gasser T., Stoltze L. (2007) Higher frequency of regulatory T cells in the elderly and increased suppressive activity in neurodegeneration. J. Neuroimmunol. 188, 117–127 [DOI] [PubMed] [Google Scholar]
- 2. Korkosz M., Bukowska-Strakova K., Sadis S., Grodzicki T., Siedlar M. (2012) Monoclonal antibodies against macrophage colony-stimulating factor diminish the number of circulating intermediate and nonclassical (CD14(++)CD16(+)/CD14(+)CD16(++)) monocytes in rheumatoid arthritis patient. Blood 119, 5329–5330 [DOI] [PubMed] [Google Scholar]
- 3. Desai A., Grolleau-Julius A., Yung R. (2010) Leukocyte function in the aging immune system. J. Leukoc. Biol. 87, 1001–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gabrilovich D. I., Nagaraj S. (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lechner M. G., Liebertz D. J., Epstein A. L. (2010) Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J. Immunol. 185, 2273–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Greten T. F., Manns M. P., Korangy F. (2011) Myeloid derived suppressor cells in human diseases. Int. Immunopharmacol. 11, 802–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kotsakis A., Harasymczuk M., Schilling B., Georgoulias V., Argiris A., Whiteside T. L. (2012) Myeloid-derived suppressor cell measurements in fresh and cryopreserved blood samples. J. Immunol. Methods 381, 14–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lechner M. G., Megiel C., Russell S. M., Bingham B., Arger N., Woo T., Epstein A. L. (2011) Functional characterization of human Cd33+ and Cd11b+ myeloid-derived suppressor cell subsets induced from peripheral blood mononuclear cells co-cultured with a diverse set of human tumor cell lines. J. Transl. Med. 9, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu C. Y., Wang Y. M., Wang C. L., Feng P. H., Ko H. W., Liu Y. H., Wu Y. C., Chu Y., Chung F. T., Kuo C. H., Lee K. Y., Lin S. M., Lin H. C., Wang C. H., Yu C. T., Kuo H. P. (2010) Population alterations of L-arginase- and inducible nitric oxide synthase-expressed CD11b+/CD14(−)/CD15+/CD33+ myeloid-derived suppressor cells and CD8+ T lymphocytes in patients with advanced-stage non-small cell lung cancer J. Cancer Res. Clin. Oncol. 136, 35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Diaz-Montero C. M., Salem M. L., Nishimura M. I., Garrett-Mayer E., Cole D. J., Montero A. J. (2009) Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol. Immunother. 58, 49–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kusmartsev S., Su Z., Heiser A., Dannull J., Eruslanov E., Kubler H., Yancey D., Dahm P., Vieweg J. (2008) Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clin. Cancer Res. 14, 8270–8278 [DOI] [PubMed] [Google Scholar]
- 12. Srivastava M. K., Bosch J. J., Thompson J. A., Ksander B. R., Edelman M. J., Ostrand-Rosenberg S. (2008) Lung cancer patients' CD4(+) T cells are activated in vitro by MHC II cell-based vaccines despite the presence of myeloid-derived suppressor cells. Cancer Immunol. Immunother. 57, 1493–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fricke I., Mirza N., Dupont J., Lockhart C., Jackson A., Lee J. H., Sosman J. A., Gabrilovich D. I. (2007) Vascular endothelial growth factor-trap overcomes defects in dendritic cell differentiation but does not improve antigen-specific immune responses. Clin. Cancer Res. 13, 4840–4848 [DOI] [PubMed] [Google Scholar]
- 14. Ko J. S., Zea A. H., Rini B. I., Ireland J. L., Elson P., Cohen P., Golshayan A., Rayman P. A., Wood L., Garcia J., Dreicer R., Bukowski R., Finke J. H. (2009) Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin. Cancer Res. 15, 2148–2157 [DOI] [PubMed] [Google Scholar]
- 15. Sun H. L., Zhou X., Xue Y. F., Wang K., Shen Y. F., Mao J. J., Guo H. F., Miao Z. N. (2012) Increased frequency and clinical significance of myeloid-derived suppressor cells in human colorectal carcinoma. World J. Gastroenterol. 18, 3303–3309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Enioutina E. Y., Bareyan D., Daynes R. A. (2011) A role for immature myeloid cells in immune senescence. J. Immunol. 186, 697–707 [DOI] [PubMed] [Google Scholar]
- 17. Yang Y., Li T., Nielsen M. E. (2012) Aging and cancer mortality: dynamics of change and sex differences. Exp. Gerontol. 47, 695–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thomas G. A., Leonard R. C. (2009) How age affects the biology of breast cancer. Clin. Oncol. (R. Coll. Radiol.) 21, 81–85 [DOI] [PubMed] [Google Scholar]
- 19. Singh T., Newman A. B. (2011) Inflammatory markers in population studies of aging. Ageing Res. Rev. 10, 319–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mundy-Bosse B. L., Young G. S., Bauer T., Binkley E., Bloomston M., Bill M. A., Bekaii-Saab T., Carson W. E., III, Lesinski G. B. (2011) Distinct myeloid suppressor cell subsets correlate with plasma IL-6 and IL-10 and reduced interferon-α signaling in CD4(+) T cells from patients with GI malignancy. Cancer Immunol. Immunother. 60, 1269–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Volk A. P., Barber B. M., Goss K. L., Ruff J. G., Heise C. K., Hook J. S., Moreland J. G. (2011) Priming of neutrophils and differentiated PLB-985 cells by pathophysiological concentrations of TNF-α is partially oxygen dependent. J. Innate Immun. 3, 298–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. U.S. Census Bureau (2001) The 65 Years and Older Population: 2000. Census 2000 Brief. U.S. Census Bureau, Washington, DC [Google Scholar]
- 23. Rockwood K., Song X., Mitnitski A. (2011) Changes in relative fitness and frailty across the adult lifespan: evidence from the Canadian National Population Health Survey. CMAJ 183, E487–E494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rockwood K., Abeysundera M. J., Mitnitski A. (2007) How should we grade frailty in nursing home patients? J. Am. Med. Dir. Assoc. 8, 595–603 [DOI] [PubMed] [Google Scholar]
- 25. Fung E., Esposito L., Todd J. A., Wicker L. S. (2010) Multiplexed immunophenotyping of human antigen-presenting cells in whole blood by polychromatic flow cytometry. Nat. Protoc. 5, 357–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Van den Biggelaar A. H., Huizinga T. W., de Craen A. J., Gussekloo J., Heijmans B. T., Frolich M., Westendorp R. G. (2004) Impaired innate immunity predicts frailty in old age. The Leiden 85-plus study. Exp. Gerontol. 39, 1407–1414 [DOI] [PubMed] [Google Scholar]
- 27. Baptista G., Dupuy A. M., Jaussent A., Durant R., Ventura E., Sauguet P., Picot M. C., Jeandel C., Cristol J. P. (2012) Low-grade chronic inflammation and superoxide anion production by NADPH oxidase are the main determinants of physical frailty in older adults. Free Radic. Res. 46, 1108–1114 [DOI] [PubMed] [Google Scholar]
- 28. Qu T., Walston J. D., Yang H., Fedarko N. S., Xue Q. L., Beamer B. A., Ferrucci L., Rose N. R., Leng S. X. (2009) Upregulated ex vivo expression of stress-responsive inflammatory pathway genes by LPS-challenged CD14(+) monocytes in frail older adults. Mech. Ageing Dev. 130, 161–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jing Y., Shaheen E., Drake R. R., Chen N., Gravenstein S., Deng Y. (2009) Aging is associated with a numerical and functional decline in plasmacytoid dendritic cells, whereas myeloid dendritic cells are relatively unaltered in human peripheral blood. Hum. Immunol. 70, 777–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hearps A. C., Martin G. E., Angelovich T. A., Cheng W. J., Maisa A., Landay A. L., Jaworowski A., Crowe S. M. (2012) Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell 11, 867–875 [DOI] [PubMed] [Google Scholar]
- 31. De M. M., Modesti M., Ginaldi L. (2004) Phenotypic and functional changes of circulating monocytes and polymorphonuclear leucocytes from elderly persons. Immunol. Cell Biol. 82, 415–420 [DOI] [PubMed] [Google Scholar]
- 32. Lin Y., Gustafson M. P., Bulur P. A., Gastineau D. A., Witzig T. E., Dietz A. B. (2011) Immunosuppressive CD14+HLA-DR(low)/− monocytes in B-cell non-Hodgkin lymphoma. Blood 117, 872–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Obermajer N., Muthuswamy R., Lesnock J., Edwards R. P., Kalinski P. (2011) Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood 118, 5498–5505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhao F., Hoechst B., Duffy A., Gamrekelashvili J., Fioravanti S., Manns M. P., Greten T. F., Korangy F. (2012) S100A9 a new marker for monocytic human myeloid-derived suppressor cells. Immunology 136, 176–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Salminen A., Kaarniranta K., Kauppinen A. (2012) Inflammaging: disturbed interplay between autophagy and inflammasomes. Aging (Albany. NY) 4, 166–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tadmor T., Fell R., Polliack A., Attias D. (2012) Absolute monocytosis at diagnosis correlates with survival in diffuse large B-cell lymphoma—possible link with monocytic myeloid-derived suppressor cells. Hematol. Oncol. doi: 10.1002/hon.2019 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37. Zhou X., McElhaney J. E. (2011) Age-related changes in memory and effector T cells responding to influenza A/H3N2 and pandemic A/H1N1 strains in humans. Vaccine 29, 2169–2177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tortorella C., Loria M. P., Piazzolla G., Schulze-Koops H., Lipsky P. E., Jirillo E., Antonaci S. (1998) Age-related impairment of T cell proliferative responses related to the decline of CD28+ T cell subsets. Arch. Gerontol. Geriatr. 26, 55–70 [DOI] [PubMed] [Google Scholar]
- 39. Zou H., Stoppani E., Volonte D., Galbiati F. (2011) Caveolin-1, cellular senescence and age-related diseases. Mech. Ageing Dev. 132, 533–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.